ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Simple and Multi-Component Synthesis of Pyrrole Heterocycles
Ayaz Mahmood Dar1,2*, Shafia Mir1, Hilal Ul Zaman1, Javid Ahmad Lone1, Hena Khanam2, Muzaffar Hussain Najar1 and Kouncer Hassan3
1Department of Chemistry, Govt. Degree College Kulgam, University of Kashmir, India
2Department of Chemistry, Aligarh Muslim University, Aligarh 202 002 UP, India
3Department of Chemistry, Amar Singh College, Srinagar, University of Kashmir, India
- *Corresponding Author:
- Ayaz Mahmood Dar
Department of Chemistry
Government Degree College Kulgam
University of Kashmir, India
Tel: +91 9286990247
E-mail: ayazchem09@gmail.com
Received Date: October 25, 2017; Accepted Date: November 28, 2017; Published Date: December 07, 2017
Citation: Dar AM, Mir S, Ul Zaman H, Lone JA, Khanam H, et al. (2017) Simple and Multi- Component Synthesis of Pyrrole Heterocycles. Synth Catal. 2:12. doi: 10.4172/2574-0431.100018
Abstract
Pyrroles is the special class of heterocyclic compounds with a broad spectrum of biological activities such as anti-inflammatory, antiproliferative, antihistaminic, anti-HIV, antifungal, antihelmintic and antiviral agents. Pyrrole is a five membered ring structure, with formula C4H4NH. The heterocyclic pyrroles are the ideal building blocks for different biologically efficient molecules including porphyrins and bile pigments. Therefore researchers are synthesizing these heterocycles through multi-stepped or single stepped pathways as target structures for biological studies. In this review, different synthetic protocols/methodologies are shown in which different entry molecules are converted into pyrrole derivatives, which are important from medicinal and pharmaceutical points of view.
Keywords
Synthesis; Pyrrole; Multicomponent; Paal-Knorr reaction
Introduction
Pyrrole is a heterocyclic aromatic organic compound, a five membered ring with formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. The existence of pyrrole in coal tar, bone oil, and in general, in products obtained by the dry distillation of proteins was first surmised by Runge [1] in 1834. The discovery that pyrrole ring was an integral part of heme and of chlorophyll molecules [2] not only created intense interest in the chemistry of pyrrole and its derivatives, but also resulted in the majority of investigations conducted during the latter part of the nineteenth century and the early years of the twentieth century being dominated by this relationship of pyrrole with naturally occurring compounds.
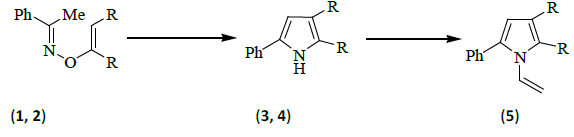
A number of years ago, Sheradsky reported [3] that when the base-catalyzed addition products [1 and its trans-isomer, R=CO2Me] of acetophenone oxime and dimethyl acetylenedicarboxylate were heated together, the pyrrole derivative (3) [R=CO2Me] was obtained. Trofimov et al. [4] have since reported that when the o-vinyl derivative (2) [R=H] of acetophenone oxime was heated with potassium hydroxide in dimethyl sulfoxide solution, 2-phenylpyrrole (4) [R=H] was obtained in high yield. The Russian workers [4] prepared compound 2 by heating acetophenone oxime, acetylene, and potassium hydroxide together in dimethyl sulfoxide solution, under conditions which can lead [5] to the formation of both 2-phenylpyrrole (4) and its 1-N-vinyl derivative (5, R=H).
Experimental
Generally, pyrroles are synthesized by the condensation of 1, 4-dicarbonyl compounds with primary amines. This condensation, known as the Paal-Knorr reaction, has a wide scope, permitting the synthesis of a wide variety of pyrrole derivatives. [6] Recently, it was found that microwaves enhance the rate of this reaction. In this communication, Rao and Jothilingam [7] reported a novel one-pot synthesis of 2, 5-di-and 1, 2, 5-trisubstituted pyrrole derivatives under microwave conditions (7) [Ar=C6H5, R=H; Ar=4-ClC6H4, R=H; Ar=4-BrC6H4, R=H; Ar=4-CH3C6H4, R=H; Ar=4- OCH3C6H4, R=H; Ar=4-Cl, 3-CH3C6H3, R=H; Ar=C6H5, R=n-C4H9; Ar=C6H5, R=C6H5; Ar=C6H5, R=CH2C6H5] from 2-butene-1, 4-diones (6) [Ar=C6H5, 4-ClC6H4, 4-BrC6H4, 4-CH3C6H4, 4-OCH3C6H4, 4-Cl, 3-CH3C6H3] through domino pathways via palladium-assisted transfer hydrogenation followed by a Paal-Knorr reaction using ammonium formate and its analogs.
The advantage of this reaction is the utility of PEG-200 as a solvent, which replaces use of high dielectric constant solvents such as water and DMF. PEG-200 is miscible with water, thereby simplifying the work-up. Furthermore, it is inexpensive and readily available in bulk quantities. But the drawback is that PEG- 400 can’t be used possibly due to low solubility of ammonium formate.
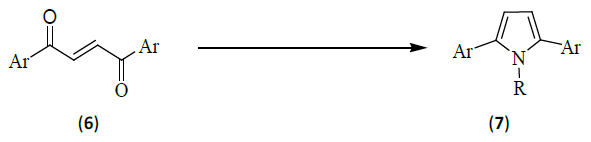
Ranu and Dey [8] carried out one-pot, three-component condensation of a carbonyl compound (8), an amine (9) and a nitroalkene (10) leading to an efficient synthesis of alkyl-substituted pyrroles (11) [R1=C8H17, CH3, CH3CH2; R2=CH3(CH2)3, C6H12, PhCH2, PhCH(CH3)2; R3=4-NO2C6H4, C7H5O2CH3, 4-ClC6H4, Ph; R4=CH3, CH3CH2] in molten tetrabutylammonium bromide. Neither a catalyst nor an organic solvent was required for that reaction and the molten ammonium salt was recyclable.
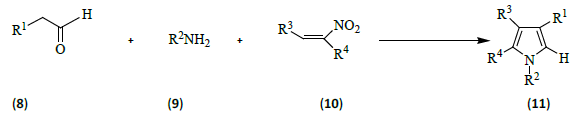
They also performed synthesis of fused pyrroles by the coupling of cycloalkanones, amines and nitroalkenes.
Nitroolefins or β-acetoxy-nitro compounds (13a-i) react with α-isocyanoesters (12a-i) in the presence of an organic base to give pyrroles (14a-i) in good yield [9].
The pyrroles produced by the present approach could be ideal building blocks for porphyrins and bile pigments since they are unsubstituted in the 5-position and the 2-position can be protected by an easily removable group such as an ester. The substituents in the 3- and 4-position originate from the nitroolefin component and can be readily varied in view of the exceptionally rich chemistry of the nitro group. In addition, the condensation to give pyrroles could also be extended to aliphatic nitro olefins. The major drawback is the difficulty in handling and storing small, very base sensitive nitro-olefins (Table 1).
| R | R1 | R2 | |
|---|---|---|---|
| (a) | t-BuO | 4-MeOC6H4 | Me |
| (b) | EtO | 4-PhCH2OC6H4 | Me |
| (c) | t-BuO | H | Me |
| (d) | MeO | H | Me |
| (e) | t-BuO | CH2CH3 | CH2CH3 |
| (f) | t-BuO | Me | CH2CH3 |
| (g) | t-BuO | CH2CH3 | Me |
| (h) | t-BuO | Me | (CH2)2CO2Me |
| (i) | Me2N | Me | CH2CH3 |
Table 1: Showing the different substituent groups R, R1, R2 in reactants and products.
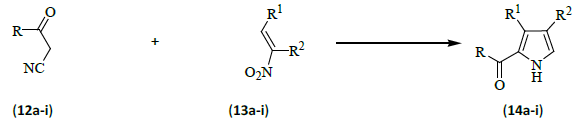
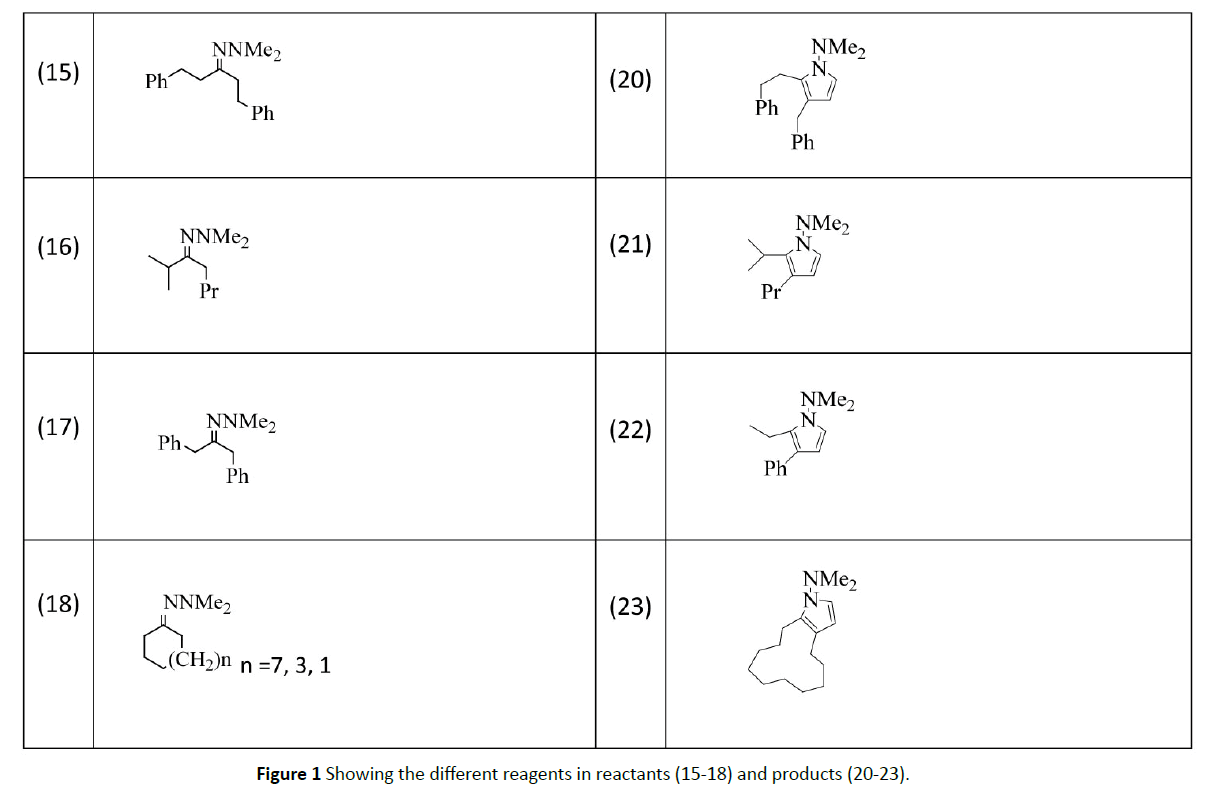
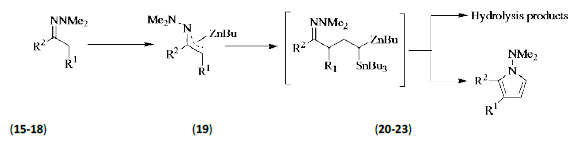
Nakamura et al. [10] reported a straightforward one-pot synthesis of 1-(dimethylamino)-1H-pyrroles (20-23) through [3+2] coupling of a ketone hydrazone (15-18) and a vinyl stannane. The keys to the development of this process are the carbometalation reaction of zincated hydrazone (19) with a vinyl stannane and efficient aerobic oxidation of the resulting functionalized gem- Zn/Sn dimetallic species under mild reaction conditions (Figure 1).
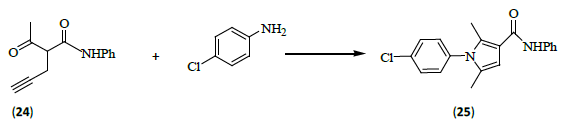
Wang et al. [11] developed a highly efficient iron-catalyzed approach to polysubstituted pyrroles (25) through the [4C+1N] cyclization of 4-acetylenic ketones (24) with primary amines, leading to the synthesis of a variety of tetra-and fully-substituted pyrroles as well as fused pyrrole derivatives in good to excellent yields.
Major advantages of the methodology is application of non-toxic iron salts as catalysts with high reaction efficiency, good to excellent yields, and use of a wide variation of substituents. Noticeably, pyrroles synthesized by this approach contain a characteristic 3-carboxamide group. The pyrrole-3-carboxamide has been found to be a key subunit in therapeutically active compounds, the well-known cholesterol reducing drug Lipitor®. Thus, present scheme could provide an efficient access to such kinds of compounds.
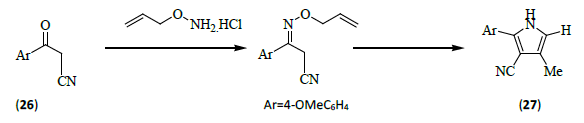
Wang et al. [12] synthesized 2, 4-and 2, 3, 4-substituted pyrroles (27) in two or three steps from commercially available ketones (26) and allyl hydroxylamine. An iridium-catalyzed isomerization reaction was developed to convert o-allyl oximes to o-vinyl oximes, which undergo a facile [3,3] rearrangement to form 1, 4-imino aldehyde Paal-Knorr intermediates that cyclized to afford the corresponding pyrroles.
This is an interesting alternative approach to the synthesis of pyrroles because it uses the Paal-Knorr sequence without requiring the synthesis of 1, 4-dicarbonyl compounds. The overall method provides a simple, selective, and functional group tolerant synthesis of substituted pyrroles.
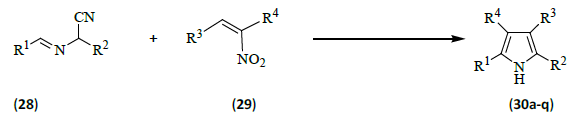
Bergner and Opatz [13] prepared 2, 3, 4, 5-tetrasubstituted pyrroles (30a-q) with high regioselectivity by a formal cycloadditions of α-(alkylideneamino) nitriles (28) [R1, R2 as defined below] and nitroolefins (29) [R3, R4 as defined below] followed by elimination of HCN and HNO2. The reaction allowed the convergent construction of the pyrrole ring in four steps from a nitroalkane and three aldehydes.
Since the pronucleophiles 28 can be obtained from two aldehydes and the electrophiles 29 can be prepared by condensation of an aldehyde and a nitroalkane, this method represents a highly modular synthesis of the pyrrole ring that is amenable to the combinatorial variation of all four substituents. While many reported pyrrole syntheses yield only acceptor-substituted products, this protocol also permits the preparation of products devoid of an electron-withdrawing substituent. On the other hand, compounds of this type can be sensitive to aerial oxidation and their longer exposure to halogenated solvents such as CDCl3 should also be avoided to prevent the formation of intensely colored oxidation products (Table 2).
| R1 | R2 | R3 | R4 | |
|---|---|---|---|---|
| (a) | 2-Naph | Me | 4-ClC6H4 | Me |
| (b) | 2-Naph | Me | 4-ClC6H4 | Me |
| (c) | 2-Naph | Me | 3,4-(MeO)2C6H3 | Me |
| (d) | 2-Naph | Me | Me | Et |
| (e) | 2-Naph | Me | Me | n-Pent |
| (f) | 2-Naph | Me | 4-CNC6H4 | Et |
| (g) | 2-Naph | Me | Ph | Ph |
| (h) | 2-Naph | Bn | 4-ClC6H4 | Me |
| (i) | 3,4-(MeO)2C6H3 | Bn | 4-ClC6H4 | Me |
| (j) | 3,4-(MeO)2C6H3 | Ph | 4-CNC6H4 | Et |
| (k) | 3,4-(MeO)2C6H3 | Ph | Ph | Ph |
| (l) | 2-Naph | Me | -(CH2)4- | |
| (m) | 2-Naph | Bn | -(CH2)4- | |
| (n) | 3,4-(MeO)2C6H3 | Bn | -(CH2)4- | |
| (o) | 3,4-(MeO)2C6H3 | Bn | -(CH2)4- | |
| (p) | 3,4-(MeO)2C6H3 | Ph | -(CH2)4- | |
| (q) | 3,4-(MeO)2C6H3 | Ph | -(CH2)4- |
Table 2: Showing the different substituent groups R1, R2, R3 and R4 in reactants and products.
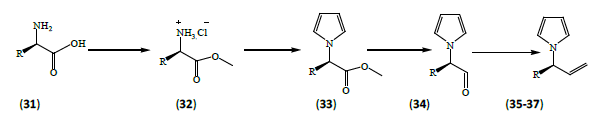
Settambolo et al. [14] reported synthesis of (3R)-3-(Pyrrol-1- yl) but-1-ene (35), (3R)-4-methyl-3-(pyrrol-1-yl) pent-1-ene (36), (3R)-3-(pyrrol-1-yl) hex-1-ene (37) in high enantiomeric excess (>92%) were prepared starting from D-α-amino acids (31). The crucial steps in the synthesis, reduction (DIBAH) of the corresponding pyrrolyl esters (33) to the corresponding pyrrolylaldehydes (34) followed by Wittig olefination proceeded without compromising the stereochemical integrity.
These synthetic schemes use inexpensive reagents and provide pure products after simple purification processes. Suitable experimental conditions to minimize the racemization process during both the ester reductions and the Wittig methylenations were set up which makes the protocol a general enantiomeric route to (3S)-or (3R)-3-(pyrrol-1-yl) alk-1-enes.
R=Me, i-Pr, n-Pr
Alizadeh et al. [15] carried out one-pot four-component reaction between two amines (38, 40) [R1, R2 as defined below] and diketene (39) in the presence of dibenzoylacetylene (41) leading to 4, 5-dihydro-1H-pyrrol-3-carboxamide derivatives (42) [R1=n- Pr, i-Bu, i-Pr, i-Bu, i-Bu, n-Pr, t-Bu, t-Bu, R2=i-Bu, n-Pr, i-Bu, i-Pr, i-Bu, n-Pr, i-Pr, n-Pr].
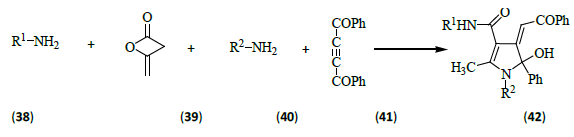
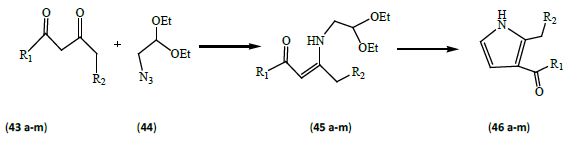
Bellur and Langer [16] synthesized a variety of pyrroles. Staudinger-aza-wittig reaction of 2-azido-1, 1-diethoxyethane (44) with 1, 3-dicarbonyl compounds (43a-m) afforded N-(2, 2-diethoxyethyl)-3-aminoalk-2-en-1-ones (45 a-m), which were subsequently transformed into functionalized pyrroles (46 a-m). This approach being multi-component/one-pot and involves the synthesis of resultant pyrroles in potential yields (Table 3).
| R1 | R2 | |
|---|---|---|
| (a) | OMe | H |
| (b) | OEt | H |
| (c) | O (CH2)2OMe | H |
| (d) | OCH2CH=CH2 | H |
| (e) | OMe | Me |
| (f) | OEt | Et |
| (g) | OEt | n-Hex |
| (h) | OEt | n-Oct |
| (i) | OEt | n-Non |
| (j) | OEt | n-Dec |
| (k) | OEt | (CH2)6Cl |
| (l) | Me | H |
| (m) | Ph | H |
Table 3: Showing the two substituents R1 and R2 involved in the above reaction.
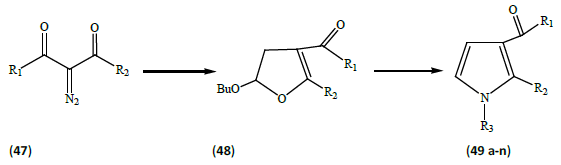
de Silva et al. [17] described an efficient one-pot synthesis of substituted pyrroles (9a-n). α-Diazocarbonyl compounds (47) were treated with a catalytic quantity of Rh(II) acetate in the presence of butyl vinyl ether to produce the corresponding 3-carbonyl-dihydrofurans (48). The reaction of the dihydrofuran intermediates with the excess of primary amines in presence of glacial acetic acid; afford the substituted pyrroles (49a-n) in moderate to good yields (Table 4).
| R1 | R2 | R3 | |
|---|---|---|---|
| (a) | OEt | Me | Benzyl |
| (b) | OEt | Me | Decyl |
| (c) | OEt | Me | Cyclohexyl |
| (d) | OEt | Me | Isopropyl |
| (e) | OEt | Me | Butyl |
| (f) | Me | Me | Decyl |
| (g) | Me | Me | Benzyl |
| (h) | Me | Me | Cyclohexyl |
| (i) | Me | Me | Isopropyl |
| (j) | Me | Me | Butyl |
| (k) | H | H | Benzyl |
| (l) | H | H | Butyl |
| (m) | H | H | Propyl |
| (n) | H | H | H |
Table 4: Showing the different substituents R1, R2 and R3 in reactants and products.
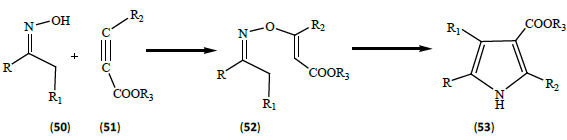
Ngwerume and Camp [18] reported gold catalyzed synthesis of highly substituted pyrroles (53) in an efficient and regiocontrolled process. First, O-vinyl oximes (52) were synthesized by the reaction of oximes (50) with electron deficient alkynes (51) using the nucleophilic catalyst DABCO. The second phase of the work was directed towards the catalytic rearrangement of O-vinyl oxime to pyrrole (53).
R=Ph, 4-NO2C6H4, 4-BrC6H4, Ph, Ph, Me, Me; R1=H, H, H, Ph, Me, Me, Et;
R2=COOMe, H; R3=Me, Et.
This method involves the gold as catalyst which later on produced pyrroles in very sufficient amounts. Since this gold catalyzed reaction is atom-economic, remarkably mild with regard to reaction conditions.
Yavari et al. [19] synthesized tetra substituted pyrrole derivatives (56a-l) by the reaction of enaminone (54) with haloketones (55), under solvent free conditions (Table 5).
| R1 | R2 | R3 | |
|---|---|---|---|
| (a) | OEt | n-Bu | 4-BrC6H4 |
| (b) | OEt | n-Bu | CO2Et |
| (c) | OEt | n-Bu | 4-MeOC6H4 |
| (d) | OEt | c-Hexyl | CO2Et |
| (e) | OEt | c-Hexyl | 4-MeOC6H4 |
| (f) | OEt | c-Hexyl | 4-BrC6H4 |
| (g) | OEt | Bn | CO2Et |
| (h) | OEt | Bn | 4-MeOC6H4 |
| (i) | OEt | Bn | 4-BrC6H4 |
| (j) | Me | Bn | CO2Et |
| (k) | Me | Bn | 4-MeOC6H4 |
| (l) | Me | Bn | 4-BrC6H4 |
Table 5: Showing the different substituent groups R1, R2 and R3 in reactants and products
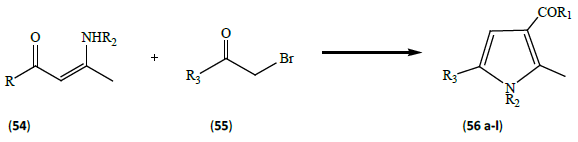
This reaction being the modification of Hantzsch’s protocol, produce high yields of products and involve solvent free conditions.
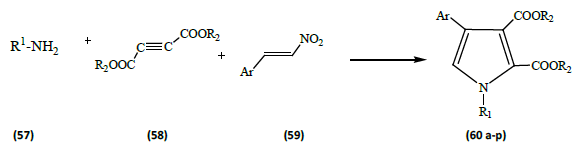
The three-component reaction of primary amines (57) [R1 as defined below], dialkyl acetylenedicarboxylate (58) [R2=Me, Et] and β-nitrostyrene derivatives (59) in the presence of Iron (III) chloride afforded 1, 2, 3, 4-tetra-substituted pyrroles (60 a-p) in high yields. These reactions could precede via domino Michel addition/cyclization process. [20] This reaction protocol being one-pot three components, fast, efficient mild and metal-free synthesis of substituted pyrrole heterocycles (Table 6).
| R1 | Ar | |
|---|---|---|
| (a) | Bn | Ph |
| (b) | Bn | Ph |
| (c) | Bn | 4-BrC6H4 |
| (d) | Bn | 4-ClC6H4 |
| (e) | Bn | 4-MeOC6H4 |
| (f) | Bn | 4-MeOC6H4 |
| (g) | Bn | 3-NO2C6H4 |
| (h) | CH3CH-Ph | Ph |
| (i) | CH3CH-Ph | 2-BrC6H6 |
| (j) | CH3CH-Ph | 4-ClC6H4 |
| (k) | CH3CH-Ph | 4-MeOC6H4 |
| (l) | CH3CH-Ph | 4-MeOC6H4 |
| (m) | CH2=CHCH2 | Ph |
| (n) | CH2=CHCH2 | Ph |
| (o) | Ph | Ph |
| (p) | 4-MeOC6H4 | Ph |
Table 6: Showing the different substituent groups R1, and Ar in the above reaction scheme.
Bandyopadhyay et al. [21] carried out the simple ultrasoundassisted eco-friendly practical method for the synthesis of N-substituted pyrroles (66) [R=Ph, 4-OMeC6H4, Py, naphthyl, tri-naphthyl, adamantly, crysenyl, pyrenyl, 1, 10-phenanthrolinyl, phenanthrenyl by reacting 2, 5-dimethoxytetrahydrofuran (64) with various amines (65) [R as defined above] in the presence of catalytic amounts (5 mol%) of bismuth nitrate pentahydrate under solvent free conditions. They also screened these new N-substituted compounds for in vitro cytotoxicity against a panel of mammalian cancer cell lines, which showed potential toxicity without being toxic to normal cancer lines.
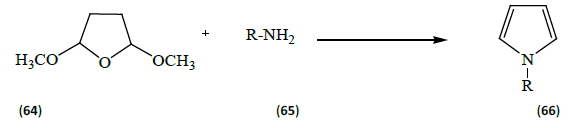
The classes of compounds synthesized by using bismuth nitrate as a catalyst are acting drugs for peptic ulcer, esophageal reflux diseases and antibacterial infections.
Zhang et al. [22] studied copper catalyzed [10 ml% of Cu(OAC)2] reaction of amines (67) [R=4-MeC6H4, 4-MeOC6H4, 3-MeOC6H4, 4-i-PrC6H4, 4-ClC6H4, 3-Br, 4-FC6H3, 4-FC6H4, 4-BrC6H4, 4-COOEtC6H4, 2, 4, 6-Me3C6H2, Benzyl-4-Me, Bn, Benzyl-4-F, n-Bu] with but-2-ynedioates (68) [R2=Et, Me] to furnish pyrrole-2, 3, 4, 5-tetracarboxylates (69) [R1, R2 as defined above] in moderate to good yields. The reaction proceeded in the presence of dioxygen with the formation of three bonds during the process.
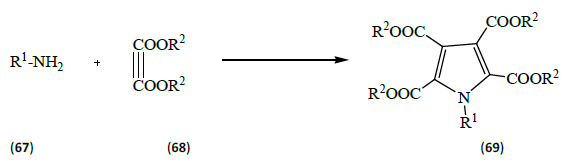
This reactions which is catalyzed by Cu (OAC)2 are highly atom-economical and environmentally benign which makes it ideal in many ways for further modifications and derivatizations.
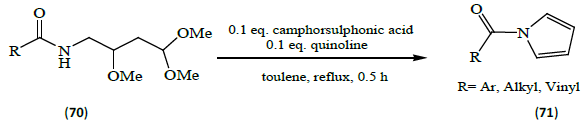
Maehara et al. [23] developed a method for the preparation of N-acylpyrroles (71) involving the condensation of carboxylic acids with 2, 4, 4-trimethoxybutan-1-amine (70), followed by acid mediated cyclization to form the pyrrole ring. The preparation procedure is highly tolerant to various functional groups.
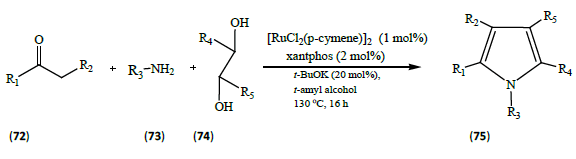
Zhang et al. [24] developed a highly regioselective synthesis of pyrroles (75) via ruthenium catalyzed three-component reaction. A variety of ketones (72), amines (73) and substituted diols (74) were supposed to react in presence of Ruthenium catalyst, potassium salt of t-butanol and t-amyl alcohol. The yields were obtained in good amounts. The utility of using the Ruthenium catalyst is it’s highly tolerance to various functional groups. The Ruthenium catalyst also allows for metathesis reactions to be performed at low temperatures, as well as for the formation of tetra substituted olefins via cross metathesis.
Kucukdisli et al. [25] developed the synthesis of disubstituted pyrroles (77) by placing a solution of cyanopyrrolene (76) in dichloromethane into a MW reaction vessel. The solvent was removed in vacuo and the vessel was flushed with argon, closed with a cap to yield the desired product.
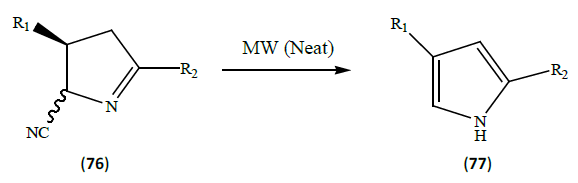
This reaction involves the microwave heating which involves the internal heat source. Microwave absorption is able to heat the target compounds without heating the entire oil bath, hence saved time and energy. Due to the Microwave assistance, the reaction got completed in few minutes. This reaction took 6 h to get completed under conventional refluxing procedure.
Results and Discussion
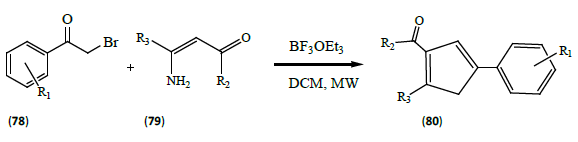
Reddy et al. [26] published a MW-activated synthetic protocol for a series of polysubstituted pyrroles (80). Added to a solution of 1 mmol of substituted phenacyl bromide (78) were 1 mmol substituted amino unsaturated ketone (79) and 10 mol% of BF3OEt3 in dichloromethane and the mixture was irradiated with MWs for 10-16 min at 130°C (250 W).
Adib et al. [27] showed the reaction of 4-nitro-1, 3-diarylbutan- 1-ones (81) and ammonium acetate (82) in the presence of morpholine and sulphur provides the corresponding 2, 4-diarylpyrroles (83) in excellent yields.
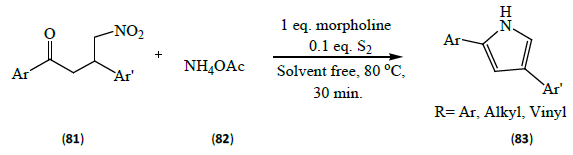
Morpholine is used to create all volatile environment and also due to its slow decomposition at high temperatures and pressures. Further its low cost and polarity leads to its common use as a solvent for chemical reaction. That is why the reaction shown above is solvent free.
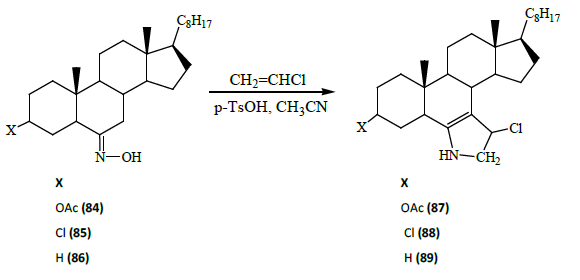
Ayaz M Dar et al. [28] revealed a convenient procedure for the synthesis of 3β-acetoxy3′-chloro-5α-cholest-6-eno [7, 6-d] 2′, 3′-dihydro-1H-pyrrole (87), 3β, 3′-dichloro-5α-cholest-6-eno [7, 6-d] 2′, 3′-dihydro-1H-pyrrole (88) and 3′-chloro-5α-cholest-6- eno [7, 6-d] 2′, 3′-dihydro-1H-pyrrole (89) has been made from steroidal oximes (84-86) under refluxing conditions.
Conclusion
These protocols provide convenient strategies to synthesize these pyrrole derivatives or to annelate different heterocyclic nuclei with widespread bioactive pyrroles thereby extending the categories of heterocyclic systems. These strategies also provide valuable information for the further design of more active biological agents through various modifications and derivatizations.
References
- Runge FF (1834) Ueber einige Produkte der Steinkohlendestillation. Ann Phys 31: 67-69.
- Hoppe FA (1868) The Chemistry of Pyrroles: Organic Chemistry: A Series of Monographs. Med Chem Untersuchungen 1871: 4-542.
- Sheradsky T (1970) The rearrangement of O-vinyloximes a new synthesis of substituted pyrroles. Tetrahedron Lett 11: 25-28.
- Trofimov BA, Korostova SE, Mikhaleva AI, Sobenina LN, Vasil’ev AN, et al. (1983) Pyrroles from ketoximes and acetylene. Acid hydrolysis of 1-vinylpyrroles. Chemischer Informationsdienst 14: 273-276.
- Mikhaleva AI, Trofimov BA, Vasil’ev AN, Zh Org Khim (1979) O-vinyl acetoxime. Org Khim 15: 602-606.
- Katrizsky AR, Rees CW, Scriven EFV, RJ Sundberg (1996) In Comprehensive Heterocyclic Chemistry II. Oxford 2: 149.
- Rao HSP, Jothilingam S (2001) One-pot synthesis of pyrrole derivatives from (E)-1, 4-diaryl-2-butene-1, 4-diones. Tetrahedron Lett 42: 6595-6598.
- Ranu BC, Dey SS (2003) An efficient synthesis of pyrroles by a one-pot, three-component condensation of a carbonyl compound, an amine and a nitroalkene in a molten ammonium salt. Tetrahedron Lett 44: 2865-2868.
- Bartona DHR, Kervagoret J, Zard SZ (1990) A useful synthesis of pyrroles from nitroolefins. Tetrahedron 46: 7587-7591.
- Nakamura M, Hara K, Sakata G, Nakamura E (1999) One-pot synthesis of pyrroles through carbometalation reaction of zincated hydrazone with vinylstannane. Org Lett 1: 1505-1508.
- Wang Y, Bi X, Li D, Liao P, Wang Y, et al. (2011) Iron-catalyzed synthesis of polysubstituted pyrrolesvia [4C+1N] cyclization of 4-acetylenic ketones with primary amines. Chem Commun 47: 809-811.
- Wang HY, Mueller DS , Sachwani RM, HN Londino, LL Anderson (2010) Carbon-carbon bond formation and pyrrole synthesis via the [3, 3] sigmatropic rearrangement of O-vinyl oxime ethers. Org Lett 12: 2290-2293.
- Bergner I, Opatz T (2007) Modular one-pot synthesis of tetrasubstituted pyrroles from α-(alkylideneamino) nitriles. J Org Chem 72: 7083-7087.
- Settambolo R, Guazzelli G, L Mengali, AMR Lazzaroni (2003) New class of optically active pyrrole derivatives: (3R)-3-(pyrrol-1-yl)alk-1-enes from d-α-aminoacids. Tetrahedron 14: 2491-2496.
- Alizadeh A, Rezvanian, Zhu GL (2008) One-pot synthesis of 4, 5-dihydro-1H-pyrrol-3-carboxamide derivatives via a four-component reaction. Tetrahedron 64: 351.
- Bellur E, Langer P (2006) Synthesis of functionalized pyrroles and 6, 7-dihydro-1H-indol-4(5H)-ones by reaction of 1, 3-dicarbonyl compounds with 2-azido-1, 1-diethoxyethane. Tetrahedron Lett 47: 2151-2154.
- de Silva FC, fonseca MG, de Rianelli RS, Cunha AC, Souza MCBV (2008) One-pot preparation of substituted pyrroles from α-diazocarbonyl compounds. J Org Chem 4: 45-49.
- Ngwerume S, Camp JE (2011) Gold-catalysed rearrangement of O-vinyl oximes for the synthesis of highly substituted pyrroles. Chem Commun 47: 1857-1860.
- Yavari I, Ghazvini M, Azad L, SanaeishoaR T (2011) A solvent-free synthesis of 1, 2, 3, 5-tetrasubstituted pyrroles from enaminones and α-haloketones Chinese. Chem Lett 22: 1219-1222.
- Ghabraie E , Balalaie S,Bararjanian M, Bijanzad HR, Rominge F (2011) An efficient one-pot synthesis of tetra-substituted pyrroles. Tetrahedron 67: 5415-5420.
- Bandyopadhyay D, Mukherjee S, Granados JC, Short JD, Banik BK (2012) Ultrasound-assisted bismuth nitrate-induced green synthesis of novel pyrrole derivatives and their biological evaluation as anticancer agents. J Med Chem 50: 209-215.
- Zhang L, Wang X, Li S, Wu J (2013) Synthesis of pyrrole-2, 3, 4, 5-tetracarboxylates via a copper-catalyzed reaction of amine with but-2-ynedioate. Tetrahedron 69: 3805-3811.
- Maehara T, Kanno R, Yokoshima S, Fukuyama T (2012) A practical preparation of highly versatile N-acylpyrroles from 2, 4, 4-trimethoxybutan-1-amine. Organic Letters 14: 1946-1948.
- Min Zhang, Fang X, Neumann H, Beller M (2013) General and regioselective synthesis of pyrroles via ruthenium-catalyzed multicomponent reactions. J Am Chem Soc 30: 11384-11388.
- Kucukdisli, Ferenc M, Heinz D, Wiebe M, Opatz C, et al. (2014) Simple two-step synthesis of 2, 4-disubstituted pyrroles and 3, 5-disubstituted pyrrole-2-carbonitriles from enones. J Org Chem 10: 466-472.
- Reddy, Reddy VH, Reddy GM, Reddy MT, YVR Reddy (2015) Microwave-assisted facile synthesis of trisubstituted pyrrole derivatives. Res Chem Intermed 41: 9805-9815.
- Adib M, Ayashi N, Heidari F, Mirzaei P (2016) Reaction between 4-Nitro-1, 3-diarylbutan-1-ones and ammonium acetate in the presence of morpholine and sulfur: An efficient synthesis of 2, 4-diarylpyrroles. Synlett 27: 1738-1742.
- Ayaz Mahmood Dar, Shafia Mir, Masrat Jan, Shamsuzzaman (2017) Spectroscopic studies of newly synthesized steroidal pyrroles. Eur Chem Bull 6: 321-324.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences