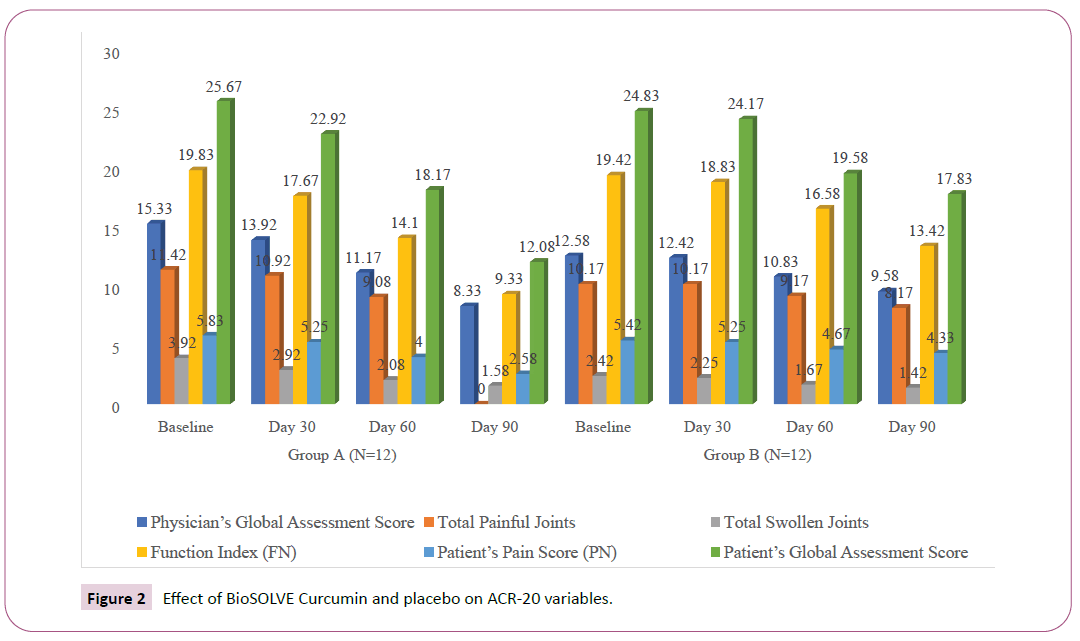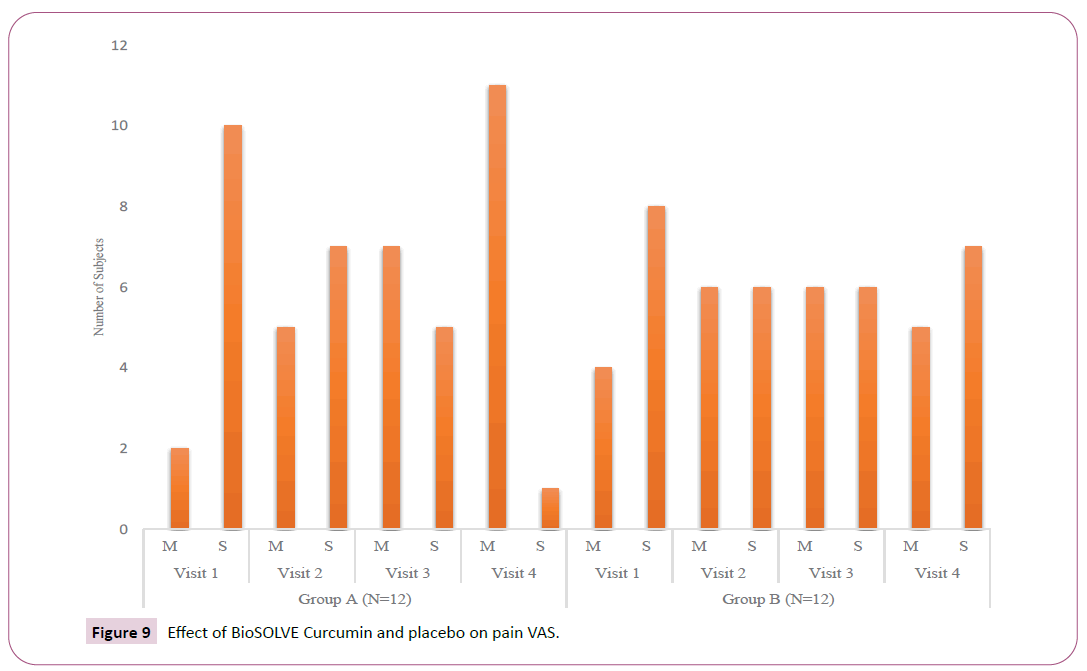ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Safety and Efficacy of Biosolve Curcumin in Active Rheumatoid Arthritis: A Randomized Double-Blind Placebo-Controlled Clinical Study
1Zeus-Hygia LifeSciences Pvt Ltd, Hyderabad, Telangana, India
2Bio Agile Therapeutics Pvt Ltd, Bengaluru, Karnataka, India
3Sri Krishna Sevashrama Hospital, Bengaluru, Karnataka, India
- *Corresponding Author:
- Shankaranarayanan Jeyakodi
ZeusHygia LifeSciences Pvt Ltd
Hyderabad, Telangana, India
Tel: + 04048539555
E-mail: ljjshankar2002@gmail.com
Received Date: November 16, 2020; Accepted Date: January 20, 2021; Published Date: January 27, 2021
Citation: Jeyakodi S, Nai AK, Divya KJ, Chandradhara P, Rani MS (2021) Safety and Efficacy of Biosolve Curcumin™ in Active Rheumatoid Arthritis: A Randomized Double-Blind Placebo-Controlled Clinical Study. Am J Phytomed Clin Ther Vol.9 No.2:5.
Abstract
Rheumatoid Arthritis (RA) is a chronic inflammatory disease. Curcumin is wellknown to alleviate the symptoms of RA. Curcumin has low aqueous solubility and bioavailability, BioSOLVE Curcumin™ is a water-soluble and bioavailable form of curcumin. The safety and efficacy of BioSOLVE Curcumin™ in the complications of RA were assessed. A total of 24 participants were randomized to BioSOLVE Curcumin™ 250 mg and placebo group Supplementation was administered twice daily after food for 12 weeks consecutively. The demographic, efficacy (ACR-20 assessments, WOMAC index and VAS pain) and safety parameters were recorded at baseline and efficacy parameters were assessed on day 30, 60, 90 and safety parameters were assessed on day 90. All participants completed the study. The superior improvement was observed in ACR-20 criteria and WOMAC index in the BioSOLVE Curcumin™ 250 mg group as compared to the placebo group (p <0.05). A significant transition of participants from severe to moderate pain categories based on VAS was observed in the intervention group. No adverse events (AEs) or serious adverse events (SAEs) occurred throughout the study. The improvement in ACR-20, WOMAC index and VAS score indicates the improved efficacy of curcumin in the complication of RA when administered as BioSOLVE Curcumin™ at the selected dose.
Keywords
Curcumin; Curcuminoids; Bioavailability; Arthritis; Rheumatoid arthritis
Abbreviations
ACR: American College of Rheumatology; AE: Adverse Events; AP-1: Activator Protein -1; CONSORT: Consolidated Standards of Reporting Trials; COX-2: Cyclooxygenase-2; CTRI: Clinical Trials Registry-India; DMARD: Disease- Modifying Anti-Rheumatic Drugs; Egr-1: Early Growth Response Protein 1; GSK3β: Glycogen Synthase Kinase 3 Beta; HAQ: Health Assessment Questionnaire; HPMC: Hydroxypropyl Methylcellulose; ITT: Intent to Treat; LOX: Lipoxygenase; MAP: Mitogen-Activated Protein; MCC: Microcrystalline Cellulose; MMP3: Matrix Metalloproteinase-3; NF-kB: Nuclear Factor Kappa-B; Nrf-2: Nuclear Factor- Erythroid-2-Related Factor 2; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; RA: Rheumatoid Arthritis; SAE: Serious Adverse Events; SJC: Swollen Joint Count; STAT: Signal Transducers And Activators Of Transcription; TJC: Tender Joint Count; TMF: Trial Master File; VAS: Visual Analogue Scale; WOMAC Index: Western Ontario and McMaster Universities Osteoarthritis Index
Introduction
Rheumatoid Arthritis (RA) is one of the prominent chronic inflammatory diseases. In RA, the clinical responses varied as the treatment changes. Treatment targets were not achieved in some of the participants due to low disease activities or disease remission. The RA modern treatment failure in many of the participants was observed due to genetic profile, joint erosion, presence or development of autoantibodies, pregnancy, and comorbidities. The existing pharmacotherapies to treat RA are disease-modifying anti-rheumatic drugs (DMARD), oral and systemic glucocorticoids, non-steroidal anti-inflammatory drugs (NSAIDs) and the newer biological treatments. These therapies are typically associated with severe side effects including osteoporosis, hypertension, gastrointestinal bleeding, hepatotoxicity, ocular toxicity, myelosuppression, hypersensitivity and risk of infection [1].
The well-known bioactive molecules such as curcumin, celastrol, thymoquinone, hesperidin etc., showed high efficacy to treat the RA symptoms in a dose-dependent manner. The anti-rheumatic activity can be attributed to their suppressive ability against various inflammatory mediators including adhesion molecules, Nuclear Factor kappa-B (NF-kB), lipoxygenase (LOX), arachidonic acid cytokines etc. [2].
Although these bioactive molecules exhibited good activity, there are several challenges to utilize them efficiently such as low aqueous solubility hence the difficulty in absorption from the gastrointestinal tract, a higher rate of first –pass metabolism resulting into poor bioavailability. However, the techniques of nano/sub-micro medicine are gaining wider attention in formulations of bioactive molecules to enhance aqueous solubility and bioavailability hence better efficacy.
Curcumin is the widely known bioactive molecule isolated from the common spice turmeric. Curcumin exhibited a wide spectrum of biological activities by modulating several transcription factors and signaling pathways. It has numerous functional groups exhibiting antioxidant activity, through modulating redoxsignaling pathways in cells. Curcumin activates the intracellular antioxidant defense system via stimulating of nuclear factorerythroid- 2- related factor 2 (Nrf-2) [3]. Curcumin is a potent and well known anti-inflammatory dietary plant component which inhibits all the inflammatory mediators such as adhesion molecules and growth factors, chemokines, cytokines, cyclooxygenase-2 (COX-2), tissue factor, inducible nitric oxide and epigenetic alterations. These anti-inflammatory abilities of curcumin are due to its ability to inhibit the NF-kB pathway and other pro-inflammatory signaling pathways such as activator protein -1 (AP-1), COX-2, Early growth response protein 1 (Egr-1), signal transducers and activators of transcription (STAT), members and mitogen-activated protein (MAP) kinases [4]. Owing to such multiple activities curcumin is currently being evaluated in several human clinical trials for several diseases, including cardiovascular diseases, type 2 diabetes, Alzheimer’s disease, rheumatoid arthritis, multiple sclerosis and a variety of human cancers [5]. In view of the overwhelming literature and promise for the potential benefits of curcumin- induced chemoprevention and treatment of several human diseases, the current pilot study was designed and executed to assess the efficacy and safety of BioSOLVE CurcuminTM in comparison with placebo for 12 weeks in participants with active rheumatoid arthritis in knees, hands and neck.
BioSOLVE Curcumin™ is water soluble/dispersible and bioavailable curcumin powder from curcumin 95% (Curcuma Longa L.) manufactured and supplied by Zeus-Hygia LifeSciences Pvt. Ltd. It is a value added and formulated nutraceutical product with the advantage of highly bioavailable curcumin for its intended pharmacological effect. Curcumin is the major bioactive component of the spice herb turmeric or Curcuma longa L., a widely used natural food product in curry powder. Curcumin being poorly water soluble; It is absorbed less from the gastrointestinal tract and gets rapidly metabolized causing faster systemic elimination [6]. The major portion of ingested curcumin is excreted through the feces as unchanged, the absorbed portion of curcumin is extensively converted to its water-soluble metabolites, glucuronides and sulfates [7]. The solubility of Curcumin 95% is relatively extremely poor, and the in vivo absorption and bioavailability is subsequently very minimal. Hence, a large amount of Curcumin must be taken orally to have some systemic effects [8]. BioSOLVE Technology is a patent filed novel platform technology from ZeusHygia which enables conversion of poorly water insoluble bioactive into highly water soluble/dispersible and bioavailable form. This technology is based up on multistage solubilizing process of active with the selected unique excipient combinations prior to proceeding with microencapsulation of active into a hydrophilic carrier system.
The current study was conducted to assess BioSOLVE Curcumin™ beneficial action and safety in the treatment of RA of knee, hands and neck based on the available literature published by various researchers worldwide [9]. BioSOLVE CurcuminTM is manufactured and marketed by ZeusHygia LifeSciences Pvt Ltd. BioSOLVE Curcumin™ is dispersible/soluble & bioavailable Curcumin powder produced from Curcumin 95% extract (Curcuma Longa). The product was administered to the participants with active RA for a period of 90 days (3 months) and the efficacy was evaluated in terms of ACR-2 [10] using subjective questionnaires.
Research Methodology
Participants
At the Sri Krishna Sevashrama Hospital, Bengaluru 24 participants with RA were enrolled from 20 August 2019 and 04 December 2019. Participants were numbered in order of admission and randomly assigned to the intervention and control groups in the ratio 1:1 (12 participants in each group). There was no participant withdrawal or dropout in either groups or all the 12 enrolled participants in each group completed the study. Written informed consent was obtained from participants. The study protocol was reviewed and approved by ACE Independent Ethics Committee before the conduct of the study on 16/07/2019. The study was registered at Clinical Trials Registry-India (CTRI) with registration no CTRI/2019/08/020535.
The inclusion criteria were participants age ranging from 18 to 65 years who are diagnosed with RA according to the revised 2010 American College of Rheumatology (ACR) criteria (with RA functional class of II), at least eight swollen joint counts (SJC) and tender joint count (TJC), recurring pain, secondary Sjogren's syndrome or limited cutaneous vasculitis. Exclusion criteria were treatment with any DMARD or NSAIDs within 30 days before enrolment, any concomitant disorder, significant systemic involvement of secondary RA, woman planning for pregnancy or in lactation period, other systemic immune disorder, history of alcohol abuse, history of hyperglycemia and motor coordination disorder, any surgical procedure related to bone, joint or synovectomy within 12 weeks before screening.
Withdrawal criteria
Participants were free to withdraw from the study at any time. Withdrawal of subject from the study was as per the investigator's discretion based on any condition or circumstances rendering the subject ineligible for further participation in the study.
Randomization and Blinding
Participants who met the conditions and signed informed consent forms were numbered according to the order of admission and randomly assigned to the intervention or control group in 1:1 ratio. The randomization sequence was generated using validated computer software by an independent statistician. The packaging and labelling of the investigational product (IP) as per randomization chart were prepared by an independent team not involved in the study conduct. Clinical operation team involved in the study, as well as the investigator, was blinded for entire randomization as well labelling process.
Intervention
The intervention used in this study was biosolve curcumintm dispersible & bioavailable curcumin powder from curcumin 95% (Curcuma Longa) manufactured and supplied by ZeusHygia Lifesciences Pvt. Ltd. Biosolve curcumintm was provided in 250 mg zero size HPMC capsule. The comparator was placebo capsule containing microcrystalline cellulose (MCC). Both the treatment and placebo capsules were without any flavor and identical in color and shape. As per the randomization, subjects received two capsules of 250 mg of placebo or treatment twice daily after food with a glass of water for 90 consecutive days. The time of administration of capsules was approximately the same for each day over the study period.
All subjects were given calculated quantity of IP at each visit. Accountability of consumed versus remaining IP for the earlier visit was done at a subsequent visit. The percentage of treatment compliance was calculated at each visit. The protocol defined criteria for treatment compliance was considered as ≥ 90% compliance.
Concomitant therapy
A list of permitted and prohibited concomitant medications was provided to each investigator before screening visit. Medical history of prior medication was taken and documented at screening visit for each subject. The same practice for concomitant medication was followed at each subsequent visit. Compliance to this list by investigator and/ or subject was monitored during each visit. Any drug or supplement which as per investigator’s opinion could impact the study results was prohibited during the study.
Allocation concealment
Individual treatment allocation code (active or placebo) was kept in a tamper-evident envelope for each subject ID with limited access at the site. The master randomization chart was sealed in an envelope and maintained in the Trial Master File (TMF).
Study design
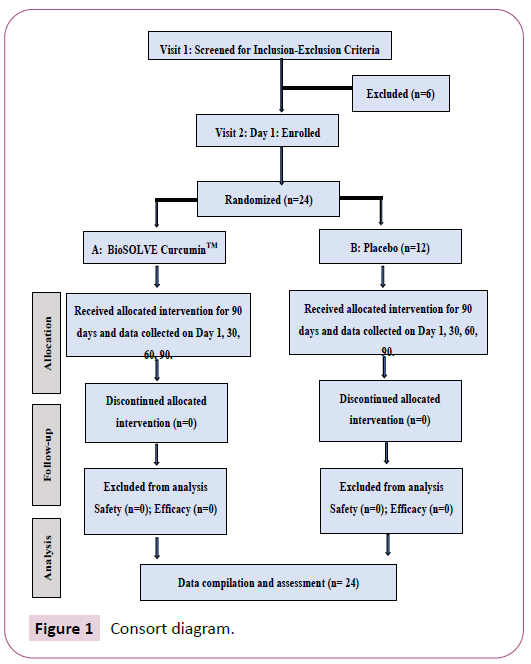
A double-blind, randomized, placebo-controlled parallel-group study conducted to evaluate the safety and efficacy of BioSOLVE CurcuminTM versus placebo for management of symptoms in participants with active rheumatoid arthritis in knees, hands, and neck. The study was conducted at two sites at Bengaluru, India. The study was conducted in accordance with the principles of Good Clinical Practice according to the International Council on Harmonization Guidelines. Ethics review procedures were conducted according to the Declaration of Helsinki and local laws and regulations. Written informed consent was obtained from all participants before participation in the study. A total of 24 eligible participants were enrolled in the study and randomized in two groups in the ratio 1:1 (n=12 in each group) to receive biosolve curcumintm or placebo for 90 days. They were evaluated over 4 visits, including screening visit, for predetermined efficacy variables. Total study duration was almost 12 weeks during which all subjects were closely monitored for protocol compliance and assessed for efficacy and safety parameters. Subjects were evaluated at 4 visits, for predetermined efficacy variables at Day – 7 to 0 (Screening / Baseline Visit), Day 30 ± 2 (Visit 2), Day 60 ± 2 (Visit 3) and Day 90 ± 2 (end of study Visit 4). All subjects were counselled regularly against the use of prohibited medications. Adverse events were monitored throughout the study period. General study participant’s flow diagram is depicted as a CONSORT Diagram (Figure 1). All 24 enrolled participants completed the study and there was no withdrawal or dropout participant in this study.
Primary efficacy endpoint
The primary efficacy endpoint was the mean change from baseline in an American college of Rheumatology 20% at each visit Day 1, 30, 60 and 90. As per the ACR-20% definition, a participant with RA must improve by at least 20% in TJC and SJC and at least 20% improvement in three out of five ACR core set measures: participant global assessment, physician global assessment, health assessment questionnaire (HAQ), an acutephase reactant, and participant’s pain assessment.
Secondary efficacy endpoints
Secondary endpoints were WOMAC index and VAS score changed from baseline. Both parameters compared the improvement from Baseline visit to Day 1, 30, 60 and 90 visits. The individual components of the WOMAC were pain, stiffness, and physical function. The scores for each subscale were summed up, with a possible score range of 0-20 for Pain (5 questions, pain while walking, climbing stairs, sleeping in the night, resting and standing), 0-8 for Stiffness (2 questions, rating stiffness at morning and evening), and 0-68 for Physical Function (17 questions evaluating the difficulties in general day to day activities).
A horizontal line indicated with unit markings from 0 to 10 was used as a VAS in this study. This scale was labelled with the pain intensity from no pain to worst pain. The units on the scale indicating the pain intensity was 0=No pain, 1 to 3=Mild pain, 4 to 6=Moderate pain, 7 to 9=Severe pain and 10=Worst pain. Subjects instructed to mark on this scale to represent a relevant amount of pain experienced by them and the value was noted in centimeter. Efficacy analysis was performed in the ITT population. Efficacy analysis of outcome variables was based on mean changes from baseline to endpoint in the ITT population.
Safety assessment
Safety variables were assessed at baseline and end of study visits. Blood pressure, pulse rate and respiratory rate monitoring were done at all visits. Subjects were screened for Adverse Events (AE) and Serious Adverse Events (SAE) at all visits. Safety assessments were performed on all participants who consumed any masked study medication.
Statistical analyses
Considering minimum 20% improvement from the test product compared to placebo and the expected standard deviation of 5 with the type I and type II error are 5% and 20% respectively, the sample size was calculated as N=24. The nominal effect size obtained in the earlier clinical studies conducted on various curcumin formulations ranged from moderate to high as 0.6 to 1. Considering the effect of perfect ‘1’, α (Type I error rate)=0.05, β (Type II error rate)=0.3, the proportion of subject in each group=0.5, and a standard deviation of ‘1’, the total sample size equals 25 with N0=13 and N1=12. This data suggested a sample size of 24 subjects was sufficient to detect the change from baseline in efficacy variables to reject the null hypothesis. The assessment of all the primary and secondary parameters was calculated in 12 participants in each group.
Statistical analyses were performed by using the software’s IBM SPSS-20 and Microsoft Excel (version 13) to evaluate the efficacy of BioSOLVE CurcuminTM in comparison with the placebo group. The data were first analyzed for normal distribution. After confirming its parametric nature, Student’s paired and unpaired t-tests were applied for assessment of the statistical significance of the study results for both treatment groups. Only the results with p ≤ 0.05 were considered statistically significant to rule out the null hypothesis.
Results
Demographic and baseline characteristics
Descriptive statistics comparing demographic variables (age and weight), baseline efficacy variables (ACR-20%, WOMAC Indextotal and WOMAC subscales assessments) and safety parameters (heart rate) are described in Table 1. There were no significant differences in demographic and baseline outcome measures between treatment and placebo groups.
| Variables | BioSOLVE Curcumin™ | Placebo | P-value |
|---|---|---|---|
| n=12 | n=12 | ||
| Demographic parameters | |||
| Age (Years) | 41.27 ± 13.27 | 43.89 ± 11.05 | 0.636 |
| Weight (Kg) | 68.42 ± 9.67 | 69.92 ± 7.65 | 0.678 |
| Safety Parameters | |||
| HR (Beats/ minute) | 76.50 ± 5.14 | 77.42 ± 4.56 | 0.649 |
| SBP (mmHg) | 123.17 ± 8.38 | 122.67 ± 4.77 | 0.859 |
| DBP (mmHg) | 81.33 ± 6.68 | 80.33 ± 4.42 | 0.67 |
| Respiratory rate (Breaths per minute) | 14.83 ± 1.75 | 15.08 ± 1.93 | 0.743 |
| ACR-20 Assessment | |||
| Physician’s Global Assessment Score | 15.33 ± 2.96 | 12.58 ± 3.26 | 0.042 |
| Total Painful Joints | 11.42 ± 2.54 | 10.17 ± 2.33 | 0.222 |
| Total Swollen Joints | 3.92 ± 1.93 | 2.42 ± 1.51 | 0.046 |
| Function Index (FN) | 19.83 ± 2.79 | 19.42 ± 2.57 | 0.708 |
| Patient’s Pain Score (PN) | 5.83 ± 1.47 | 5.42 ± 1.31 | 0.471 |
| Patient’s Global Assessment Score | 25.67 ± 2.93 | 24.83 ± 3.66 | 0.545 |
| WOMAC Scale Assessment | |||
| WOMAC-Pain | 11.33 ± 1.72 | 10.42 ± 1.78 | 0.214 |
| WOMAC-Stiffness | 4.83 ± 0.83 | 4.08 ± 0.79 | 0.034 |
| WOMAC-Physical Function | 39.08 ± 4.78 | 34.58 ± 5.98 | 0.054 |
| WOMAC Index-Total | 54.50 ± 6.16 | 50.33 ± 6.60 | 0.124 |
| Pain VAS (%) | |||
| Moderate | 16.67% | 33.32% | - |
| Severe | 83.33% | 66.64% | - |
Table 1: Demographic and baseline characteristics of intervention and control groups at baseline.
Primary efficacy analysis
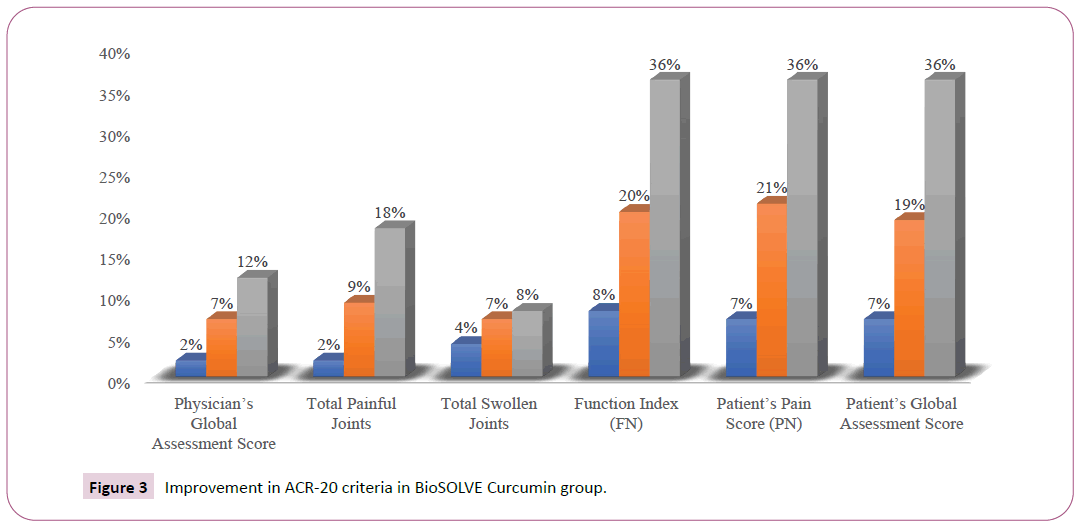
A significant improvement in ACR-20 criteria was observed in both intervention and placebo groups, although the improvement was superior in the intervention group. A minor placebo effect was observed on the three parameters assessed by participant viz. participant pain assessment, participant global assessment and participant self-assessed disability. In intervention, group improvement was observed in total painful joints (18%), function index (36%), participant pain score (36%) and participants global assessment score (36%). In placebo group only function index (20%), participant pain score (20%) and participant’s global assessment score (22%) showed the improvement which might be purely due to the placebo effect. At the end of the treatment (Day 90), intervention group exhibited an improvement of 12% in as compared to an improvement of 5% placebo group in RA symptoms as assessed by physician’s global assessment. At end of study visit (Day 90), the total number of painful joints and swollen joints were decreased by 18% and 8% respectively in the intervention group as compared to 8% and 4% decrease respectively, in the placebo group. An improvement of 20% and 10% in function index was observed in intervention and placebo group respectively, at Visit 3 (Day 60). The function index was further improved to 36% and 20% in intervention and placebo group respectively at the end of the study visit (Day 90). Similarly, an improvement of 19% and 10% in RA symptoms as assessed by the participant’s global assessment score was observed in intervention and placebo group respectively, at Visit 3 (Day 60). The participant’s global assessment score was further improved to 36% and 20% in intervention and placebo group respectively, at the end of the study visit (Day 90) (Table 2, Figures 2, 3, and 4).
| BioSOLVE Curcumin TM | Placebo | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameters | Visit 1 (Baseline) | Visit 2 | Visit 3 | Visit 4 | Visit 1 (Baseline) | Visit 2 | Visit 3 | Visit 4 |
| (n=12) | (Day 30) | (Day 60) | (Day 90) | (n=12) | (Day 30) | (Day 60) | (Day 90) | |
| (n=12) | (n=12) | (n=12) | (n=12) | (n=12) | (n=12) | |||
| Physician’s Global Assessment Score | 15.33 ± 2.96 | 13.92 ± 2.91 | 12.58 ± 3.26 | |||||
| (27 ± 5%) | (25 ± 5 %) | 11.17 ± 2.69 | 8.33 ± 2.10 | (22 ± 6%) | 12.42 ± 3.20 | 10.83 ± 2.69 | 9.58 ± 2.07 | |
| (20 ± 5%) | (15± 4%) | (22± 6%) | (19 ± 5%) | (17 ± 4%) | ||||
| % change | - | 2% | 7% | 12% | - | 0% | 3% | 5% |
| Total Painful Joints | 11.42 ± 2.54 | 10.92 ± 2.54 | 9.08 ± 2.50 | 1.75 ± 2.26 | 10.17 ± 2.33 | 10.17 ± 2.33 | 9.17 ± 2.29 | 8.17 ± 1.75 |
| (41 ± 9%) | (39 ± 9 %) | (32 ± 9%) | (23 ± 10%) | (36 ± 8%) | (36 ± 8%) | (33 ± 8%) | (28 ± 8%) | |
| % change | - | 2% | 9% | 18% | - | 0% | 3% | 8% |
| Total Swollen Joints | 3.92 ± 1.93 | 2.92 ± 1.51 | 2.08 ± 1.08 | 1.58 ± 0.67 | 2.42 ± 1.51 (9 ± 5%) | 2.25 ± 1.42 | 1.67 ± 1.07 | 1.42 ± 0.90 |
| (14 ± 7%) | (10 ± 5%) | (7 ± 4%) | (6 ± 2%) | (8 ± 5%) | (6 ± 4%) | (5 ± 3%) | ||
| % change | - | 4% | 7% | 8% | - | 1% | 3% | 4% |
| Function Index (FN) | 19.83 ± 2.79 | 17.67 ± 3.14 | 14.10 ± 2.66 | 9.33 ± 1.44 | 19.42 ± 2.57 | 18.83 ± 2.48 | 16.58 ± 1.93 | 13.42 ± 2.02 |
| (67 ± 12%) | (59 ± 10 %) | (47 ± 9%) | (31 ± 5%) | (65 ± 8%) | (63 ± 8%) | (55 ± 6%) | (45 ± 7%) | |
| % change | - | 8% | 20% | 36% | - | 2% | 10% | 20% |
| Patient’s Pain Score (PN) | 5.83 ± 1.47 | 5.25 ± 1.48 | 4.00 ± 1.04 | 2.58 ± 0.67 | 5.42 ± 1.31 (60 ± 15%) | 5.25 ± 1.29 | 4.67 ± 1.07 | 4.33 ± 2.64 |
| (65 ± 16%) | (58 ± 16%) | (44 ± 12%) | (29 ± 7%) | (58 ±14 %) | (52 ± 12%) | (40 ± 12%) | ||
| % change | - | 7% | 21% | 36% | - | 2% | 8% | 20% |
| Patient’s Global Assessment Score | 25.67 ± 2.93 | 22.92 ± 3.32 | 24.83 ± 3.66 | |||||
| (66 ± 8%) | (59± 8%) | 18.17 ± 3.07 | 12.08 ± 1.62 | (64 ± 9%) | 24.17 ± 3.38 | 19.58 ± 5.20 | 17.83 ± 2.86 | |
| (47 ± 8%) | (30 ± 10%) | (62 ± 9%) | (54 ± 7%) | (40 ± 13%) | ||||
| % change | - | 7% | 19% | 36% | - | 2% | 10% | 22% |
Table 2: Effect of BioSOLVE Curcumin and placebo on ACR-20 variables.
Secondary efficacy analysis
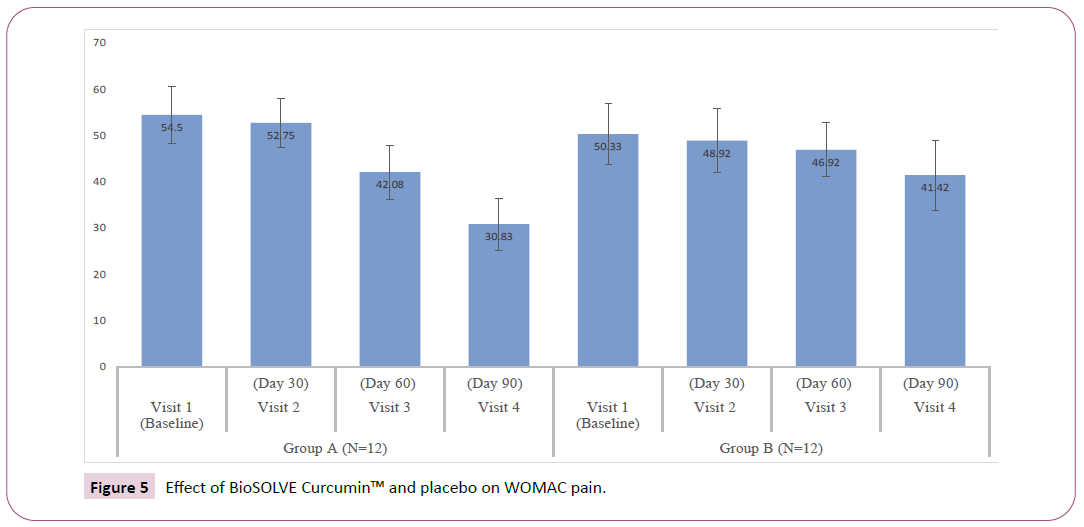
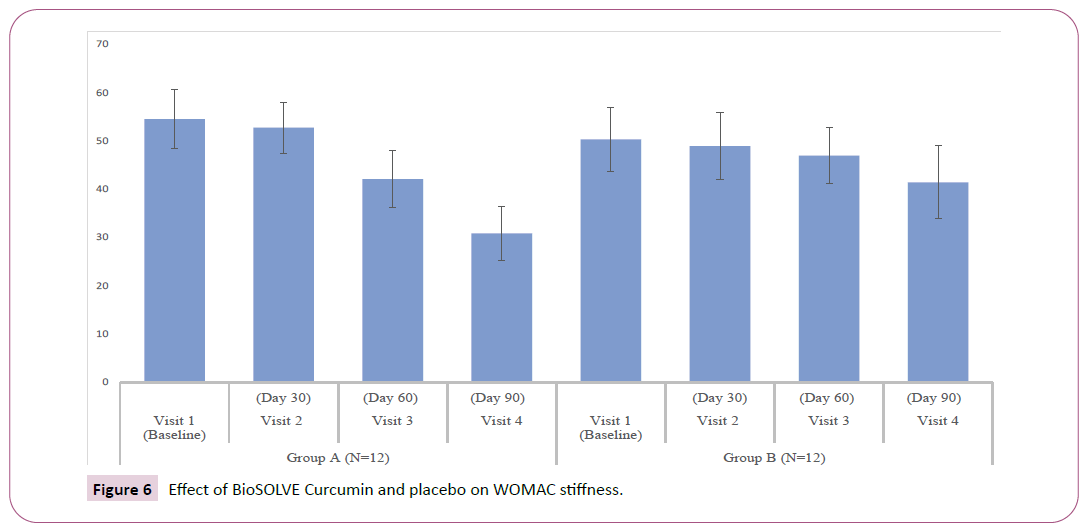
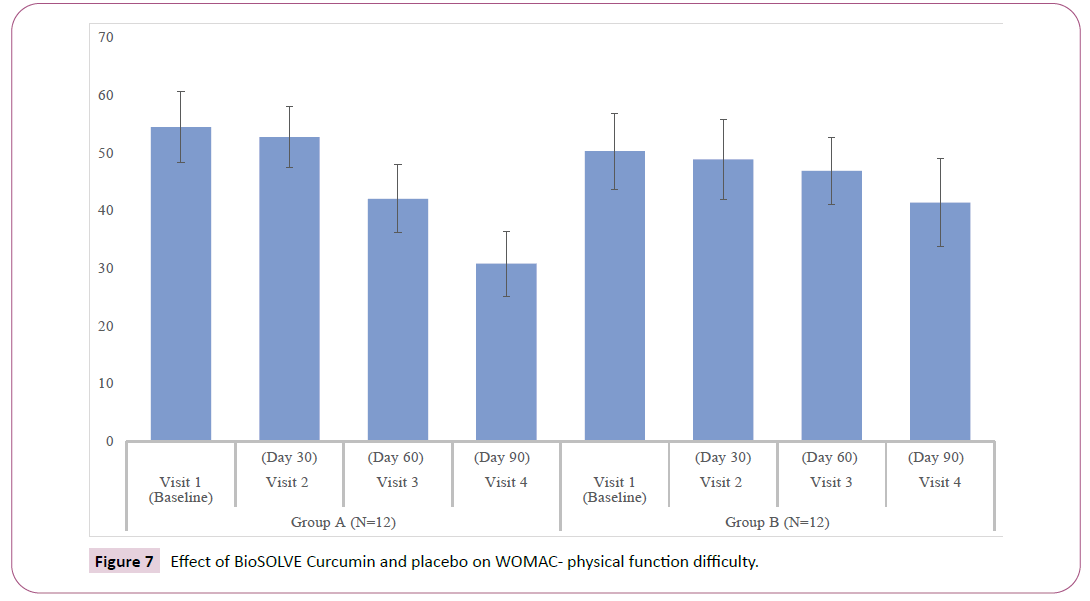
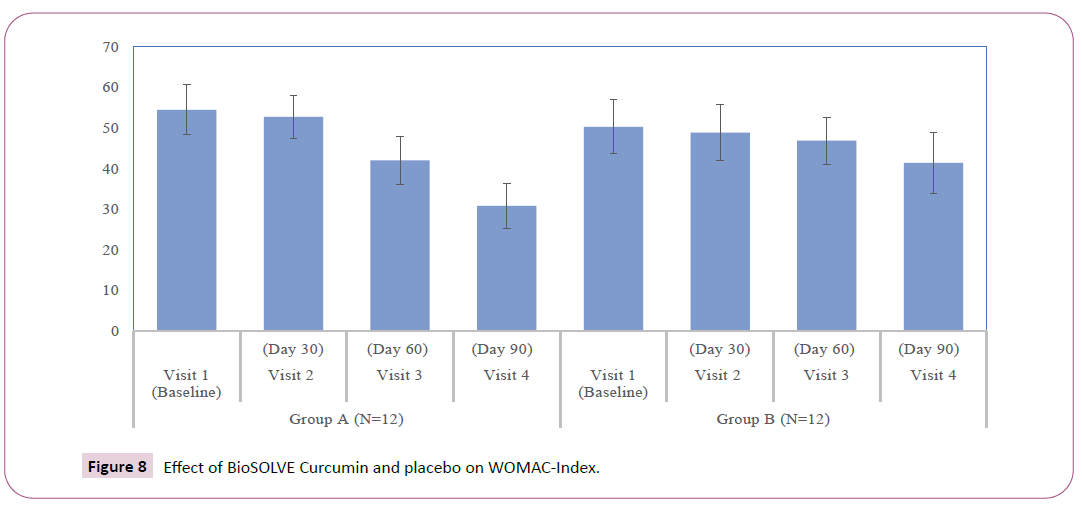
WOMAC index and subscales: The interventional group showed progressive decrease in WOMAC subscale scores as compared Baseline. A statistically significant reduction in pain score and stiffness intensity was observed within group at Day 30 (p<0.05), 60 (p<0.05) and 90 (p <0.05) (Figures 5 and 6). The significant reduction in difficulty in physical function was observed within group at Day 60 (p<0.05) and 90 (p<0.05) (Figure 7). In placebo group statically significant reduction was observed in stiffness intensity (p=0.017) and difficulty related to physical function (p<0.05) at Day 90. The statistically significant reduction in total WOMAC index was observed in both intervention and placebo group on Day 60 (p<0.05) and 90 (p<0.05) (Figure 8). In the placebo group, the reduction in pain score was insignificant at all the visits. The p-values of intergroup comparisons for the subscales are described in Table 3.
| BioSOLVE Curcumin™ | Placebo | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Visit 1 (Baseline) | Visit 2 | Visit 3 | Visit 4 | Visit 1 (Baseline) | Visit 2 | Visit 3 | Visit 4 | Intergroup p at EOS |
| (n=12) | (Day 30) | (Day 60) | (Day 90) | (n=12) | (Day 30) | (Day 60) | (Day 90) | ||
| (n=12) | (n=12) | (n=12) | (n=12) | (n=12) | (n=12) | ||||
| WOMAC Pain | 11.33 ± 1.72 | 10.00 ± 1.48 | 7.50 ± 1.00 | 5.00 ± | 10.42 ± 1.78 | 10.42 ± | 9.67 ± | 8.75 ± | < 0.05* |
| 1.54 | 1.78 | 1.3 | 2.26 | ||||||
| Change from baseline | - | 1.33 ± 0.49 | 3.83 ± | 6.33 ± | - | 0.00 ± 1.28 | 0.75 ± | 1.67 ± | < 0.05* |
| 1.53 | 1.67 | 1.96 | 3.11 | ||||||
| Within-group p | - | < 0.05* | < 0.05* | < 0.05* | - | 1 | 0.211 | 0.09 | - |
| WOMAC Stiffness | 4.83 ± 0.83 | 4.33 ± 0.98 | 3.50 ± | 2.50 ± | 4.08 ± 0.79 | 4.00 ± 0.85 | 3.92 ± | 3.25 ± | < 0.05* |
| 0.67 | 0.52 | 0.9 | 0.45 | ||||||
| Change from baseline | - | 0.50 ± 0.52 | 1.33 ± | 2.33 ± | - | 0.08 ± 0.29 | 0.17 ± | 0.83 ± | < 0.05* |
| 0.65 | 0.98 | 0.39 | 1.03 | ||||||
| Within-group p | - | < 0.05* | < 0.05* | < 0.05* | - | 0.338 | 0.166 | < 0.05* | - |
| WOMAC Physical Function | 39.08 ± 4.78 | 38.08 ± 4.76 | 31.17 ± | 23.75 ± | 34.58 ± 5.98 | 34.92 ± 4.74 | 33.42 ± | 28.92 ± | < 0.05* |
| 5.37 | 4.59 | 4.87 | 5.79 | ||||||
| Change from baseline | - | 1.00 ± 2.92 | 7.92 ± | 15.33 ± | - | -0.33 ± 4.36 | 1.17 ± | 5.67 ± | < 0.05* |
| 4.12 | 5.18 | 5.04 | 4.42 | ||||||
| Within-group p | - | 0.26 | < 0.05* | < 0.05* | - | 0.795 | 0.439 | < 0.05* | - |
| WOMAC Index | 54.50 ± 6.16 | 52.75 ± 5.29 | 42.08 ± | 30.83 ± | 50.33 ± 6.60 | 48.92 ± 6.95 | 46.92 ± | 41.42 ± | < 0.05* |
| 5.87 | 5.62 | 5.81 | 7.56 | ||||||
| Change from baseline | - | 1.75 ± 4.20 | 12.42 ± | 23.67 ± | - | 1.42 ± 2.31 | 3.42 ± | 8.92 ± | < 0.05* |
| 5.28 | 7.2 | 1.78 | 6.67 | ||||||
| Within-group p | - | 0.176 | <0.05* | <0.05* | - | 0.057 | <0.05* | <0.05* | - |
Table 3: Effect of BioSOLVE Curcumin™ and Placebo on pain VAS.
Change in VAS: The intervention group showed a cumulative transition of 25.02%, 41.68% and 75% subjects from severe pain category to moderate pain category at Day 30, 60 and 90, respectively. In placebo group a cumulative transition of 16.66%, 16.66% and 8.33% subjects from severe pain category to moderate pain category at Day 30, 60 and 90 respectively (Table 4 and Figure 9).
| Treatment group | Visit | Subjects with moderate pain, n (%) | Subjects with severe pain, | Percent change from Baseline in subjects with | Percent change from Baseline in subjects with |
|---|---|---|---|---|---|
| n (%) | moderate pain | severe pain | |||
| BioSOLVE Curcumin™ | Visit 1 (Baseline) | 2 (16.67%) | 10 (83.33%) | - | - |
| (N=12) | Visit 2 | 5 (41.65%) | 7 (58.31%) | 24.98% | -25.02% |
| (Day 30) | |||||
| Visit 3 | 7 (58.31%) | 5 (41.65%) | 41.64% | -41.68% | |
| (Day 60) | |||||
| Visit 4 | 11 (91.63) | 1 (8.33) | 74.96% | -75% | |
| (Day 90) | |||||
| Placebo | Visit 1 (Baseline) | 4 (33.32%) | 8 (66.64%) | - | - |
| (N=12) | Visit 2 | 6 (49.98%) | 6 (49.98%) | 16.66% | -16.66% |
| (Day 30) | |||||
| Visit 3 | 6 (49.98%) | 6 (49.98%) | 16.66% | -16.66% | |
| (Day 60) | |||||
| Visit 4 | 5 (41.65%) | 7 (58.31%) | 8.33% | -8.33% | |
| (Day 90) |
Table 4: Effect of BioSOLVE Curcumin™ and placebo on vital signs.
Safety assessments: No adverse events (AEs) or serious adverse events (SAEs) occurred throughout the study duration. There were no notable changes in vital parameters (Table 5).
| Parameters | BioSOLVE Curcumin™ | Placebo | ||
|---|---|---|---|---|
| Baseline | End of Study | Baseline | End of Study | |
| (N = 12) | (N = 12) | (N = 12) | (N = 12) | |
| Systolic BP | 123.17 ± 8.38 | 120.17 ± 7.36 | 122.67 ± 4.77 | 119.08 ± 2.81 |
| (mm Hg) | ||||
| Diastolic BP | 81.33 ± 6.68 | 78.92 ± 7.79 | 80.33 ± 4.42 | 74.67 ± 3.73 |
| (mm Hg) | ||||
| Heart rate (Beats per minute) | 76.50 ± 5.14 | 84.17 ± 6.58 | 77.42 ± 4.56 | 86.75 ± 0.97 |
| Respiratory rate (Breaths per minute) | 14.83 ± 1.75 | 14.67 ± 0.65 | 15.08 ± 1.93 | 14.58 ± 0.67 |
Table 5: Effect of BioSOLVE Curcumin™ and placebo on vital signs.
Discussion
Curcumin modulates inflammation through many cellular targets, although the exact mechanisms are unclear [11]. Curcumin must first undergo oxidative activation to exert its oxidative and anti-inflammatory abili [12]. Through a series of conjugations, reductions, and metabolic pathways, various curcumin metabolites are produced. Most studies indicate that curcumin exerts its effects by binding to proteins COX-2, lipoxygenase, and GSK3β. One main pathway curcumin affects is arachidonic acid metabolism, leading to decreased COX-2 expression and subsequent prostaglandin and thromboxane synthesis, and inhibition of 5-LOX activity and subsequent leukotriene synthesis [13]. Decreased expression of these bioactive chemical mediators’ attributes to the anti-inflammatory effects of curcumin. The mechanism of action of curcumin is like the mechanism of NSAIDs, a common treatment for osteoarthritis. NSAIDs such as celecoxib inhibit the inducible COX-2 isoenzyme, which modulates inflammation. However, the cardiovascular safety of COX-2 inhibitors and non-specific attributes of NSAIDs have become more concerning as trials suggest that COX-2 inhibitors reduce prostacyclin production, an antithrombotic product [14]. Thus, the use of curcumin in inflammatory diseases is a plausible alternative due to decreased side effects. Other studies report that curcumin decreases inflammation in osteoarthritis synovial cells by inhibiting matrix metalloproteinase-3 (MMP3) expression. MMP3 breaks down extracellular matrix proteins in normal physiological processes as well as disease processes such as arthritis and tumor proliferation. By inhibiting MMP3 in osteoarthritis, inflammation and cartilage degradation should decrease [15]. Curcumin has been used in Indian and Asian medicine for its therapeutic abilities for thousands of years. Recent studies provide evidence that increased curcumin levels can modify cellular disease mechanisms toward a more antiinflammatory profile. This mainly occurs by inhibition of the COX and lipoxygenase pathway [16]. Additionally, curcumin suppresses the NF-KB pathway to decrease inflammation [17]. In addition to its anti-inflammatory effects, curcumin also exerts anti-oxidative capacity [18]. The involvement of these pathways in normal rheumatic disease progression could possibly explain curcumin’s therapeutic effects targeting these disease processes [19].
Though the curcumin is widely consumed in the regular diet through the turmeric powder it is clear from the earlier studies, a major portion of ingested curcumin is excreted through feces due to lower absorption. A very small portion absorbed is extensively converted to its water-soluble metabolites, glucuronides and sulfates [20]. Limited bioavailability possesses the biggest limitation of high quantities daily intake of curcumin to reflect its systemic effects [21].
Curcumin is poorly water-soluble, chemically unstable and with the low pharmacokinetic profile. These properties of curcumin affect the bioavailability of curcumin in human and directly influence the therapeutic potentials of curcumin [22]. The Low bioavailability of curcumin is mainly attributed to pharmacokinetic factors including low absorption at the small intestine, extensive phase I and II conjugative and reductive biotransformation at liver and immediate elimination through the gall bladder. Apart from these pharmacokinetics properties, the transformation of curcumin is affected due to enterocyte protein binding which leads to modification of structure [23]. In current study intervention group was treated with BioSOLVE Curcumin™, the value-added nutraceutical highly bioavailable curcumin. In the current study, significant improvement in ACR-20 criteria, WOMAC index and subscale and VAS pain was observed in the intervention group at a relatively lower daily dose of 500 mg could be attributed towards the increased bioavailability of BioSOLVE Curcumin™.
From the results of the current studies it was observed that ACR-20 criteria were significantly improved in both intervention and placebo groups; however, the intervention group showed superior improvement in ACR-20 criterion. In the placebo group, the ACR-20 parameters in which improvement was observed were participant pain assessment, participant global assessment and participant self-assessed disability. Significant improvement in function index was observed at Day 60 and 90 in the intervention group, which was observed only at the 90 days in the placebo group. A significant reduction in the total number of painful joints and swollen joints were observed as compared to the placebo group; however, the reduction in joint pain was within 20%. Thus, apart from a remarkable reduction in the number of painful and swollen joints, the intervention group exhibited an improvement of ≥ 20% in 4 out of 5 ACR-20 criteria related to subjective assessment by physician or participant [24-26].
In intervention group a significant reduction in WOMAC pain and stiffness intensity was observed at all the post-treatment visits at Day 30, 60 and 90 while, the considerable reduction in physical function difficulty and total WOMAC index was observed at Day 60 and 90. The improvement in these parameters was progressive from Day 30 to 90. In the placebo group the reduction in WOMAC pain, stiffness intensity and A significant reduction in WOMAC pain, stiffness intensity, physical function difficulty and total WOMAC index was observed only on Day 90 (the end of the study), which can be attributed to placebo effect observed in such type of study tools as WOMAC which is widely reported. Similarly, VAS was reduced in the intervention group shifting the subjects from severe pain category to moderate pain category right from Day 30 assessment. This shifting of the subject was progressive and observed till Day 90 visit. The VAS data suggests that almost 75% of subjects were benefited from the treatment.
Conclusion
In this clinical trial, the efficacy and safety of the value-added nutraceutical product BioSOLVE Curcumin™ were studied at a dose of one capsule of 250 mg twice in a day for 12 weeks in the management of symptoms of RA. The improvement in ACR-20 criteria, WOMAC index and VAS pain score indicates the improved efficacy of BioSOLVE CurcuminTM at the selected dose. The formulation notably took the edge off the associated pain and stiffness of RA and ameliorated physical function in healthy middle-aged and older RA participants. Thus, the trial revealed that the value-added soluble curcumin nutraceutical formulation significantly increased bioavailability of curcumin and hence contributed to better management of RA symptoms. As a virtue of increased bioavailability, faster onset of therapeutic action of curcumin was achieved. Further, it indicated improved safety along with the upgraded efficacy of BioSOLVE Curcumin™ formulation.
Although our present data are very encouraging, they are a platform only for future planning of long-term studies with the drug in combination with existing standard therapies in RA. It will provide newer avenues of curcumin in pharmacology thus featuring the complete utility of BioSOLVE CurcuminTM in disease management.
Conflict of Interest
The authors declare no conflict of interest.
Author’s Contributions
Shankaranarayanan Jeyakodi contributed to the design of the project, and data analysis and was primarily responsible for writing the manuscript. Shubha Rani M and Divya Chandradhara contributed to the patient recruitment and management and data collection. Arunkanth Krishnakumaran Nair, Kalyan Janjanam were involved in developing the patented formulation and the active drug product.
References
- Ak T, Gulcin I (2008) Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 174: 27-37.
- Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Bioavailability of curcumin: Problems and promises. Mol Pharm 4: 807-818.
- Arnett FC, Edworthy SM, Bloch DA, Mcshane DJ, Fries JF, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism: Official J Amer C Rheumatol 31: 315-324.
- Chandran B, Goel A (2012) A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 26: 1719-1725.
- Conigliaro P, Triggianese P, De Martino E, Fonti GL, Chimenti MS, et al. (2019) Challenges in the treatment of rheumatoid arthritis. Autoimmun Rev 18: 706-713.
- Dcodhar SD, Sethi R, Srimal RC (2013) Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian J Med Res 138: 170-172.
- Drazen JM (2005) COX-2 inhibitors--A lesson in unexpected problems. NEJM 352: 1131-1132.
- Edwards RL, Luis PB, Varuzza PV, Joseph AI, Presley SH, et al. (2017) The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J Biol Chem 292: 21243-21252.
- Hassaninasab A, Hashimoto Y, Tomita-Yokotani K, Kobayashi M (2011) Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. PNAS 108: 6615-6620.
- He Y, Yue Y, Zheng X, Zhang K, Chen S,et al. (2015) Curcumin, inflammation, and chronic diseases: how are they linked? Molecules 20: 9183-9213.
- Heger M, van Golen RF, Broekgaarden M, Michel MC (2014) The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev 66: 222-307.
- Hong J, Bose M, Ju J, Ryu JH, Chen X, et al. (2004) Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A 2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 25: 1671-1679.
- Kang G, Kong PJ, Yuh YJ, Lim SY, Yim SV, et al. (2004) Curcumin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression by inhibiting activator protein 1 and nuclear factor κB bindings in BV2 microglial cells. J Pharmacol Sci 94: 325-328.
- Lao CD, Ruffin MT, Normolle D, Heath DD, Murray SI, et al. (2006) Dose escalation of a curcuminoid formulation. BMC Compl Alternative Med 6: 14.
- Park C, Moon DO, Choi IW, Choi BT, Nam TJ, et al. (2007) Curcumin induces apoptosis and inhibits prostaglandin E2 production in synovial fibroblasts of patients with rheumatoid arthritis. Int J Mol Med 20: 365-372.
- Rahman M, Beg S, Verma A, Al-Abbasi FA, Anwar F, et al. (2017) Phytoconstituents as pharmacotherapeutics in rheumatoid arthritis: challenges and scope of nano/submicromedicine in its effective delivery. Journal of Pharmacy and Pharmacology 69(1): 1–14.
- Rao CV (2007) Regulation of COX and LOX by curcumin. In The molecular targets and therapeutic uses of curcumin in health and disease. Springer, UK. p. 213-226.
- Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, et al. (2004) Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 10: 6847-6854.
- Shehzad A, Lee YS (2010) Curcumin: Multiple molecular targets mediate multiple pharmacological actions: A review. Drugs Fut 35: 113.
- Shishodia S, Singh T, Chaturvedi MM (2007) Modulation of transcription factors by curcumin. In The molecular targets and therapeutic uses of curcumin in health and disease. Springer, UK. p. 127-148.
- Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, (2008) Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. CEPB 17: 1411-1417.
- Wahlström B, Blennow G (1978) A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol 43: 86-92.
- Waterfield J, Sim J (1996) Clinical assessment of pain by the visual analogue scale. BJTR 3: 94-97.
- Wolfe F, Kong SX (1999) Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis 58: 563-568.
- Zeng J, Wang H, Shen Z, Yao X, Wu C, et al. (2019) Curcumin inhibits proliferation of synovial cells by downregulating expression of matrix metalloproteinase‐3 in osteoarthritis. Orthop Surg 11: 117-125.
- Zhou H, Beevers CS, Huang S (2011) The targets of curcumin. Curr Drug Targets 12: 332-347.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences