ISSN : 0976-8505
Der Chemica Sinica
SiO2.TTC: Efficient Catalyst for Synthesis of 3,4-Dihydropyrimidine-2(1H)- ones/Thiones
Vishvanath D Patil*, Prathamesh V Gidh and Amruta M Salve
Organic Chemistry Research Laboratory, Department of Chemistry, C.K.Thakur A.C.S. College New Panvel, Raigad, Maharashtra, India
Abstract
Efficient method has been developed to synthesize various 3,4-dihydropyrimidinones. Dihydropyrimidinone derivatives were synthesized with excellent yield by using aldehyde, ethylacetoacetate and urea or thiourea with silica supported Titanium trichloride. The reaction carr ied out under room temperature. This method is a very easy and rapid for synthesis of dihydropyrimidinone derivatives.
Keywords
SiO2.TTC, 3,4-Dihydropyrimidin-2(1H)-ones, Urea/thiourea.
Introduction
Dihydropyrimidone and their derivatives have been useful for natural, synthetic, pharmacological, therapeutic and bioorganic chemistry mainly due to their wide range of biological activities and its applications [1-7]. Research in multicomponent reactions (MCRs) is an encoring and hot topic in organic chemistry, as there advantages in preparations of heterocyclic compounds and in drugs discovery procedures [8]. These organic compounds show different activities as calcium channel blocks, antihypertensive agents, alpha antagonists of neuropeptids etc. Various synthetic methods have been reported by different pharmacists such as acids [9-13], microwave variants [14-27], Mn(OAc)2 [28-32] LiBr [33,34], ammonium salt [35], on reagents like CAN and clay [36]. But most of the methods have limitations in terms of yields, catalyst loading, stability, long reaction time etc. causing disposal. In continuation of our research on the introduction of more efficient solid supportive catalyst in organic synthesis.
Materials and Methods
All the chemicals and solvents were purchased from Merckand Sigma Aldrich. All reagents are received which are purified by distillation and recrystallization. The reaction was monitored by TLC.
The spot were detected either under UV light or by placing in an iodine chamber. Melting points were determined in pen capillaries methods.
General procedure for synthesis of 3,4-Dihydropyrimidin-2(1H)-ones/thiones
Aromatic aldehyde (1 mmol), ethylacetoacetate (1 mmol), urea/thiourea (1 mmol) and SiO2.TTC (10%) were stirred in round bottom flask for 5-30 min with solvent Chloroform (1 ml) at room temperature. The development of reaction was monitored by thin-layer chromatography. The completion of reaction confirmed with TLC. After drying it was purified by recrystallization from hot alcohol and further purification by column chromatography. The product obtained after the usual workup were characterised using spectroscopic techniques Scheme 1.
Results and Discussion
The optimum condition for the synthesis of 3,4-Dihydropyrimidon-2(1H)-ones or thiones derivatives were established by considering a reaction between aldehyde, ethylacetoacetate, urea or thiourea with SiO2.TTC (Silica supported titanium trichloride) using chloroform as a solvent. The product obtained was confirmed by melting points and spectral data.
The effectiveness of solid supportive catalyst SiO2.TCC was determined with respect to its leading amounts. There was no improvement in yield with increasing amount from 0.10 mmol of catalyst (Table 1, Entry 3).
Thus, the most appropriate amount for anhydrous SiO2.TTC as a catalyst was found to be 0.1 mmol as per results summarized in Table 1.
| Entry | SiO2.TTC (mmol %) | Time (min.) | Yielda (%) |
|---|---|---|---|
| 1 | 0.01 | 15 | 55 |
| 2 | 0.05 | 15 | 65 |
| 3 | 0.10 | 05 | 92 |
| 4 | 0.15 | 05 | 92 |
| 5 | 0.20 | 05 | 92 |
aIsolated yield of corresponding product
Table 1: Investigation of catalytic effects for synthesis of 3,4-Dihydropyrimidine -2(1H) ones/thiones.
As summarized in Table 2, aromatic/aliphatic aldehydes with electron-donating or electron withdrawing groups, ethylacetoacetate and urea/thiourea in presence of SiO2.TTC were reacted, resulting in corresponding 3,4-dihydropyrimidin-2(1H)-ones/thiones in good to excellent yields.
| Entry | Aldehydea | X | Productb | Time (Min) |
Yieldc (%) |
|---|---|---|---|---|---|
| 1 | 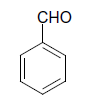 |
O | 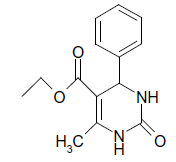 |
05 | 92 |
| 2 | 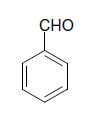 |
S | 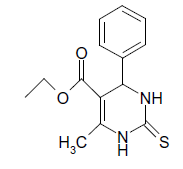 |
30 | 90 |
| 3 | 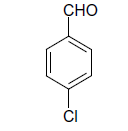 |
O | 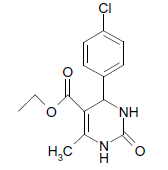 |
05 | 91 |
| 4 | 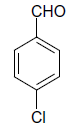 |
S | 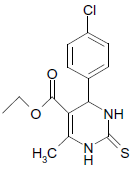 |
25 | 89 |
| 5 | 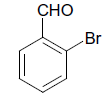 |
O | 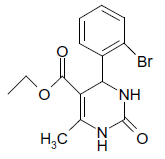 |
25 | 90 |
| 6 | 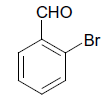 |
S | 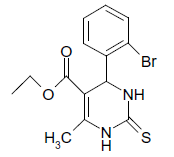 |
25 | 88 |
| 7 | 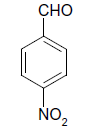 |
O | 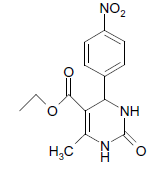 |
05 | 95 |
| 8 | 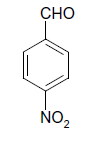 |
S | 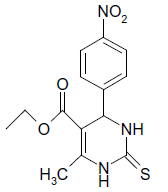 |
25 | 84 |
| 9 | 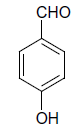 |
S | 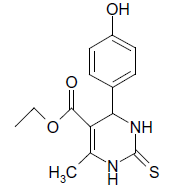 |
25 | 92 |
| 10 | 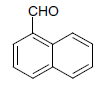 |
O | 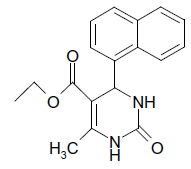 |
25 | 90 |
| 11 | 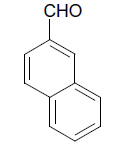 |
O | 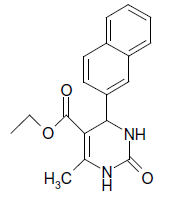 |
25 | 89 |
| 12 |  |
O | 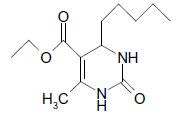 |
50 | 80 |
| 13 |  |
O | 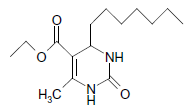 |
50 | 84 |
| 14 | 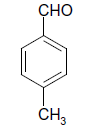 |
O | 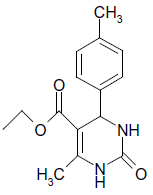 |
15 | 92 |
| 15 | 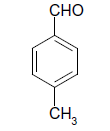 |
S | 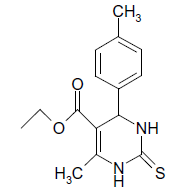 |
25 | 90 |
| 16 | 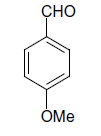 |
O | 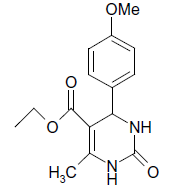 |
15 | 90 |
| 17 | 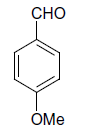 |
S | 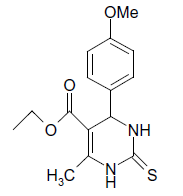 |
25 | 88 |
aThe substrate was treated with aldehyde (1mmol), Ethylacetoacetate (1 mmol),Urea/Thiourea (1 mmol) in presence of anhydrous SiO2.TTC as a catalyst at room temperature.
bAll products were identified by their IR and 1HNMR spectra.
cIsolated Yields.
Table 2: Synthesis of diydrohyropyrimidones catalysed by SiO2.TTC.
Three components reacted smoothly in presence of solid supportive SiO2.TTC with CHCl3 gave good yields of corresponding products (Table 2). SiO2.TTC was proved to be an efficient catalyst under mild conditions. Various aromatic aldehydes containing either electron donating or withdrawing substituents at different position reacted well under present reaction condition (Table 2) proving the wide scope and generality of the protocol. The nature and substitution pattern of different substituents affected the course of reaction in terms of time and yields. These aromatic aldehyde having electron withdrawing groups gave excellent yields, in short reaction time (Table 2, Entry 7) but those with electron donating substitution gave comparatively low yields with slow reaction rate (Table 2, Entry 3,14,16). Further aldehydes with o-substituents were found to be less reactive (Table 2, Entry 5,6) aliphatic aldehydes were less reactive even at more time (Table 2, Entry 12,13). The products obtained from thiourea gives moderate yields (Table 2, Entry 2,4,6,8,9,15,17). This proved wide scopes and generality of the present protocol. The categorization date of various 1H, NMR, IR achieved for various compounds are given in Table 2.
Characterization
(i) 4-(4-Chlorophenyl) 3,4-dihydro-6-phenylpyrimidin-2(1H)-one (Table 2, Entry 3): Melting point 266°C;
1HNMR (400 MHz, DMSO); δ =8.65 (s, 1H, NH), 8.08 (s, 1H, NH), 7.53 7.33 m, (m, 9H, Ar H), 5.45(d, J 2.8 Hz, 1H, CH), 5.18 (d, 1H, J 2.8 Hz, CH)
IR (KBr) cm-1 3232, 2936, 1685, 1573 and 1465
(ii) 3,4-Dihydro-4-(4-hydroxyphenyl)-6-phenylpyrimidin- 2(1H)-one (Table 2, Entry 9): Melting point 256°C
1HNMR (400 MHz, DMSO) δ=9.20 (s, 1H, NH), 8.14 7.53 (m, 9H, Ar H), 7.35 7.31 (s, 1H, NH), 7.28 (d, J 8.8 Hz, 1H, CH), 5.56 (s, 1H, OH) 5.15 (d, 1H, J 8.8 Hz, CH);
IR (KBr) cm-1 3386, 2922, 1626, 1519 and 1445
(iii) 3,4-Dihydro-4-(4-methoxyphenyl)-6-phenylpyrimidin-2(1H)-one (Table 2, Entry 16):
1HNMR (400 MHz, DMSO) δ=12.06 (s, 1H, NH), 9.35 (s, 1H, NH), 8.38 7.22 (m, 9H, Ar H), 6.95 (d, J 8.7 Hz, 1H, CH), 5.47 (d, 1H, J 8.7 Hz, CH), 3.75 (s, 3H, OCH3);
IR (KBr) cm-1 3382, 2936, 1614, 1522, 1418
Conclusion
In this research paper, we successfully developed a simple and highly efficient one pot synthesis of 3,4-Dihydropyrimidin- 2(1H)-ones or thiones derivatives from easily available starting materials using SiO2.TTC. We developed multi components reaction of ethyl acetoacetate, aromatic aldehydes, and urea/thiourea using SiO2.TTC catalyst. This protocol is attractive in terms economy, short reaction times, simple easy workup make this procedure a useful addition to modern synthetic methods are few of the advantages of this procedure.
Acknowledgement
The authors acknowledge the kind support to this work by Principal Dr. S.T. Gadade C.K.Thakhur A.C.S. College, New Panvel, Raigad, Maharaahtra, India.
References
- Meshram GA, Patil VD (2009) A simple and efficient method for sulfonylation of amines, alcohols and phenols with cupric oxide under mild conditions. Tetrahedron Lett 50: 1117-1121.
- Patil VD, Sutar NR, Patil KP (2016) Synthesis of 2,4,5-Triaryl-1H-Imidazoles using anhydrous PbCl2. J Chem Pharm Res 8: 728-732.
- Domling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106: 17-89.
- Akritopoulou-Zanze I, Djuric SW (2007) Recent advances in the development and application of post-Ugi transformation. Heterocycles 73: 125-147.
- Akritopoulou-Zanze I (2008) Isocyanide-based multicomponent reactions in drug discovery. Curr Opinion Chem Biol 12: 324-331.
- Hulme, C (2005) Applications of Multicomponent Reactions in Drug Discovery-Lead Generation to Process Development. In Multicomponent Reactions. Zhu J; Bienayme H, Eds., Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, pp: 311-341.
- Hulme C, Gore V (2003) “Multi-component reactions : emerging chemistry in drug discovery" 'from xylocain to crixivan'. Current Med Chem 10: 51-80.
- Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, et al. (1991) Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J Med Chem 34: 806-811.
- Biginelli P (1893) Synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Gazz chim Ital 23: 360-416.
- Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, et al. (1995) Novel Alkaloids from the Sponge Batzellasp: Inhibitors of HIV gp120-Human CD4 Binding. Eur J Org Chem 60: 1182-1188.
- Heys L, Moorea CG, Murphy PJ (2000) The guanidine metabolites of Ptilocaulisspiculifer and related compounds; isolation and synthesis. J Chem Soc Rev 29: 57.
- Tu S, Fang F, Miao C, Jiang H, Feng Y, et al. (2003) One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using boric acid as catalyst. Tetrahedron Lett 44: 6153.
- Hu EH, Sidler DR, Dolling Ulf-H (1998) Unprecedented Catalytic Three Component One-Pot Condensation Reaction: An Efficient Synthesis of 5-Alkoxycarbonyl- 4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J Org Chem 63: 3454-3457.
- Ranu BC, Hazra A, Jana UJ (2000) Indium (III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1, 3-dicarbonyl compounds, aldehydes, and urea: an improved procedure for the Biginelli reaction. J Org Chem 65: 6270-6272.
- Yadav JS, Reddy BVS, Srinivas R, Venugopal C, Ramalingam T (2001) LiClO4-catalyzed one-pot synthesis of dihydropyrimidinones: an improved protocol for Biginelli reaction. Synthesis 2001: 1341-1345.
- Ramalinga K, Vijayalakshmi P, Kaimal TNB (2001) Bismuth(III)-catalyzed synthesis of dihydropyrimidinones: improved protocol conditions for the Biginelli reaction. Synlett 2001: 863-865.
- Reddy V, Mahesh M, Raju PVK, Babu TR, Reddy VVN (2002) Zirconium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett 43: 2657-2659.
- Fu NY, Yuan YF, Cao Z, Wang SW, Wang JT, et al. (2002) Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: improved protocol conditions for the Biginelli reaction. Tetrahedron 58: 4801-4807.
- Fan X, Zhang X, Zhang Y (2002) Samarium chloride catalysed Biginelli reaction: one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. J Chem Res 2002: 436-438.
- Lu J, Bai Y (2002) Catalysis of the Biginelli Reaction by Ferric and Nickel Chloride Hexahydrates. One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones. Synthesis 2002: 466-470.
- Varela R, Alam MM, Adapa SR (2003) Bismuth triflate catalyzed one-pot synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones: an improved protocol for the Biginelli reaction. Synlett 2003: 67-70.
- Bose DS, Fatima L, Meryala HB (2003) Green Chemistry Approaches to the Synthesis of 5-Alkoxycarbonyl-4-aryl-3,4- dihydropyrimidin-2(1H)-ones by a Three-Component Coupling of One-Pot Condensation Reaction: Comparison of Ethanol, Water, and Solvent-free Conditions. J Org Chem 68: 587-590.
- Sabitha G, Reddy GSKK, Reddy CS, Yadav JS (2003) One-pot synthesis of dihydropyrimidinones using iodotrimethylsilane. Facile and new improved protocol for the Biginelli reaction at room temperature. Synlett 2003: 858-860.
- Paraskar AS, Dewkar GK, Sudalai A (2003) 3,4-Dihydropyrimidinones/thiones and their derivatives are synthesized via Biginelli routes involving an aldehyde, 1,3-dicarbonyl compound. Tetrahedron Lett 44: 3305.
- Yadav JS, Subba Reddy BV, Reddy JE, Ramalingam T (2000) Microwave-assisted efficient synthesis of dihydro pyrimidines: improved high yielding protocol for the Biginelli reaction. J Chem Res 2000: 354-355.
- Stadler A, Kappe CO (2000) Atroposelective attack of nucleophiles on 2-formyl-1-naphthamides and their derivatives: chelation and non-chelation control. J Chem Soc Perkin Trans 1 2000: 1363-1378.
- Kappe CO, Kumar D, Varma R (1999) Microwave-assisted high-speed parallel synthesis of 4-aryl-3,4-dihydropyrimidine-2(1H)-ones using a solventless Biginelli condensation protocol. Synthesis 1799-1803.
- Kidwai M, Saxena S, Mohan R, Venkataramanan R (2002) A novel one pot synthesis of nitrogen containing heterocycles: An alternative methodology to the Biginelli and Hantzsch reactions. R J Chem Soc Perkin Trans 1: 1845-1846.
- Donodoni A, Massi A (2001) Parallel synthesis of dihydropyrimidinones using Yb(III)-resin and polymer-supported scavengers under solvent free conditions. A green chemistry approach to the Biginelli reaction. Tetrahedron Lett 42: 7975-7978.
- Malti G, Kundu P, Guin C (2003) One pot synthesis of dihydropyrimidinone catalyzed by lithium bromide: an improved procedure for the Biginelli reaction. Tetrahedron Lett 44: 2757-2758.
- Reddy KR, Reddy CV, Mahesh M, Raju PVK, Reddy N (2003) New environmentally friendly solvent-free synthesis of dihydropyrimidinones catalysed byN-butyl-N,N-dimethylphenylethylammonium bromide. Tetrahedron Lett 44: 8173-8175.
- Bigi F, Carloni S, Frullanti B, Maggi R, Sartori G (1999) A revision of the Biginelli reaction under solid acid catalysis. Solvent-free synthesis of dihydropyrimidines over montmorillonite KSF. Tetrahedron Lett 40: 3465-3468.
- Wipf P, Cunningham AA (1995) A solid phase protocol of the Biginelli dihydropyrimidine synthesis suitable for combinatorial chemistry. Tetrahedron lett 36: 7819-7822.
- Salehi P, Dabiri M, Zolfigol MA, Fard MAB (2003) Silica sulfuric acid: an efficient and reusable catalyst for the one-pot synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones. Tetrahedron Lett 44: 2889-2891.
- Yadav JS, Subbareddy BV, Reddy KB, Raj KS, Prasad AR (2001) Ultrasound-accelerated synthesis of 3, 4-dihydropyrimidin-2 (1 H)-ones with ceric ammonium nitrate. J Chem Soc Perkin Trans 1 2001: 1939-1941.
- Mitra AK, Banerjee K (2003) Clay catalysed synthesis of Dihydropyrimidiniones under Solvent-free conditions. Synlett 10: 1509-1511.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences