ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Role of Quetiapine in an Adult Critical Care Practice
Vikram Anumakonda*
Dudley Group of Hospitals NHS Foundation Trust Birmingham, West Midlands, UK
Abstract
Delirium is basically inattention and confusion that represents the brain temporarily failing. Delirium increases the risk of longer stays in the hospital, higher cost of care, death, and more long-term cognitive impairment up to one year later. Hyperactive delirium (HD) patients may become physically aggressive at any moment, and become dangerous to other patients and staff. Hyperactive delirium increases risk of increase clinical and nursing interventions & increased morbidity and mortality subsequently.
Keywords
Delirium, Dexmedetomidine, Antipsychotic, Dopamine.
Introduction
Delirium, an acute and fluctuating disturbance of consciousness and cognition, is a common manifestation of acute brain dysfunction in critically ill patients, occurring in up to 80% of the sickest intensive care unit (ICU) populations. Patients with delirium have longer hospital stays and lower 6-month survival than do patients without delirium [1]. Management of ICU delirium is always challenging in this cohort of patients. RASS scores for sedation have been used regularly by nursing staff and clinicians to ensure patients were not over or under-sedated. Dexmedetomidine, clonidine and remifentanil are used to manage delirium traditionally [2-6].
Quetiapine is a dibenzothiazepine derivative with antipsychotic property. Quetiapine fumarate antagonizes serotonin activity mediated by 5-HT 1A and 5-HT2 receptors. With a lower affinity, this agent also reversibly binds to dopamine D1 and D2 receptors in the mesolimbic and mesocortical areas of the brain leading to decreased psychotic effects, such as hallucinations and delusions. In addition, quetiapine also binds to other alpha-1, alpha-2 adrenergic and histamine H1 receptors.
To investigate role of quetiapine, a second-generation antipsychotic in management of hyperactive delirium in this cohort of patients admitted to ICU [7,8]. As administration of modified release quetiapine tablets was difficult to administer through a nasogastric tube, non-sustained formulation was used.
Material and Methods
A retrospective review of all the patients over a period of 20 months with HD who were treated with quetiapine were included and audited its clinical outcomes and efficiency. Nursing staff daily recorded patient RAAS score and assessed for delirium during daily sedation breaks. ICU consultant would assess patient’s neurology during their ward round and review sedative twice a day. Quetiapine was started by ICU physicians as it was deemed clinically indicated in all these patients.
Patient data were entered prospectively by nursing staff and clinicians during ICU stay on to an electronic patient records(EPR). Data collected was obtained by using SQL from ICIP-EPR.
Medications prescription, dosing instruction were recorded on e-prescribing. Nursing staff recorded dosage escalation and de-escalation every hour. Sedation score were recorded every hour by nursing staff. SQL, Excel, and SPSS were used to analyze data sets. This is a service improvement project, henceforth ethics approval was not needed.
Results and Discussion
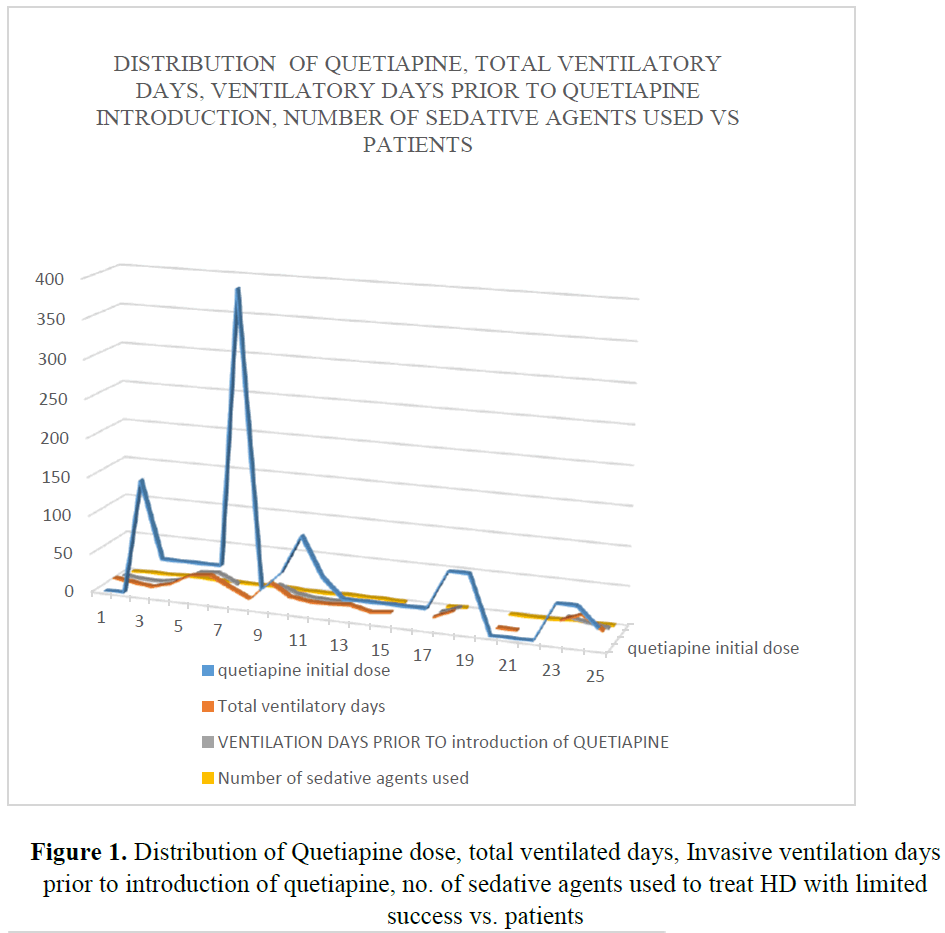
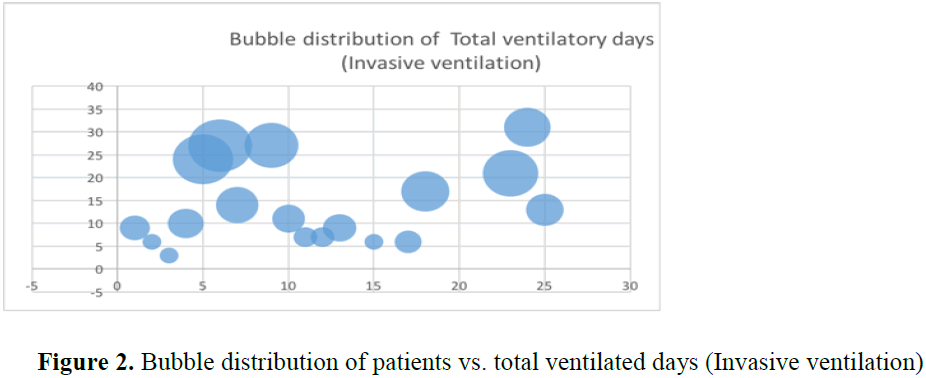
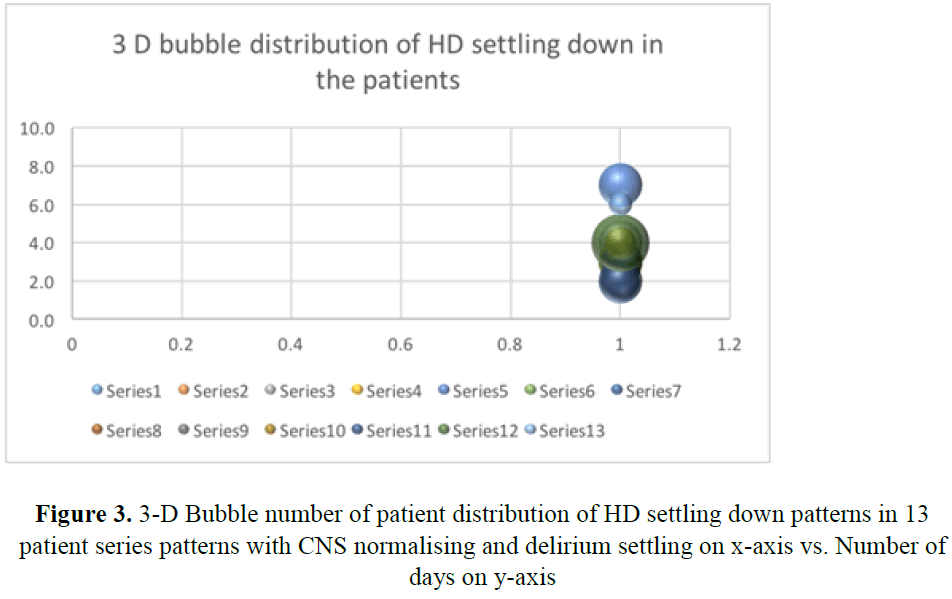
A total of 34 patients were prescribed quetiapine during their ICU stay. Of these nine were excluded from study, as they received less than two dose of quetiapine. A total of twenty-five patients were included in this review. Amongst them, seven were female patients. Average age of the patient in this cohort was fifty-four years (F:52.17 vs M: 54.62 yrs.). HD settles in all those patients within 3.7 days (Figure 1). However, two patients who were previously on quetiapine on PRN basis, HD did settle within 3 days. From third day after introduction of quetiapine, weaning of sedatives was possible very rapidly. Only one patient needed dexmedetomidine for 24 hours after extubating to tolerate non-invasive ventilation. Quetiapine decreased number of ventilator days (p-value=0.006) (Figures 2 and 3) (Table 1). It has a role in patients requiring multiple agents for HD (p-value=0.0005) and minimized need for reintubation for HD (p-value=0.0019). Patients above forty years could not be extubated and needed invasive ventilation before introduction of quetiapine (Table 1). significantly Use of ad-hoc haloperidol use was also reduced significantly (p value=0.001) [10] (Table 2).
| Age in years | No: of HD patients in the review group | Average No. of days patients were intubated prior to introduction of quetiapine | No. of patients requiring re-intubation for Hyperactive deiirium | Average number of days before CNS examination/ HD started to settle | Average Duration of quetiapine treatment in days |
|---|---|---|---|---|---|
| >40 | 5 | 1.4 | 1 | 2.8 | 3 |
| 41-50 | 9 | 11.44 | 3 | 4.5 | 10.14 |
| 51-60 | 3 | 7.55 | 2 | 3.33 | 7 |
| 61-70 | 5 | 6.25 | 3 | 3.4 | 8.5 |
| >70 | 3 | 17.33 | 2 | 4 | 9 |
Table 1: Age in years vs. various variables associated with quetiapine use
| Extubation after introduction of quetiapine: | p-value=0.002 |
| Total ventilatory days (Invasive ventilation) | p-value=0.006 |
| Invasive ventilation in days prior to introduction of QUETIAPINE | p-value=0.004 |
| No. of sedative agents used to manage HD prior to Quetiapine introduction | p-value=0.0005 |
| Weaning of midazolam associated with quetiapine | p-value=0.0018 |
| Weaning of clonidine associated with quetiapine | p-value=0.0018 |
| Synergistic action with dexmedethomidaine + remifentanyl | p-value=0.0018 |
| Need for Reintubation after quetiapine treatment after day 3 post quetiapine exposure | p value=0.0019 |
Table 2: T-test result of various variables associated with quetiapine initial administration dose
Number of agents used for HD prior to introduction of quetiapine were at least 3 at maximum dosage. In all these patients, maximum dose of remifentanil dexmedethomidaine, and propofol to manage their hyperactive delirium, before quetiapine was considered. Clonidine was used in three cases and midazolam infusion was used in two cases for a brief period as a rescue to treat their HD.
Quetiapine was well tolerated in our cohort. However, it was stopped in one patient following prolongation of QTc interval on fourth day. In another case, patient developed a wide spread drug rash. Quetiapine was discontinued on advice of dermatologist on day 8 of his stay. Interestingly, in both these patients, HD settled before drug was stopped.
Quetiapine is a selective monoaminergic antagonist with high affinity for the serotonin Type 2 (5HT2), and dopamine type 2 (D2) receptors. Quetiapine is an antagonist at serotonin 5-HT1A and 5HT2, dopamine D1 and D2, histamine H1, and adrenergic alpha 1 and alpha 2 receptors. Quetiapine has no significant affinity for cholinergic muscarinic or benzodiazepine receptors. Drowsiness and orthostatic hypotension associated with use of quetiapine may be explained by its antagonism of histamine H1 and adrenergic alpha 1 receptors, respectively. Quetiapine's antagonism of adrenergic a1 receptors may explain the orthostatic hypotension observed with this drug.
Pharmacokinetics
Quetiapine fumarate is rapidly absorbed after oral administration, reaching peak plasma concentrations in 1.5 hours. The tablet formulation is 100% bioavailable relative to solution. The bioavailability of quetiapine is marginally affected by administration with food, with Cmax and AUC values increased by 25% and 15%, respectively.
Steady state concentrations are expected to be achieved within two days of dosing.
Quetiapine is widely distributed throughout the body with an apparent volume of distribution of 10 +/-4 L/kg. It is 83% bound to plasma proteins at therapeutic concentrations. Quetiapine appears to be distributed into milk in humans in relatively small amounts.
Following oral administration of a single dose of quetiapine, approximately 73 and 20% of the dose is excreted in urine and feces, respectively; less than 1% of the dose is excreted unchanged.
Quetiapine is extensively metabolized in the liver principally via sulfoxidation and oxidation to inactive metabolites. The major metabolic pathways are sulfoxidation, mediated by cytochrome P450 3A4 (CYP3A4), and oxidation of the terminal alcohol to a carboxylic acid. The mean terminal half-life of quetiapine is about 6 hours.
The apparently low incidence of extrapyramidal effects associated with quetiapine therapy suggests that the drug is more active in the mesolimbic than in the neostriatal dopaminergic system. Quetiapine does not cause sustained elevations in serum prolactin concentrations and therefore is unlikely to produce adverse effects such as amenorrhea, galactorrhea, and impotence [9].
Interactions
Increased doses of quetiapine may be required in patients receiving quetiapine and phenytoin, or other hepatic enzyme inducers (e.g., carbamazepine, barbiturates, rifampin, glucocorticoids). Caution should be taken if phenytoin is withdrawn and replaced with a non-inducer (e.g., valproate). Caution is indicated when quetiapine is administered with ketoconazole and other inhibitors of cytochrome P450 3A (e.g., itraconazole, fluconazole, and erythromycin) in view of reduced clearance [9,10].
Thioridazine increases oral clearance of quetiapine by 65%. Administration of multiple daily doses of cimetidine resulted in a 20% decrease in the mean oral clearance of quetiapine.
Co-administration of fluoxetine; imipramine, haloperidol, or risperidone with quetiapine did not alter the steady-state pharmacokinetics of quetiapine.
Conclusion
HD in ICU patient is very challenging to manage and treat effectively. Quetiapine can effectively treat HD in such challenging patients who are difficult to treat with traditional weaning agents. It decreases morbidity, number of sedative agents used, length of ventilatory days and associated complications. We are aware of small sample size of this review. However, large multicenter prospective clinical trial is needed to do address dose specific effectiveness of quetiapine and effective combination of sedatives in hyperactive delirium in view of our significant findings.
References
- Yamaguchi T., Tsukioka E., Kishi Y., et al. Outcomes after delirium in a Japanese intensive care unit. General Hospital Psychiatry. 2014;36:634-36.
- Tan JA., Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: A meta-analysis. Eur J Anaesthesiol. 2011;28:3-6.
- Jones GM., Murphy CV., Gerlach AT., et al. High-dose dexmedetomidine for sedation in the intensive care unit. Ann Pharmacother. 2011;45:740-47.
- Kanazi GE., Aouad MT., Jabbour-Khoury SI., et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222-27.
- Sudheesh K., Harsoor SS. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323-24.
- Rosenzweig AB., Sittambalam CD. A new approach to the prevention and treatment of delirium in elderly patients in the intensive care unit. J Community Hosp Intern Med Perspect. 2015;5:27950.
- Devlin JW., Roberts RJ., Fong JJ., et al. Efficacy and safety of quetiapine in critically ill patients with delirium: A prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Critical care medicine. 2009;38:419-27.
- Sasaki Y., Matsuyama T., Inoue S., et al. A Prospective, open-label, flexible-dose study of quetiapine in the treatment of delirium. The Journal of Clinical Psychiatry. 2003;64:1316-21.
- De-Vane CL., Nemeroff CB. Clinical pharmacokinetics of quetiapine: an atypical antipsychotic. Clin Pharmacokinet. 2001;40:509-22.
- Grover S., Mahajan S., Chakrabarti S., et al. Comparative effectiveness of quetiapine and haloperidol in delirium: A single blind randomized controlled study. World J Psychiatr. 2016;6:365-37.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences