Role of Apoproteins, Lactate, Lipoproteins, Magnesium, Plasma Glucose, Glycosylated Hemoglobin in Oral Squamous Cell Carcinoma: A Case Control Study
Vignesh Kanneboyina1, Krishna Sumanth Nallagangula1, Hemalatha Ananthramaiah Shashidhar2, Vinay Babu S3 and Shashidhar Kurpad Nagaraj1*
1Department of Biochemistry, Sri Devaraj Urs Medical College, SDUAHER, Karnataka, India
2Department of Pathology, Sri Devaraj Urs Medical College, SDUAHER, Karnataka, India
3Department of ENT, Sri Devaraj Urs Medical College, SDUAHER, Karnataka, India
- *Corresponding Author:
- Shashidhar K Nagaraj
Professor, Department of Biochemistry
Sri Devaraj Urs Medical College
SDUAHER, Tamaka, Kolar, Karnataka, India
Tel: +91 9845248742
E-mail: drshashikn1971@yahoo.co.in
Received Date: 17 January 2018; Accepted Date: 29 March 2018; Published Date: 05 April 2018
Citation: Kanneboyina V, Nallagangula KS, Hemalatha AS, Babu VS, Shashidhar KN (2018) Role of Apoproteins, Lactate, Lipoproteins, Magnesium, Plasma Glucose, Glycosylated Hemoglobin in Oral Squamous Cell Carcinoma: A Case Control Study. Endocrinol Res Metab Vol. 2 No 1:9.
Copyright: © 2018 Kanneboyina V et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Oral squamous cell carcinoma (OSCC) of oral cavity is more complex with multi step progression which involves combination of acquired and inherited alterations in DNA sequence. Deregulated proliferation of cancer cells with altered energy metabolism and glucose is the main source of energy which converts to lactate even under highly aerobic conditions is hall mark of cancer. Cancer cells have high gluttony towards lipids and cholesterol for carcinogenic process including cell growth, cell migration and metastasis formation. Aim: To find out the role of Apoproteins, lactate, lipoproteins, magnesium, glucose, glycosylated hemoglobin in OSCC.Materials and methods: Blood samples were collected from 68 subjects (34 OSCC subjects and 34 age and gender matched healthy subjects) from R.L.Jalappa Hospital and Research Centre, Kolar, Karnataka, India. The biochemical parameters were analyzed by using dry chemistry fully automated VITROS 5.1 FS analyzer. Glycosylated haemoglobin (HbA1C) was analyzed by Bio- Rad D10. Statistical analysis was done by using institutional licensed version of IBM SPSS 20. Results: Significant reduction in plasma glucose, serum magnesium with significant increase in serum lactate levels in OSCC were observed compared to healthy subjects. There was no significant change in HbA1C. Total cholesterol, triglycerides, LDL and Apo A were significantly decreased in patients. No significant alteration in HDL and Apo B was noted in OSCC patients. Conclusion: Reprogrammed energy metabolism in cancer cells with significant elevation of serum lactic acid levels with significant reduction of plasma glucose and serum magnesium levels strongly suggest cancer aggravation which promotes angiogenesis. Disruption of lipid metabolic pathways and lipid homeostasis play an important role in progression of cancer. These abnormal levels of lactate and lipids are associated with poor progression of OSCC.
Keywords
Oral squamous cell carcinoma; Lactic acid; Reprogrammed energy metabolism; Lipid rafts; Apoproteins
Introduction
Oral squamous cell carcinoma (OSCC), a malignant neoplasia of oral cavity is one of the most common and challenging malignancies of head and neck region with more than half million patients being affected each year worldwide [1]. Cancers of all regions of the oral cavity and pharynx are grouped and OSCC is the sixth most common cancer globally with two to three times more prevalence in men than women. International Agency for Research on Cancer report states that annual incidence is increasing globally around 300,000 diagnosed cases with annual mortality of 145,000 [2]. In India; OSCC represents around 40% of all cancers. According to surveillance, epidemiology and end results program, if diagnosed early, the 5 year survival rate is 80% compared to late stage which is 20% [3]. Resistances to chemotherapy, recurrence and pain may be the cause for high mortality and morbidity [4]. More than 90% of OSCC are attributed by exogenous factors viz tobacco smoking and chewing and alcohol abuse. Other risk factors include immune defects, genetic factors and virus like Human papilomavirus, Epstein- Barr virus etc. [5,6].
Oral carcinogenesis is more complex and mainly involves the tongue, oropharynx, floor of the mouth and buccal mucosa. Normal cells transform into pre-neoplastic cells and then to cancer after a series of clinical and histopathological stages involving genetic and molecular changes. The multi-step progression of cancer involves combination of acquired and inherited alterations in DNA sequence. Genetic changes in keratinocytes leads to progressive acquisition of a malignant phenotype from premalignant to cancer. The overexpression of oncogenes causes a disruption in the cell cycle which leads to abnormal cell proliferation. Risk factors can lead genetic and epigenetic alterations of gene expression through aberrant DNA methylation, histone modifications and expressions of micro RNAs [5-7].
Deregulated proliferation of cancer cell is associated with altered energy metabolism. Glucose is a primary source of energy; normal cells metabolize glucose to pyruvate by aerobic glycolysis and to lactate by anaerobic glycolysis in cytosol. Cancer cells primarily derive energy from glucose via glycolysis to lactate even under highly aerobic conditions. It is accompanied by marked enhance in glucose uptake and consumption by cancer cells [8]. Aerobic glycolysis with reprogrammed energy metabolism is a hallmark of cancer [9]. OSCC cells have an increase in glycolysis leading to enhanced glucose uptake and lactate formation. Molecular mechanism for up-regulation of glycolysis in oral cancer is not well understood. It is proposed that it may be due to defective cellular respiration, oncogenic changes and over expression of glycolytic enzyme, hexokinase. Magnesium (Mg), a micronutrient is required for the regulation of hexokinase activity. Lactate, an end product of glycolysis is a crucial regulator of cancer development, maintenance and metastasis and unfavors the clinical outcome in OSCC [10,11]. Lactate is the powerful fuel and signaling molecule continuously produced and circulates through the body. Lactate may facilitate cancer cell intrinsic effect on metabolism and induces tumorigenesis. Being a signaling molecule, it may promote inflammation and tumor angiogenesis [12].
Highly proliferative cancer cells show high avidity to lipids and cholesterol which exerts different biochemical functions during cancer development [13]. Survival and metastatic spreading of cancer cells rely on exogenous fatty acid uptake and consumption by β-oxidation pathway [14]. Complex lipid structure in cells alters membrane fluidity and protein dynamics [15]. Bioactive lipid mediators regulate a variety of carcinogenic process including cell growth, cell migration and metastasis formation. Up-regulation of lipogenesis and cholesterol synthesis pathway are essential for development and progression of cancer [16]. Excessive lipids are stored in the form of lipid droplets in cancer cells which are considered as hallmarks of cancer aggressiveness [13]. Even though studies show lipoprotein role in cancer, apolipoproteins role is not well established. Hence, in the present study we made an attempt to understand the role of glucose, lactate, magnesium, total cholesterol, triglycerides, low density lipoproteins (LDL), high density lipoproteins (HDL), Apolipoprotein A (Apo A) and Apolipoprotein B (Apo B) in OSCC.
Materials and Methods
Subjects for this study (n=34) were selected from the patients registered in the Head and Neck Oncosurgery unit, Department of ENT, R.L. Jalappa Hospital and Research Centre, attached to Sri Devaraj Urs Medical College, A constituent of Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India. Patients with complications of myocardial infarction, acute and chronic renal failure, community acquired pneumonia, arthritis, inflammatory diseases, patients who are on hypolipidemic medications and magnesium supplementation and patients who were on ventilation with altered acid base balance were excluded from the study. Age and gender matched healthy subjects; not having any history of acute and chronic diseases viz., diabetes, myocardial infarction, acute and chronic renal failure, cancer and pneumonia and not on any medications were included in the study.
Collection of blood samples from post-operative (stopped medication 48Hr before surgery) OSCC and healthy subjects (34 OSCC subjects and 34 healthy subjects) were carried out after obtaining informed consent and the study is approved by institutional ethical committee (DMC/KLR/IEC/129/2016-17). Fasting (8 hrs fast) 5 ml of venous blood from antecubital vein in comfortable position of patient under aseptic precautions was collected; 2.5 ml of sample was transferred into sodium citrate and serum separator tubes. Samples were stored at - 20°C until further analysis.
Biochemical parameters Apo A, Apo B, total cholesterol, direct HDL, direct LDL, triglycerides, glucose, lactate and magnesium were analyzed by using dry chemistry fully automated VITROS 5.1 FS analyzer (reflectance photometry, Ortho Clinic Diagnostics, New Jersey, United States). Glycosylated haemoglobin was analyzed by Bio-Rad D10 (High Performance Liquid Chromatography method, Bio-Rad Laboratories, California, US). Statistical analysis was done by using institutional licensed version of IBM SPSS Statistics 20. Descriptive statistics were presented as mean and standard deviation. Comparison of biochemical parameters between groups was done using student t-test.
Results
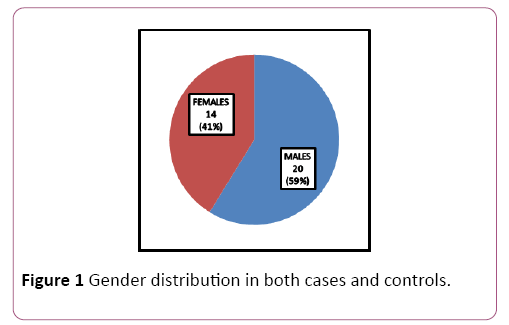
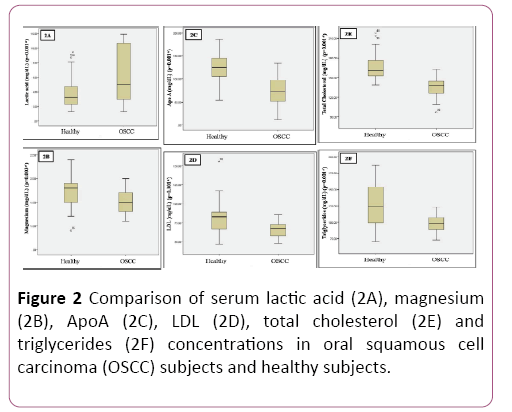
Patients for the study were in the age group of 30-50 years with a mean age of 40.9 ± 5.74; 59% (n=20) were males and 41% (n=14) were females (Table 1 and Figure 1). Significant reduction in plasma glucose levels was observed in OSCC compared to healthy subjects with a significant increase in serum lactate levels. Serum levels of magnesium significantly reduced in OSCC when compared to healthy subjects. There was no significant change in HbA1C. Total cholesterol, triglycerides, LDL and Apo A were significantly decreased in patients compared to healthy subjects. Even though reduction of HDL was noted in OSCC subject; was not significant. No significant alteration in Apo B was observed (Table 2 and Figure 2).
Table 1 Comparison of weight and BMI between oral squamous cell carcinoma and healthy subjects.
| Variables | Gender | OSCC Subjects | Healthy Subjects |
|---|---|---|---|
| Weight (Kg) | Males (n=20) | 54.68 ± 10.81 | 60.81 ± 11.56 |
| Females (n=14) | 48.01 ± 9.87 | 54.45 ± 10.34 | |
| Body Mass Index (BMI) (kg/m2) | Males (n=20) | 21.43 ± 3.78 | 24.72 ± 4.06 |
| Females (n=14) | 20.87 ± 3.14 | 22.69 ± 5.41 |
Table 2 Statistical analysis of biochemical parameters (n=34 both oral squamous cell carcinoma and healthy subjects).
| Variables | Groups | Mean ± SD | SEM | t-Value | p-Value |
|---|---|---|---|---|---|
| Lactic acid (mg/dL) | I | 3.85 ± 2.02 | 0.35 | -3.42 | 0.01* |
| II | 6.45 ± 3.92 | 0.67 | -3.42 | ||
| Apo A (mg/dL) | I | 125.13 ± 29.48 | 5.05 | 6.82 | 0.01* |
| II | 71.85 ± 34.68 | 5.94 | 6.82 | ||
| Apo B (mg/dL) | I | 104.47 ± 39.85 | 6.83 | -0.08 | 0.932 |
| II | 105.16 ± 25.71 | 4.41 | -0.08 | ||
| Magnesium (mg/dL) | I | 1.70 ± 0.30 | 0.05 | 3.08 | 0.03* |
| II | 1.50 ± 0.24 | 0.04 | 3.08 | ||
| Glucose (mg/dL) | I | 94.88 ± 10.62 | 1.82 | 4.44 | 0.01* |
| II | 83.79 ± 9.95 | 1.7 | 4.44 | ||
| HbA1C (%) | I | 5.25 ± 0.83 | 0.14 | 0.77 | 0.442 |
| II | 5.11 ± 0.66 | 0.11 | 0.77 | ||
| TC (mg/dL) | I | 166.35 ± 20.02 | 3.43 | 6.84 | 0.01* |
| II | 136.26 ± 16.02 | 2.74 | 6.84 | ||
| TG (mg/dL) | I | 124.85 ± 32.05 | 5.49 | 4.54 | 0.01* |
| II | 97.85 ± 13.14 | 2.25 | 4.54 | ||
| HDL (mg/dL) | I | 32.00 ± 3.79 | 0.65 | 0.8 | 0.424 |
| II | 30.79 ± 7.87 | 1.35 | 0.8 | ||
| LDL (mg/dL) | I | 80.64 ± 19.75 | 3.38 | 3.88 | 0.01* |
| II | 66.20 ± 8.99 | 1.54 | 3.88 |
Note: Group I: Healthy subjects; Group II: Clinically Proven Oral Squamous Cell Carcinoma subjects; SD: Standard Deviation; SEM: Standard Error Mean; TC: Total cholesterol; TG: Triglyceride; HDL: High Density Lipoprotein; LDL: Low Density Lipoprotein.
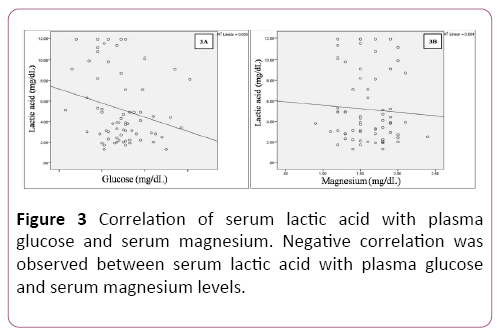
Pearson’s correlation analysis showed negative correlation between serum lactate levels with plasma glucose and serum magnesium levels (Table 3 and Figure 3).
Table 3 Correlation of serum lactic acid with plasma glucose and serum magnesium levels.
| Correlations | |||
|---|---|---|---|
| Glucose | Magnesium | ||
| Lactic acid (mg/dL) | Pearson Correlation | -0.237 | -0.06 |
| Sig. (2-tailed) | 0.051 | 0.626 | |
| N | 68 | 68 | |
**Correlation is significant at the 0.01 level (2-tailed)
*Correlation is significant at the 0.05 level (2-tailed)
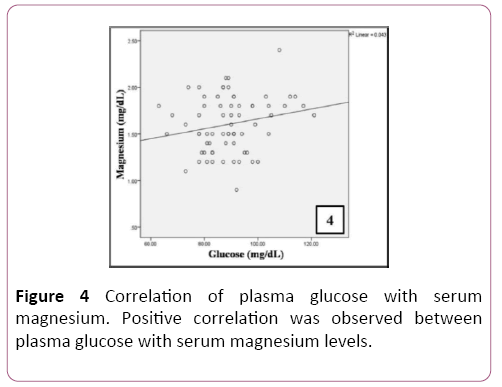
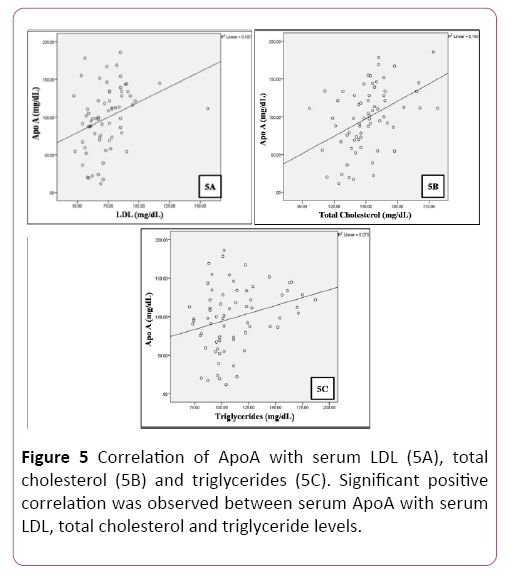
A positive correlation was observed between plasma glucose levels and serum magnesium in patients (Table 4 and Figure 4). A significant positive correlation was observed between serum levels of total cholesterol, LDL, triglycerides and Apo A (Table 5 and Figure 5).
Table 4 Correlation of plasma glucose withserum magnesium levels.
| Correlations | ||
|---|---|---|
| Magnesium | ||
| Glucose (mg/dL) | Pearson Correlation | 0.208 |
| Sig. (2-tailed) | 0.088 | |
| N | 68 | |
** Correlation is significant at the 0.01 level (2-tailed)
* Correlation is significant at the 0.05 level (2-tailed)
Table 5 Correlation of serum Apo A with serum LDL, total cholesterol and triglyceride levels.
| Correlations | ||||
|---|---|---|---|---|
| LDL | Total cholesterol | Triglycerides | ||
| Apo A (mg/dL) | Pearson Correlation | 0.327** | 0.435** | 0.275* |
| Sig. (2-tailed) | 0.007 | 0.001 | 0.023 | |
| N | 68 | 68 | 68 | |
** Correlation is significant at the 0.01 level (2-tailed)
* Correlation is significant at the 0.05 level (2-tailed)
Discussion
In the present study, negative correlation between plasma glucose and lactate (reduced glucose and increased lactate) with positive correlation between plasma glucose and magnesium levels is an indicator of enhanced glucose uptake by cancer cells. Enhanced glycolysis represents reprogrammed energy metabolism and it is a key metabolic hallmark of cancer which results in metabolic acidosis [17,18]. Lactate (2- hydroxypropanoic acid), 3 carbon hydroxylic acid, is produced in the cytoplasm by anaerobic glycolysis. Serum concentration of lactate represents equilibrium between production and metabolism. Normal physiological conditions, lactate is cleared by liver and kidneys [10]. In OSCC, augmented cellular glucose uptake leads to enhanced cellular glycolytic pathway which results in formation of lactate even under normal oxygen conditions [19]. To meet cancer cell proliferation requirements, the glycolytic switch is related to increased glucose consumption and lactate accumulation for energy generation for biosynthetic process [20]. Defective cellular respiration, oncogenic changes and over expression of glycolytic enzymes, enhanced activator for hexokinase enzyme might be the underlying causes for up-regulation of glycolysis [21].
High concentration of lactate is unfavored for clinical outcome of OSCC. Increased lactate alters microenvironment and fuels cancer cells resulting in metabolic acidosis, inflammation, angiogenesis and immunosuppresion [22]. Studies have revealed that cancer cells can uptake lactate and use it for energy production and amino acid formation. Lactate is the fuel for oxidative metabolism of oxygenated cancer cells and also acts as a signal mediator [23]. In OSCC, high levels of lactate is known to have harmful effect on tumor infiltrating immune cells results in loss of immune system ability to effectively eradicate aberrant cells [24]. Lactateosis leads to over expression of matrix metalloproteinase-9, vascular endothelial growth factor-A (VEGF-A), transforming growth factor-β (TGF- β) and IL-8 which enhance cellular proliferation [25]. High lactate levels in OSCC may promote resistance to radiation because of its antioxidant property which leads to chemoresistance. High tumor lactate levels are predictive of metastasis and restricted patient survival [8,26].
Significant reduction in serum total cholesterol, triglycerides, LDL and Apo A in the present study reveal significant uptake of these lipids by cancer cells. Studies revealed the inverse relation between abnormal levels of lipids and cancer. Cancer is the consequence of alterations in lipid metabolic pathways [27]. Highly proliferative cancer cells are known to have strong avidity for lipids and cholesterol either by enhanced endogenous synthesis or by increasing uptake of exogenous lipids and lipoproteins. Accumulated lipids and cholesterol are stored in the form of lipid droplets in cancer cells [28]. These high lipid droplets and stored cholesteryl esters are main cancer aggravators and exert multiple biochemical functions during cancer development [27].
Lipids are a class of biomolecules with unique chemical structure in terms of fatty acid chain number, length and location of double bond [15,27]. Cell membranes contain different classes of lipids viz., cholesterol and sphingolipids which form specific planar microdomains called as lipid rafts. In cancer cells, a wide range of signaling proteins and receptors regulate pro-oncogenic and apoptotic pathways in lipid rafts [29]. Disruption of lipid metabolic pathways leads to imbalance of lipid homeostasis which induces tumor regression in OSCC and it is due to changes in lipid rafts composition [27]. Lipid rafts regulate cancer cell migration by regulating cytoskeletal reorganization and focal adhesion dynamics [30]. The reservoir of triglycerides in cancer cells provides free fatty acids which are taken up and used as energy source for cancer cells proliferation [31]. Accumulation of membrane lipids disturb endoplasmic reticulum (ER) structure which impairs ER homeostasis results in the formation of ER stress; imbalanced cholesterol homeostasis results in free cholesterol overload which also induces ER stress. Complex lipid and cholesterol imbalanced homeostasis induced ER stress enhances cancer cell apoptosis. Increased risk of cancer with high mortality is associated with low levels of lipid profile in OSCC patients [32,33].
Limitations
Body weight and BMI are important parameters for the metabolism of glucose and lipids, OSCC and healthy subjects should be matched properly for validation studies with large sample number. OSCC subjects included in the present study were on Cisplatin, Methotrexate, Flurouracil; it needs to be documented the effects from these medications which may alter the results.
Conclusion
Significant elevation of serum lactate levels with significant reduction of plasma glucose and serum magnesium levels strongly suggest that there will be reprogrammed energy metabolism in cancer cells which induces cancer aggravation. Altered glucose metabolism is an aspect of tumor metabolism. Lactate generated from cancer cells plays a vital role to promote angiogenesis by immunosuppression. Significant reduction in lipid profile and Apo A reveals enhanced lipid uptake by cancer cells. Disruption of lipid metabolic pathways and lipid homeostasis play an important role in progression of cancer. These abnormal levels of lactate and lipids are associated with development and poor prognosis of OSCC which needs further evaluation with large study population.
Funding
This research has been funded by Sri Devaraj Urs Academy of Higher Education and Research (SDUAHER), Kolar, Karnataka, India.
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
References
- Haddad RI, Shin DM (2008) Recent advances in head and neck cancer. N Engl J Med 11: 1143-1154.
- Cesar R (2015) Essentials of oral cancer. Int J Clin ExpPathol 8: 11884-11894.
- Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, et al. (2008) SEER cancer statistics review, 1975-2005. National Cancer Institute, Bethesda, MD.
- Vishakha G, Anoop K, Komal S, Kaur G (2016) Chronic inflammation and carcinogenesis – emerging role of chronic inflammatory periodontal disease. Cancer Research Fronties 2: 200-225.
- Scully C, Bagan J (2009) Oral cell squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis 15: 388-399.
- Corso GD, Villa A, Tarsitano A, Gohel A (2016) Current trends in oral cancer: A review. Cancer Cell Microenviron3: e1332.
- Gasche JA, Goel A (2012) Epigenetic mechanisms in oral carcinogenesis. Future Oncol 8: 1407-1425.
- Choi SYC, Collins CC, Gout PW, Wang Y (2013) Cancer-generated lactate: A regulatory, immunosuppressive metabolite? J Pathol 230: 350-355.
- Daye D, Wellen KE (2012) Metabolic reprogramming in cancer: Unravellingthe role of glutamine in tumorigenisis. Semin Cell Div Biol 23: 362-369.
- Wu Y, Dong Y, Atefi M, Liu Y, Elshimali Y, et al. (2016) Lactate, a neglected factor for diabetes and cancer interaction. Mediators Inflamm.
- Preeti TB, Satya R, Mohsina H (2016) Nutritional aspects of essential trace elements in oral health and disease: An extensive review. Scientifica.
- Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123: 3685-3692.
- Guillaumond F, Bidaut G, Ouaissi M, Servais S, Gouirand V, et al. (2015) Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc Natl Acad Sci USA 112: 2473-2478.
- Daniels VW, Smans K, Royaux I, Chypre M, Swinnen JV, et al. (2014) Cancer cells differentially activate and thrive on de novo lipid synthesis pathways in a low lipid environment. PloS ONE 9: e106913.
- Staubach S, Hanisch FG (2011) Lipid rafts: signalling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics 8: 263-277.
- Zaidi N, Swinnen JV, Smans K (2012) ATP-citrate lyase: a key player in cancer metabolism. Cancer Res 72: 3709-3714.
- Feron O (2009) Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radio Ther Oncol 92: 329-333.
- Hsu PP, Sabatini D (2008) Cancer cell metabolism: Warburg and beyond. Cell 134: 703-707.
- Hirschhaeuser F, Sattler UGA, Mueller-Klieser W (2011) Lactate: a metabolic key player in cancer. Cancer Res 71: 6921-6925.
- Dhup S, Dadhich RK, Porporate E, Sonveaux P (2012) Multiple biological activities of lactate in cancer: influences on tumor growth, angiogenesis and metastasis. Curr Pharm Des 18: 1319-1330.
- DeBerardinis RJ (2008) Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genet Med10: 767-777.
- Hussain Z, Seth P, Sukhatme VP (2013) Tumor-derived lactate and myeloid-derived suppressor cells: linking metabolism to cancer immunology. Oncoimmunology 2:e26383.
- Bonuccelli GA, Tsirigos DA, Whitaker-Menezes D, Pavlides S, Pestell RG, et al. (2010) Ketones and lactate fuel tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle 9: 3506-3514.
- Fischer K, Hoffmann SP, Voelkl S, Meidenbauer N, Ammer J, et al. (2007) Inhibitory effect of tumor cell-derived lactate on human T cells. Blood 89: 877-885.
- Baumann P, Leukel F, Doerfelt A, Beier CP, Dettmer K, et al. (2009) Lactate promotes glioma migration by TGF-β2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol 11: 368-380.
- Sattler UGA, Mueller-Klieser W (2009) The antioxidant capacity of tumor glycolysis. Int J Radiat Biol 85: 169-175.
- Beloribi-Djefaflia S, Vasser S, Guilloaumond F (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5: e189.
- Abramczyk H, Surmacki J, Kopec M, Olejnik AK, Lubecka-Pietruszewska K, et al. (2015) The role of lipid droplets and adipocytes in cancer. Raman imaging of cell cultures: MCF10A, MCF7 and MDA-MB-231 compared to adipocytes in cancerous human breast tissue. Analyst 140: 2224-2235.
- Mollinedo F, Gajate C (2015) Lipid rafts as major platforms for signalling regulation in cancer. Adv Biol Regul 57: 130-146.
- Wang R, Bi J, Ampah KK, Zhang C, Li Z, et al. (2013) Lipid rafts regulates the initial spreading of melanoma A375 cells by modulating beta1 integrin clustering. Int J Biochem Cell Biol 45: 1679-1689.
- Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, et al. (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumour growth. Nat Med 17: 1498-1503.
- Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, et al. (2006) Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res 47: 2726-2737.
- Rios-Marco P, Martin-Fernandez M, Soria-Bretones I, Ríos A, Carrasco MP, et al. (2013) Alkylphospholipids deregulates cholesterol metabolism and induce cell-cycle arrest and autophagy in U-87 MG glioblastoma cells. BiochemBiophysActa 1831: 1322-1334.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences