Some Metal Elements Concentrations in Snout Fish (Mormyrus rume: Valenciennes 1847) from Obubra Region of Cross River, Cross River State, Nigeria
1Department of Fisheries and Aquatic Science , Faculty of Agriculture and Forestry, Cross River University of Technology, Calabar, PMB 102 Obubra Campus, Obubra Cross River State. Nigeria
2College of Fisheries, Henan Normal University, Xinxiang, 453007, P.R. China
- *Corresponding Author:
- Fidelis Bekeh Ada
Department of Fisheries and Aquatic Science
Faculty of Agriculture and Forestry
Cross River University of Technology
Calabar, PMB 102 Obubra Campus
Obubra Cross River State, Nigeria
Tel: +234 9096417140
E-mail: fbekehada@yahoo.com
Received date: August 07, 2018; Accepted date: June 05, 2018; Published date: June 12, 2018
Citation: Ada FB (2018) Some Metal Elements Concentrations in Snout Fish (Mormyrus rume: Valenciennes 1847) from Obubra Region of Cross River, Cross River State, Nigeria. Insights Aquac Cult Biotechnol Vol.2 No.1:4
Abstract
Mormyridae are important in Fisheries in Nigeria. Twenty six species are found in Nigerian waters and the largest of the species, Mormyrus rume, capable of growing up to 100 cm is common in the middle reaches of Cross River. Being common in the catches, heavy metal concentrations in its tissues were investigated in order to find out if the concentrations are above permissible levels for human consumption. Fish specimens were obtained from local fishers that fish with gill net, cast net as well as local traps. The gills, muscle, liver, heart and spleen were dissected out for analysis of metal concentrations. Metals analysed were lead, aluminium, zinc, iron, cadmium, chromium, cobalt and copper. Lead and aluminium were not detectable in any of the tissues. The highest mean concentrations of zinc was 0.042 ppm, iron was 0.12 ppm, chromium was 0.031 ppm, cobalt was 0.032 ppm, copper was 0.035 ppm and cadmium was 0.055 ppm. Statistical analysis showed variations in concentrations of metals in various tissues. All these were lower than maximum permissible levels in foods by World Health Organisation (WHO), Federal Environmental Protection Agency of Nigeria (FEPA) and United State Environmental Protection Agency (USEPA). Although this fish leads benthic lifestyle, where these metals are expected at higher concentrations, it was seen not to bio accumulate heavy metals to intolerant levels.
Keywords
Heavy metal; Mormyrus rume; Middle Cross River; Fisheries
Introduction
Twenty six species of the family Mormyridae occur all over Nigeria. Out of these, eight (namely: M. rume, Petrocephalus simus, Mormyrops deliciosus, Isichthys henryi, Gnathonamus petersi, Gnathonamus deboensus, and Marcusenus branchyistius) are found in Cross River system [1,2]. M. rume has seventy two to ninety fin rays in the dorsal fin and sixteen to twenty one rays in the anal fin (D72-96, A16-21). It has a trunk like snout [3,4]. Mormyridae are common in most West African rivers. The fish is distributed in Gambia, Senegal, Niger, Nigeria, Volta and Chad basins. Fishbase.org reported that the fish can be found in Sahelo-Soudanian basins and the coastal basins of rivers Cavally, Bandama, Sassandra, Comoe, Mono, Oueme, Ogun and Culufi. It has two sub species; M. rume found in West Africa, Chad System and M. rume proboscirostris found in Congo River. M. Rume is harvested in the Cross River where it forms a high proportion of catch. It can grow to 1000 mm and is the largest member of the mormyridae family. It has a fine taste and has few bones. The bones are soft and easy to eat. It is highly priced and preferred by the common population. The fish forms a high proportion in the catch of artisan fishers in the Cross River, therefore in the diet of the people. Based on the plausible attribute listed above, M. rume needs to be seriously investigated since it is heavily consumed in the region. So an investigation into the level of heavy metal concentration in its tissues needs be carried out to point out if the concentration is above tolerant limits since it is highly consumed by the people of the region. Given that is highly consumed, it may be seen as a candidate for Aquaculture in the Cross River Basin and Nigeria as a whole. If the level of metals concentration is known, this may be compared with values elsewhere including aquaculture enclosures in future studies. At present, results of this work will inform the consumers on its safety with respect to heavy metals toxicity.
Metals with specific gravity of more than five times that of water at temperature 1°C are called heavy metals [5].There are 35 metals that concern us and 23 of these are heavy metal: antimony (121.75), arsenic (74.9216), bismuth (208.9806), cadmium (112.40), cerium (140.12), chromium (51.996), cobalt (58.9332), copper (63.546), gallium (69.72), gold (196.9665), iron (55.847), lead (207.2), manganese (54.9380), mercury (200.59), nickel (58.71), platinum (195.09), silver (107.868), tellurium (127.60), thallium (204.37), tin (118.69), uranium (238.029), vanadium (50.9414) and zinc (65.37) [6-8]. Heavy metals are hardly metabolised and are often bio accumulated. Although some heavy metals are non-toxic and found in many bio-systems where they enhance physiological reaction as well as forming components of many important pigments, others are highly toxic [9,10]. Some metals like Cobalt (Co), Copper (Cu), Chromium (Cr), Iron (Fe), Magnesium (Mg), Manganese (Mn), Molybdenum (Mo), Nickel (Ni), Selenium (Se) and Zinc (Zn) are useful in biological systems [11-13]. Some of these metals are not only useful in organic systems; they are equally used in many manufacturing processes in industries which form their route of entry into aquatic ecosystems. At above optimum concentrations, even the useful metals become toxic. For instance, Lead is highly poisonous but it is used as a radiation shield around X-ray equipment. It is part of electroplating materials, pesticides, batteries, alloys, textile dyes and steel [14]. Most heavy metal distribution and contamination are caused by anthropogenic activities. They are found occurring naturally. They have been found to cause health hazard in aquatic organisms and in man. Some of them form the top of the list of most harmful substances in the world, namely arsenic (1), lead (2), mercury (3), and cadmium (7) [15]. Heavy metals generally pose pollution problems because of their ability to bio accumulate; they are persistent due to their non-degradable nature [16]. Intake of heavy metals into aquatic fauna is principally through tissues with contact with the ambient water and Often through these are the gills, skin and gut [17]. Oguzie noted that diffusion facilitated absorption is a pathway of metals uptake [18].
Materials and Methods
Study area
Cross River has origin from the Cameroons [19]. Its course goes through Itung, Ikom, Obubra, Biase, Akamkpa, Odukpani and Calabar local governments of Cross River State (Globally, it is located at 7o30'-10o00' E and 4o00'-8o00' N.). It supplies water for domestic and Agricultural uses. It provides food in form of aquatic resources to the riparian population living in its basin. The middle reaches of the river is in Obubra Local Government of Cross River State where it forms high proportion of catch by artisan fishers.
Sampling
Samples were taking from landings of the local fishers who use available gears in the area. These gears include gill nets, cast nets, hooks and traps (baited and unbaited) [20-22]. Three beaches in the middle reaches of the river were sampled. These are Apiapum, Beach, Ovonum Beach and Ahaha Beach. Three specimens each were bought from each beach in each of the three visits which was carried out bimonthly from March to August of 2016. Specimens were dissected to remove desired tissues. These were acidified to below pH 2 with concentrated HNO3 in wait for metal analysis [23].
Metal element analysis
The dissected tissues were oven dried at 105°C using (MAMMART) to constant weight. These were grounded using mortar and pestle to fine powder. The powders were sieved using a 2 mm mesh size filter. Samples were digested in 0.02 M HNO3 and HCl in the ratio of 1: 3 at 80°C in a fume cupboard. Samples were analysed for metal elements (Pb, Al, Zn, Fe, Cd, Cr Co and Cu) concentrations using atomic absorption spectrophotometer (AAS MODEL: GBC- 902 Australia [17,24,25]. Statistical analysis was performed using analysis of variance (ANOVA) in a computer software SPSS (statistics for political and social sciences) version 16. The post hoc analysis was carried out using Dumcan’s multiple range tests.
Results
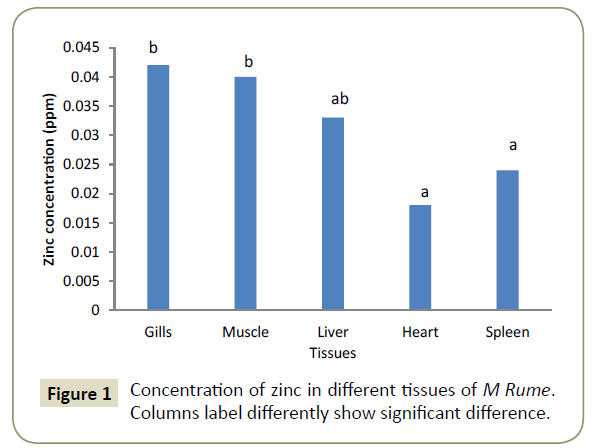
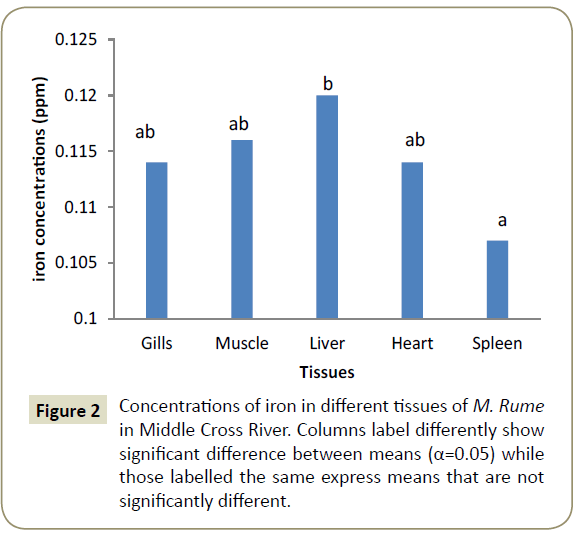
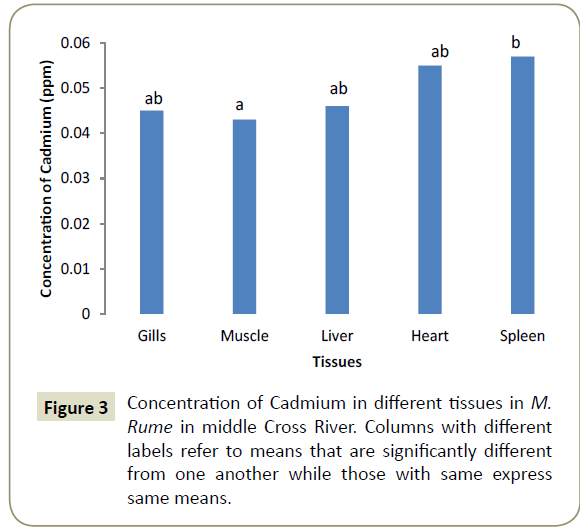
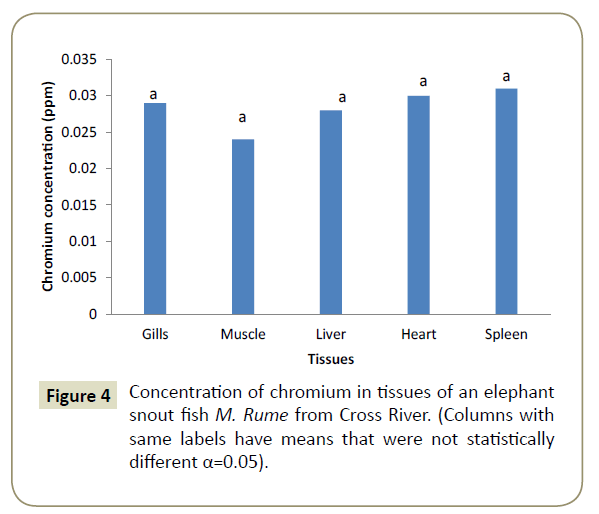
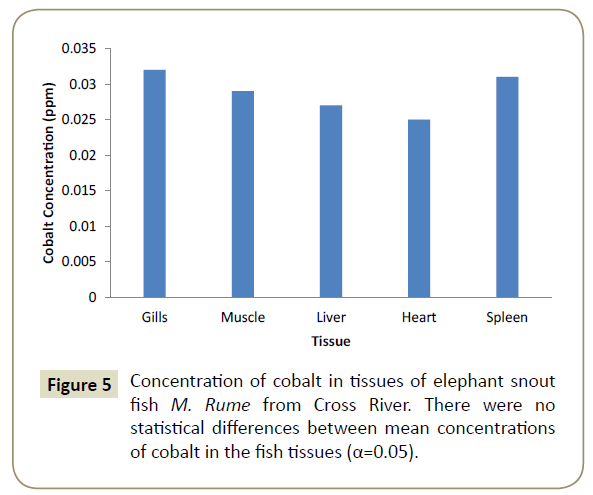
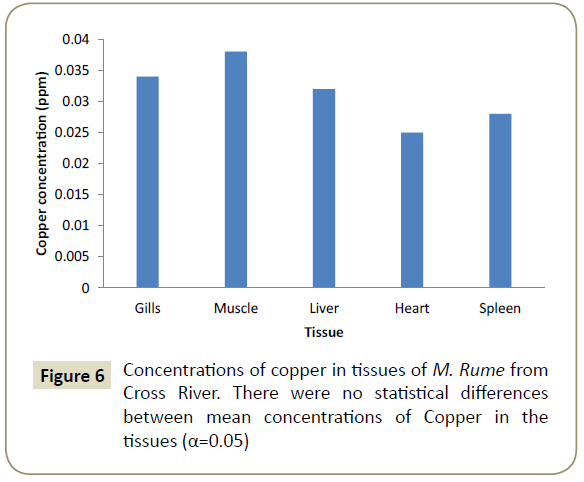
Observations showed that lead and aluminium were not detected in any of the tissues analysed for heavy metals. Zinc showed significant variation (α=0.05) in concentration from one tissue to another and was in the range of 0.024 ppm in the heart (cardiac tissue to 0.042 ppm in gills). This is shown pictorially in Figure 1. Similarly, iron was differentially concentrated (α=0.05) in the different tissues with highest concentration of 0.117 ppm in liver and lowest concentration of 0.107 ppm in spleen (Figure 2). Cadmium also showed significant differences in concentration (α=0.05) in the different fish tissues of the fish ranging from 0.042 ppm in muscles to 0.055 ppm in heart tissues as displayed in Figure 3. Chromium, Cobalt and Copper showed concentrations that were not significantly different (α=0.05) in different tissues. The concentration of chromium was between 0.024 ppm in muscle to 0.037 in liver as shown on Figure 4, while Figures 5 and 6 shows the concentrations of cobalt and copper.
Discussion
In this work, Lead (Pb) and aluminium (Al) were below detectable limit in the organs of Mormyrus species. This may not be necessarily due to their absence in the water ecosystem, because in an earlier experiment, Ayotunde et al. found lead in tissues of Chrysichthys nigrodigitatus ranging from 0.0 to 0.067 ppm. It is worthwhile to note that tissue accumulation of metals equally depends on the niche of the aquatic organisms and not only dependent on the presence of concentration of the metal in the ecosystem [26]. Metal concentration levels in tissues of fish is also dependent on ecological needs, chemical characteristics of water, fish feeding habits and season of sampling [17]. Season is particularly important because concentration is a resultant of intake and excretion [27].
Zinc is used for activation of several enzymes including carbonic anhydrase. It is needed for the production of insulin [28]. Concentrations as low as 1.0 ppm are capable of causing mortality in fresh water fish especially in poorly aerated water as reported by Nemesok et al. [29] The concentration found in various tissues of this fish in Cross River Central (Obubra) ranged between 0.018 ppm in the heart, 0.024 ppm in spleen, 0.033 ppm in the liver, 0.04 in muscle (myotomes) and 0.042 in the gills.
Iron has biological functions in tissues of organisms. It is the back bone of the haemoglobin a pigment that serves as vehicle for transport of gases in the body. Iron was seen to be more concentrated the liver (1.2 ppm compared to other organs. This is not strange, for the liver acts as a detoxifying centre. Old blood cells are destroyed in the liver, breaking down haemoglobin into components namely an iron globin complex and an iron less haem group. The Iron globin complex is further metabolised in the liver [28]. Iron bill for further blood production is attached to Iron stored in the liver cells as ferritin and haemosiderin [30].
Heavy metals concentration in aquatic environment is responsible in part to fish production losses because they lead to polluted habitats. These pollutants hinder physiological processes in fish including reproduction and growth as well as development. On consumption by man, who is at the end of food chain heavy metals do bio-accumulate (bio magnification) and are capable of causing damage to vital organs in the body. Although the concentration of metals in this fish were lower than the maximum acceptable limit agreed by USEPA (1987), WHO (1983) and FEPA (2003) as presented in Table 1, it is not a guarantee that the metals will not cause problem to man as a final consumer [31]. Chemicals that are not easily degraded like soil active herbicides and especially heavy metal remain in the bodies of the consumer and bio magnify with trophic level [32,33]. So at present concentrations, the fish is safe for human consumption in this area. It is worthy to note that it is bio-accumulation, which is influenced by the rate of intake as well as rate of excretion that determines the concentration in the final consumer, which at a long run is man. More investigations involving the survival of this species in captivity will be carried out in future.
| Metal element | Zinc (Zn) (ppm) | Iron (Fe) (ppm) | Chromium (Cr) (ppm) | Cobalt (Co) (ppm) | Copper (Cu) (ppm) | Cadmium (Cd) (ppm) | Lead (Pb) (ppm) | Aluminium (Al) (ppm) |
|---|---|---|---|---|---|---|---|---|
| Organ where highest concentration was found | Gills | Liver | Spleen | Gills | Gill | Heart | - | - |
| Concentration in Mormyrus rume | 0.042 +0.011 |
0.12 +0.09 |
0.031 +0.012 |
0.032 +0.008 | 0.035 +0.07 | 0.055 +0.022 |
0.0 | 0/0 |
| WHO (WHO,1983) specification | 5.00 | 0.30 | 0.15 | - | 0.05 | 3.00 | 0.005 | - |
| FEPE (FEPA, 1991, 2003) specification | <1.0 | 20 | < 1.0 | - | < 1.0 | < 1.0 | < 1.0 | - |
| USEPA (USEPA, 1987) specification | 0.0766 | 0.1 | 0.05 | - | 1.00 | 0.008 | 0.0058 | - |
Table 1: Highest concentrations of various metal elements in Mormyrus rume obtained from middle Cross River, Cross River State, Nigeria, Compared to world standards as recommended by WHO, FEPE and USEPA
Acknowledgement
We are grateful to Mr Igri, Igri I. of the Central Laboratory of the Faculty of Agriculture, University of Calabar, Nigeria, for carrying out elemental element concentration for this work.
References
- Eli (2007) List of fresh water fishes of Nigeria. Accessed 11/01/2017.
- FAO (2017) Fish species diversity in rivers, lakes, and reservoirs of Nigeria. www.fao.org/docrep/005/T1230E04.htm
- Holden M, Reed W (1972) West African Fresh water fish: West African Nature Books. Longman group Ltd. London. 68pp.
- Fishbase.org 2016. https://www.fishbase.org/summary/2397
- Sienko M J, Plane R A (1976) Chemistry. Fifth edition. Magrow Hill Kogakusha ltd. New Delhi. 623pp
- Pacyna J M (1996) Monitoring and assessment of metal contaminants in the air. In: Chang LW, Magos L, Suzuli T, editors. Toxicology of Metals Boca Raton, FL: CRC Press; pp. 9-28.
- Arruti A, Fernandez-Olmo I, Irabien A (2010) Evaluation of the contribution of local sources to trace metals levels in urban PM2.5 and PM10 in the Cantabria region (Northern Spain) Journal of Environmental Monitoring 12(7):1451-1458.
- Strater E, Westbeld A, Klemm O (2010) Pollution in coastal fog at Alto Patache, Northern Chile. Environmental Science and Pollution Resources Intervention.
- Ruffus H J (2002) Heavy metals-a meaningless term? Pure and Applied Chemistry 74(5): 793 – 807
- He ZL, Yang XE, Stoffella PG (2005) Trace elements in agroecosystemss and the impact on the environment. Journal of trace elements and medical biology 19: 125-140.
- Tchounwou PB, Yedjou CG, Patlolla A, Sutton DJ (2012) Heavy Metals Toxicity and the Environment EXS. 101: 133–164.
- WHO/FAO/IAEA (1996) World Health Organisation Switzerland: Geneva; Trace Elements in Human Nutrition and Health
- Wurts WA (2017) Understanding water hardness. World Aquaculture 24(1): 18.
- Roberts JR (1999) Metal Toxicity in children. In Training manual on paediatric Environmental Health: Putting it into Practice. Children’s Environmental Health Network.
- ATSDR (2001) Agency for Toxic Substances and Disease Registry (ATSDR). A Department of the U.S. Health and Human Services.
- Tam NFY, Wong YS (2000) Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environmental Pollution110: 23-39
- Ayotunde EO, Offem BO, Ada FB(2011) Heavy Metal profile of Cross River: Cross River State, Nigeria: Using Bioindicators Indian Journal of Animal Research 45(4): 232-246.
- Oguzie FA (1996) Heavy metals in fish, water and effluents of the lower Ikpoba River in Benin. Ph.D Thesis. University of Benin, Nigeria. Page 136.
- Nawa IG (1980) Prospect for mariculture in the Cross River estuary. Proceedings of the Cross River State Fisheries Conference Calabar pp 66-68.
- Fawole OO (2002) Morphometry and diet of Mormyrus rume in the Lekki lagoon, Nigeria Rev. Biol. Trop. 50(2): 689-694.
- Imaobong EE, Mandu AE, Bello O(2014) Temporal and seasonal abundance of fish species in Ikot Ebom, a tropical rainforest river, Nigeria. Global Advanced Research Journal of Food Science and Technology 3(3): 90-98.
- Onimisi MM, Shittu OA (2015) Some Aspects of the Biology of African Electric Fish Mormyrus rume Valenciennes, 1846 (Osteoglossiformes; Mormyridae) in Lower River Niger at Idah, Kogi State, Central Nigeria International Journal of Innovative Research and Development 4(8) 45 – 48.
- Khadse GK(2010) Collection and Preservation of Samples and Field Analysis Presented at : Training Programme on QAQC in Water Quality Monitoring, Assessment
- APHA (1998) Standard Methods for the Examination of Water and Wastewater. 20th Ed., American Public Health Association, New York, USA.
- Udosen ED, Offiong NO, John BE (2016) Distribution of trace metals in surface water and sediments of Imo River Estuary (Nigeria): Health risk assessment, seasonal and physicochemical variability Journal of Environmental Chemistry and Ecotoxicology 8(1): 1-8.
- Ada FB, Ekpenyong E, Bayim PB (2012) Heavy metals concentration in some fishes (Chrysichthys nigrodigitatus, Clarias gariepinus and Oreochromis niloticus) in the Great Kwa River, Cross River State, Nigeria. Global Advanced Research Journal of Environmental Science and Toxicology 1(7): 183-189.
- Bull KR, Dearstly AF, Inskip, MH(1981) Growth and mercury content of Rutilus rutilus L. Perch (PercaFluviallis) and Pike (Esox, Lusius, L.) living in sewage effluents. Environmental Pollution (Series A) 25: 229-240.
- Simpkins J, Williams JI (1989) Advanced Biology 3rd edition. English Language Book Society, Unwin, London.
- Nemesok JI, Orban B, Asztalos VE (1987) Accumulation of pesticides in the organs of carp (Cyprinus carpio L) at 4°C and 29°C. Bulletin ofEnvironmetal Contamination Toxicololgy 39(3): 370-378.
- Ochei J, Kolhatkar A (2000) Medical Laboratory Science; Theory and Practice. Tata Mcgraw-HillPoblishing Company Limited, New Delhi.
- USEPA (1987) Quality Criteria for Water. EPA Publication 440/5-86- 001. U.S. Gov. Prin. Office, Washington D.C., USA.
- National Library of Medicine HSDB Database. (2010).
- Suwanketnicom R, Toopornsiri M, Kasetsart J (1992). Butachlor Concentration in Effluents. Natural Science 26: 81–89.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences