ISSN : 0976-8505
Der Chemica Sinica
Simultaneous Determination of Fluconazole and Tinidazole in Combined Dose Tablet using High Performance Thin Layer Chromatography
Dhananjay B Meshram1*, Priyanka Mishra1, Sapna D Desai1 and Madhukar R Tajne2
1Pioneer Pharmacy Degree College, Vadodara, Gujarat, India
2Department of Pharmaceutical Sciences, RTM Nagpur University Nagpur, India
Abstract
This paper presents an HPTLC method, validated for simultaneous determination of fluconazole (FLZ) and tinidazole (TNZ) in a combined dose tablet. Chromatography was performed on silica gel 60F254 plates with 250 μm thickness, using Toluene: Ethyl acetate: Chloroform: Methanol (1.2:3:2:0.8 v/v) as a mobile phase. For the proposed method, linearity (FLZ-r2 > 0.996; TNZ-r2 > 0.997), sensitivity (FLZ-3.04 ng/spot; TNZ-11.12 ng/spot), recovery (FLZ- 98.66%-99.13%; TNZ- 99.26%-100.54%), and repeatability were found to be satisfactory. The HPTLC method offers a simple, sensitive, rapid and cost effective technique that can be applied successfully in routine quality control of formulations containing FLZ and TNZ.
Keywords
Fluconazole, Tinidazole, HPTLC
Introduction
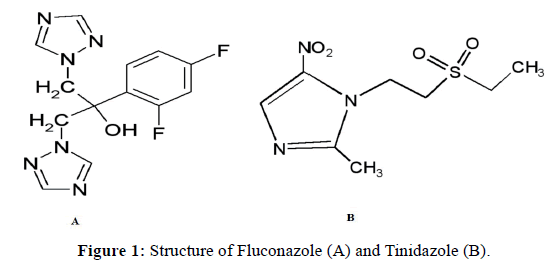
Fluconazole (FLZ), 2-(2,4-Difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-yl) propan-2-ol) (Figure 1A), is a triazole antifungal drug which in sensitive fungi, inhibits a cytochrome P450 dependent enzyme resulting in impairment of ergosterol synthesis in fungal cell membranes. It is widely used as a broad-spectrum antifungal agent for the treatment and prophylaxis of fungal infections of a deep organ. Tinidazole (TNZ), 1-[2-(Ethylsulphonyl) ethyl]-2-methyl-5- nitroimidazole (Figure 1B) is widely used to treat infectious diseases such as amoebiasis, giardiasis, trichomoniasis and those caused by anaerobic bacteria. To treat systemic fungal infection, a combination of these drugs is used either as two different tablets in a form of a kit or a combination of two drugs as a single tablet. Literature cited various methods to estimate FLZ in biological fluids, similarly, few methods such as I.R. spectroscopy [1], UV spectrophotometry [2-6], microbiological assays [7] and TLC [8] for determination of FLZ in pharmaceutical formulations.
The literature survey revealed HPTLC [9,10] and HPLC [11,12] methods to estimate TNZ from dosage forms, the study of TNZ hydrolysis [13] and its degradation behavior [14] by HPLC along with various methods such as UV [15], Micellar HPLC and UV derivative [16] and RP-HPLC [17-19] methods for simultaneous determination of TNZ and FLZ in dosage forms. However, the literature did not cite any method for simultaneous determination of FLZ and TNZ by HPTLC. Therefore, we have attempted to develop an HPTLC method for determination of FLZ and TNZ from combined tablet dosage form.
High-performance thin-layer chromatography (HPTLC) is a simple, rapid, and highly efficient method, which allows analysis of several samples simultaneously using a small amount of solvent. This reduces the time, cost of analysis, and decreases the possibility of environmental pollution.
Materials and Methods
Experimental
Materials and reagents: Zim Labs Nagpur, kindly gifted FLZ and Suven laboratories, Chennai, kindly supplied TNZ. All other chemicals and solvent were of analytical grade and purchased locally.
Flucoti tablets (labeled claim: tinidazole 1000 mg and fluconazole 75 mg) manufactured by Fourrts (India) Pvt. Ltd., Chennai, were purchased from local pharmacy.
Standard solutions
Stock solutions of individual drug and a mixed standard stock solution (containing FLZ 500 μg/ml and TNZ 6.65 mg/ mL) were prepared separately in methanol and stored at 4°C. An accurately measured, 3.0 ml mixed stock standard solution was diluted to 10.0 ml with methanol to give working standard solution (conc: FLZ-150 μg/mL; TNZ-1.995 mg/mL).
Sample solutions
A sample of 20 tablets were accurately weighed and powdered. Tablet powder equivalent to about 12.5 mg of FLZ and 166.63 mg of TNZ was accurately weighed, dissolved using 15 mL of methanol and sonicated for about 15 minutes. The volume was made up to 25.0 ml and then filtered. 3.0 ml of the clear filtrate was diluted to 10.0 ml with methanol.
Procedure
This procedure was finalized based on trial and error. Working standard solution was accurately applied as a band 4 mm wide with a Camag 100 μl syringe (Hamilton, Bonaduz, Switzerland) on a pre-coated silica gel 60F254, (10 cm × 10 cm with 250 μm thickness; E. Merck, Darmstadt, Germany) HPTLC plates, using a CAMAG Linomat V (Switzerland) sample applicator. Precoated HPTLC plates were washed with methanol and activated at 110°C for 5 min prior to chromatography. Spots were applied at a constant rate of 5.0 s/μl and space between two bands was 4 mm. The linear ascending development was performed in a 20 cm × 10 cm twin trough glass chamber (Camag, Muttenz, Switzerland) saturated with the mobile phase Toluene: Ethyl acetate: Chloroform: Methanol (1.2:3:2:0.8 v/v) for 10 min at room temperature (25°C ± 2) at a relative humidity of 60% ± 5. The length of chromatogram run was 70 mm. Camag TLC scanner III performed densitometric scanning in the reflectance-absorbance mode at 205 nm.
Validation of the developed method
The proposed method was validated, for linearity, accuracy, precision, and specificity.
Linearity
Mixed standard solutions containing FLZ in the range of 0.45 μg/ml-1.2 μg/ml and TNZ in the range of 5.99 μg/ ml-15.99 μg/ml were analysed to obtain the calibration curve. Correlation coefficient (r2) was estimated to determine linearity.
Precision
The system repeatability (intraday precision, percentage RSD) was assessed by six determinations of sample solutions and standard solutions. Intermediate precision was considered by three different analysts on different days (day 1, day 3 and day 5).
Accuracy
Accuracy was estimated by standard addition method by evaluating percentage recoveries of the known quantities of combination of FLZ and TNZ added to the solutions with marketed formulations.
Specificity
Specificity of the method towards the drugs was established by attempting deliberate degradation of the two drugs with exposure to stress conditions like acidic (0.1 M HCl), alkali (0.1 M NaOH), an oxidizing agent (3% H2O2), heat (60°C) and UV rays. After 24 hours, samples were diluted with methanol and analysed by proposed method.
Applicability of the developed method to marketed formulation
Accurately, 6 μl of the sample solution (FLZ: 0.75 μg/spot, TNZ: 9.99 μg/spot) was spotted on the HPTLC plate followed by development and scanning as described in the experimental section.
Results and Discussion
Optimization of HPTLC method
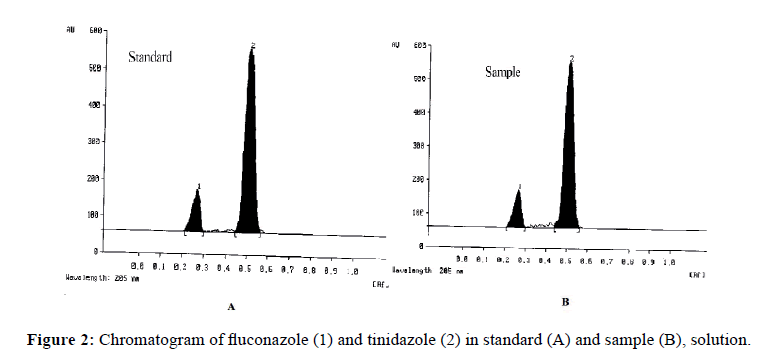
To develop a robust mobile phase, the mixture of Toluene: Ethyl acetate: Chloroform: Methanol (1.2:3:2:0.8 v/v) was optimized resulting in well-resolved and compact spots free of tailing with better sensitivity. The observed Rf values for FLZ and TNZ were 0.27 ± 0.012 and 0.51 ± 0.022 respectively. Figure 2 depicts the chromatograms obtained from standard and sample drug solutions.
Method validation
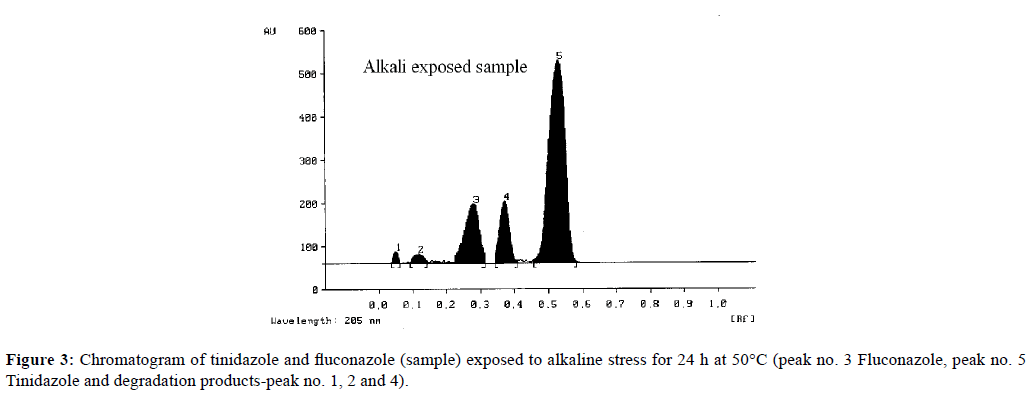
The response was linear in the range, from 450 ng/band to 1050 ng/band for FLZ and 5985 ng/band to 13965 ng/band for TNZ. Correlation coefficients (r) value were >0.99 for both the drugs. The limit of detection for TNZ and FLZ were 11.12 and 3.04 ng/spot respectively, and limit of quantitation values were 37.32 and 14.24 ng/spot. The effect of random events on the precision of the analytical method due to a different day and different analyst variation or intermediate precision, expressed in terms of percentage RSD, shows that the method is precise (Table 1). The result obtained (Table 2) suggest the accuracy of the method without any interference by additives in tablet formulation. The results of specificity studies have shown that the sample undergoes degradation on exposure to alkaline conditions as depicted in (Figure 3). The chromatogram shows extra peaks at 0.07, 0.12 and 0.37 (Rf). The degradation products are well resolved from the target analyte, thus affording precise determination of FLZ and TNZ in stressed conditions.
| Parameter | Tinidazole | Fluconazole |
|---|---|---|
| By Peak Area | By Peak Area | |
| Analyst-1 [percentage estimated] |
99.63 | 99.09 |
| Analyst-2 [percentage estimated] |
100.89 | 99.14 |
| Analyst-3 [percentage estimated] |
98.87 | 100.12 |
| Interday precision [percentage RSD, n=6] |
0.69 | 0.36 |
| Intraday precision [percentage RSD, n=6] |
0.42 | 0.64 |
Table 1: Results from study of intermediate precision.
| Drug | Amount of standard drug added (mg) |
Amount of standard drug recovered |
Recovery (%) |
|---|---|---|---|
| TNZ | 16.66 50.02 83.42 |
16.54 49.73 83.87 |
99.26 99.43 100.54 |
| FLZ | 1.25 3.75 6.25 |
1.24 3.67 6.19 |
99.13 98.66 99.07 |
Table 2: Recovery studies of tinidazole and fluconazole (n=6).
No degradation was observed in the case of, samples exposed to other stress conditions. Applicability of the method was verified by determination of FLZ and TNZ in a combined dose tablet. The results obtained (Table 3) suggest suitability of the method for routine analysis.
| Sample | Constituents Estimated |
Labeled Claim (mg/tab) |
Mean Amount Estimated (mg/tab) |
Mean Percent of labeled Claim ± SD, (%RSD) |
|---|---|---|---|---|
| By Peak Area | ||||
| Tablet Formulation |
Fluconazole | 75 | 75.62 | 100.82 ± 0.92, (0.91) |
| Tinidazole | 1000 | 993.4 | 99.34 ± 0.33, (0.33) |
Table 3: Summary of results of estimation in tablet formulation (n=6)
The matrix did not affect the quantitative determination of two drugs. This has been established by the recovery studies where, finely powdered tablet was spiked with standard drug and amount of recovery calculated. The percentage recovery obtained indicates the efficacy of the method to quantify FLZ and TNZ in combined tablet dosage form.
Conclusion
The proposed chromatographic method enables quantitative determination of FLZ and TNZ without the interference of degradation products of TNZ. HPTLC combined with densitometry is a technique, which is a simple and low-cost method of drug assay. The proposed method is rapid, sensitive, and suitable for routine control of fluconazole and tinidazole in pharmaceuticals.
The developed method can be applicable to quantify FLZ and TNZ in their single dosage form. Moreover, it is able to resolve TNZ from its degradation products; hence it can be used as a stability indicating analytical method for TNZ.
Acknowledgements
The authors are thankful to Dr. N. J. Gaikwad, Professor and Head, Department of Pharmaceutical Sciences, R.T.M. Nagpur University, Nagpur for providing laboratory facilities. Authors are also thankful to Zim Laboratories, Nagpur India, for providing gift sample of fluconazole and Suven laboratories Chennai for providing tinidazole as a gift sample. One of the authors is thankful to University Grant Commission, New Delhi India, for supporting the project financially, under Rajiv Gandhi National Fellowship Scheme.
References
- Atay O, Selcuk F (1996) Quantitative determination of fluconazole by infrared spectrophotometry. Anal Letter 29: 2163-2176.
- Lu F, Fang H, Wu Y (1996) Investigation of drug compatibility by Convolution Spectrometry. Zhongguo Yaoxue Zazhi 16: 505-506.
- He D, Dongzhou X (1997) Determination of Fluconazole content in Fluconazole injection by UV Spectrophotometry. Guangdong Yaoxueyuan Xuebao 13: 107-108.
- Goger NG, Aboul-Enein HY (2001) Quantitative determination of fluconazole in capsules and IV solutions by UV spectrophotometric methods. Anal Letter 34: 2089-2098.
- Aboul-Enein HY, Goger NG, Turkalp A (2002) Quantitative determination of fluconazole in syrups by first order derivative spectrophotometry. Anal Letters 35: 1193-1204.
- Anand RM, Kumar TR, Nandakumar N, Saravanan D, Lalitha KG, et al. (2006) Spectrophotometric method for estimation of fluconazole in capsules. Indian Drugs 43: 320-322.
- Sane RT, Fulay A, Joshi AA (1994) Quantification of fluconazole from its pharmaceutical preparations by simple microbiological method. Indian Drugs 31: 207-210.
- Meshram DB, Bagad SB, Tajne MR (2008) A simple TLC method for analysis of fluconazole in pharmaceutical dosage forms. J Planar Chromatogr 21: 191-195.
- Salomies H (1992) Determination of Tinidazole in dosage forms by quantitative HPTLC. J Planar Chromatogr 5: 291-293.
- Patel RB, Patel AA, Gandhi TP, Patel PR, Patel VC (1980) New methods for the estimation of Tinidazole. Indian Drugs 18: 76-78.
- Menouer M, Guermauche S, Guermauche MH (1987) Analysis of tinidazole in tablets and plasma by liquid chromatography. J Pharm Belg 42: 243-246.
- Bhatia MS, Kaskhedikar SG, Chaturvedi SC (1999) High performance liquid chromatographic estimation of ciprofloxacin hydrochloride and tinidazole from tablets. J Pharm Sci 61: 311-312.
- Salomies H, Salo JP (1993) An HPLC study of tinidazole hydrolysis. Chomatographia 36: 79-82.
- Bakshi M, Singh S (2004) HPLC and LC-MS studies on stress degradation behavior of tinidazole and development of a validated specific stability indicating HPLC assay method. J Pharm Biomed Anal 34: 11-18.
- Pandey S, Pandey P, Dubey S, Chaturvedi U, Rai AK (2012) Facile derivative UV spectroscopy method: simultaneous estimation of tinidazole and fluconazole in combined tablet dosage form. Thai J Pharm Sci 36: 55-62.
- Belal F, Sharaf El-Din MK, Eid MI, El-Gamal RM (2014) Micellar HPLC and derivative spectrophotometric methods for the simultaneous determination of fluconazole and tinidazole in pharmaceuticals and biological fluids. J Chrom Sci 52: 298-309.
- Meshram DB, Bagade SB, Tajne MR (2009) Simple HPLC method for simultaneous estimation of fluconazole and tinidazole in combined dose tablet. J Chrom Sci 47: 885-888.
- Bodepudi C, Bantu S, Reddy KOM, Shanmugasundaram P, Aanandhi MV (2011) Novel Reverse Phase HPLC Method development and validation of Fluconazole and Tinidazole in a combined tablet dosage form. Int J Chem Tech Res 3: 1309-1317.
- Sathiyasundar R, Veeramanikandan P, Arcot AS, Rajaganapathy K (2014) Analytical method development and method validation of simultaneous determination of tinidazole and fluconazole by RP-HPLC. Int J Chem Pharm Sci 5: 106-109.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences