Retrospective Analysis of Venous Thromboembolism Prophylaxis Prescribing Practices and Outcomes at Two Academic Medical Centers
Christine Gillis1*, Marjan Sadegh1, Kevin Day1, Jessica Rimsans1 and Scott Shikora2
1Department of Pharmacy, Brigham and Women’s Hospital, Francis Street Boston, MA 02115, USA
2Department of Bariatric Surgery, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA
*Corresponding Author:
Christine Gillis
Department of Pharmacy
Brigham and Women’s Hospital
Francis Street Boston, MA 02115
USA
Tel: 857-919-0820
Fax: (617) 566-2396
E-mail: cgillis@bwh.harvard.edu
Received Date: 30 December 2019; Accepted Date: 13 January 2020; Published Date: 20 January 2020
Citation: Gillis C, Sadegh M, Day K, Rimsans J, Shikora S (2020) Retrospective Analysis of Venous Thromboembolism Prophylaxis Prescribing Practices and Outcomes at Two Academic Medical Centers. J Pharma Prac Edu Vol.3 No.1. DOI: 10.36648/pharmacy-practice.3.1.19
Copyright: © Gillis C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Bariatric surgery is the most effective treatment for morbid obesity, resulting in sustained weight loss as well as pronounced beneficial effects on obesityrelated comorbidities. However, postoperative venous thromboembolism (VTE) remains a leading cause of morbidity and mortality post Laparoscopic Sleeve Gastrectomy (LSG).
Objective: To evaluate inpatient and outpatient VTE prophylaxis prescribing trends in relation to VTE and bleeding outcomes post LSG.
Setting: Two academic medical centers.
Method: Retrospective chart review was performed of all adult patients who underwent primary LSG at two academic medical centers between May 30, 2015 and April 30, 2017.
Results: Majority of patients received perioperative VTE prophylaxis with unfractionated heparin (UFH) and was continued throughout hospital admission. On discharge, only 9% of patients were prescribed VTE chemoprophylaxis. Based on a regression analysis, a Body Mass Index (BMI) of greater than or equal to 50 kg/m2 (odds ratio [95%CI], 59.44 [27.6-127.9]) and a history of VTE (odds ratio [95%CI], 18.0 [5.35-60.36]) demonstrated a trend towards physicians prescribing VTE prophylaxis more at discharge. Rates of VTE and bleeding were low (0.5% and 0.8% respectively).
Conclusion: Chemoprophylaxis for high risk patients, such as those with BMI greater than or equal to 50 kg/m2 or a history of VTE event, may be considered for a 28-day regimen of therapy.
Keywords
Laparascopic sleeve gastrectomy; Venous thromboembolism events; Bleeding; ProphylaxisAbbreviations: Kg: Kilogram; SD: Standard Deviation; VTE: Venous Thromboembolism event; DVT: Deep Venous Thromboembolism; PE: Pulmonary Embolism; LMWH: Low Molecular Weight Heparin
Background
In the United States, the prevalence of obesity is estimated to be about 39.8% of adults or approximately 93.3 million patients [1]. Bariatric surgery has demonstrated effectiveness in sustained weight loss and significant positive impact on obesityrelated complications. The number of procedures performed worldwide has increased dramatically during the last decade with the popularity of the LSG, accounting for approximately 75% of all procedures [2]. Laparoscopic surgery, as utilized with the Laparoscopic Sleeve Gastrectomy (LSG) has conclusively demonstrated accelerated hospital recovery and a reduction in the likelihood of postoperative complications [3].
Despite the proven safety of LSG, postoperative venous thromboembolism (VTE) remains a leading cause of morbidity and mortality post LSG. Rates of VTE for bariatric surgery patients range from 0-6.4% [4-5]. Currently, available literature on the utilization and impact of VTE chemoprophylaxis in this patient population is both limited and differs greatly in terms of patient variability, type and duration of VTE prophylaxis making the optimal approach for outpatient post-surgery chemoprophylaxis unclear [6-8]. As a result, it is difficult to draw conclusions to standardize VTE protocols.
The objective of this analysis was to evaluate VTE prophylaxis prescribing trends in relation to VTE and bleeding outcomes post LSG. Furthermore, to propose a safe and effective guideline for our institution, and ultimately for implementation in our bariatric enhanced recovery after surgery (ERAS) protocol.
Materials and Methods
A retrospective chart review was performed in all adult patients who underwent primary LSG two academic medical centers between May 30, 2015 and April 30, 2017. The seven bariatric surgeons included in the analysis performed surgeries at both institutions and followed a standard surgical care protocol. The protocol encompassed VTE prophylaxis including chemoprophylaxis, early post-operative ambulation and pneumatic compression boots were implemented for all patients. The computerized physician order entry (CPOE) system utilized a standardized order set that provided the surgeons with different chemoprophylaxis agents and dosing to select pre and post operatively.
This retrospective chart review was approved by the institutions’ Institutional Review Board.
Patient identification and data collection
All patients were identified utilizing the Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) Data Registry. The date of surgery, type of surgery and body mass index (BMI) at the time of surgery was provided to the research team. The patient’s electronic medical records were reviewed which included past medical history, laboratory work, physician and periprocedural notes, and medication administration records for additional patient information.
Data endpoints
Data collected included type, dose, and route of administration of VTE chemoprophylaxis during the hospitalization and on discharge. The timing of initiation (i.e. day of surgery defined as post-operative day (POD) 0 vs. POD 1) and duration of VTE prophylaxis given perioperatively, during hospitalization and on discharge were also recorded. Rates of VTE and bleeding were assessed 30 days post-surgery. A VTE event was defined as evidence of a deep venous thromboembolism (DVT) on lower extremity non-invasive test (LENI) or ultrasound doppler or pulmonary embolism (PE) on chest computerized axial tomography (CAT) or angiography. Portal vein thrombosis was defined as evidence on abdominal Computed Tomography (CT). Significant bleeding was defined based on a modification of the International Society on Thrombosis and Haemostasis (ISTH) surgical bleeding definition [9]. Itwas defined as either fatal bleeding, bleeding requiring reoperation, bleeding causing a fall in hemoglobin level of 2 g/dL or more, or bleeding leading to transfusion of two or more units of whole blood or red cells within 24 to 48 hours related to the bleeding [9].
Statistical analysis
Descriptive statistics were utilized to describe prescribing trends for chemoprophylaxis surrounding surgery. A multivariable logistic regression analysis of all included patients was applied to determine if certain patient characteristics were independently correlated to prescribing VTE prophylaxis on discharge. Variables of interest included BMI, history of VTE event and smoking history. These patient characteristics were chosen as they are known risk factors for VTE events.
Results
A total of 772 patients were included in the analysis. Table 1 lists the baseline characteristics of those who were included. Majority of patients were female, with 19% being former smokers, 12% receiving current oral contraceptives at the time of surgery, and 4% had a previous VTE event.
| Variable | Female | Male | Total patients |
|---|---|---|---|
| (N=600) | (N=172) | (N=772) | |
| Age, years | |||
| Mean ± SD | 41.7 ± 13.0 | 45.0 ± 12.2 | 42.8 ± 13.0 |
| Actual body weight, kg | |||
| Mean ± SD | 117 ± 42.2 | 146.1 ± 33.3 | 123.3 ± 42.2 |
| Ideal body weight, kg | |||
| Mean ± SD | 55.0 ± 6.8 | 70.5 ± 8.1 | 58.3 ± 9.5 |
| Body mass index, kg/m2 | |||
| Mean ± SD | 43.5 ± 7.1 | 45.9 ± 8.5 | 44.1 ± 7.4 |
| PMH, n (%) | |||
| Previous smoker | 102 (17.0) | 41 (23.8) | 143 (18.5) |
| Oral contraceptive use | 94 (15.7) | 0 (0.0) | 94 (12.2) |
| Previous VTE | 22 (3.7) | 8 (4.6) | 30 (3.9) |
| DVT | 13 (2.2) | 4 (2.3) | 17 (2.2) |
| PE | 9 (1.5) | 4 (2.3) | 13 (1.7) |
| Atrial fibrillation/atrial flutter | 12 (2.0) | 11 (6.4) | 23 (3.0) |
| Factor V Leiden | 3 (0.5) | 1 (0.6) | 4 (0.5) |
| Von Willebrand disease | 1 (0.2) | 2 (1.2) | 3 (0.4) |
| Antiphospholipid Antibody Syndrome | 2 (0.3) | 0 (0.0) | 2 (0.3) |
| Protein C & S Deficiency | 2 (0.3) | 0 (0.0) | 2 (0.3) |
| Any valve replacement | 1 (0.2) | 0 (0.0) | 1 (0.1) |
| Hemophilia | 0 (0.0) | 1 (0.6) | 1 (0.1) |
Table 1: Patient Demographics.
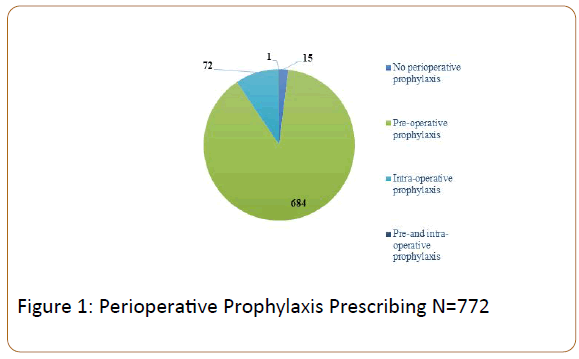
Perioperatively, majority (98%) of patients received prophylaxis prior to surgery (Figure 1) . The agent of choice for prophylaxis was unfractionated heparin (UFH) 5,000 units administered subcutaneously (SC) before the operating room. Approximately 87% of patients continued on UFH 5000 units SC every 8 hours for the duration of the hospitalization. A small percentage (2%) of patients was transitioned back to therapeutic anticoagulation prior to discharge.
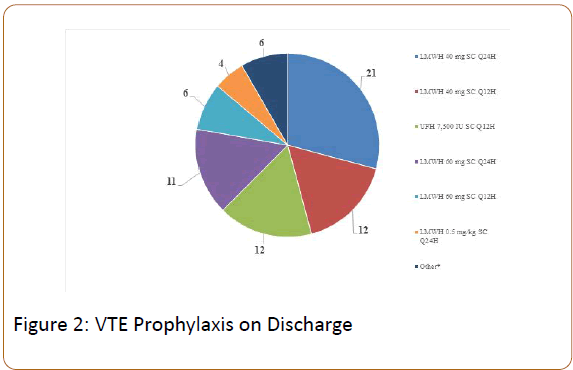
Seventy-two (9%) patients received DVT prophylaxis on discharge. The most commonly prescribed regimen was enoxaparin 40 mg SC once daily for 28 days (median 28 days; range 10-60 days). See Figure 2 for more details. Based on a regression analysis, a body mass index of greater than 50 kg/m2 (odds ratio [95% CI], 59.44 [27.6-127.9]) and a history of VTE (odds ratio [95% CI], 18.0 [5.35-60.36]) were significantly associated with physicians prescribing chemoprophylaxis on discharge.
*Other=LMWH 0.5 mg/kg SC Q12H=2, UFH 10,000 IU SC Q8H=1, LMWH 1 mg/kg SC Q24H=1, apixaban=1, dabigatran=1; LMWH=low molecular weight heparin
Bleeding events
The overall incidence of bleeding was low (0.8%) and most commonly occurred on POD 0 (range intra-operatively to POD 5). There was no fatal bleeding or bleeding that required reoperation. Sites of bleeding were observed at right sided port site, tissue between spleen and upper stomach and intraabdominal hematoma. All patients that experienced bleeding received a pre-operative dose of UFH 5,000 IU SC x 1 and were initiated on UFH 5,000 IU SC Q8H post-operatively with the exception of patient number 1. Patient number 1 had presence of a ventricular assist device implanted and was on warfarin that was reversed with a 4-factor prothrombin complex concentrate prior to surgery. Two units of packed red blood cells (PRBCs) were administered for an observed drop of more than 2 points in hemoglobin. No further intervention was needed, and patient was re-initiated on a therapeutic heparin infusion with aPTT goal 60-80. Patient number 2 only had an estimated blood loss (EBL) of 10 milliliters (mL), however had a 4 point drop in hemoglobin post-operatively and patient became tachycardic on POD 0. Imaging of the abdomen demonstrated bleeding that was likely originating from the right sided port site. Total of 4 units of PRBCs was given administered and hemoglobin normalized without any further intervention. Patient number 4 had a history of DVT and protein C&S deficiency who received chemoprophylaxis immediately post-operatively and was discharged home on therapeutic enoxaparin with warfarin bridge (INR goal 2-3). Patient re-presented to the hospital on POD 5 and was found with have an intra-abdominal hematoma. Patient was given 2 units PRBCs and initiated on heparin infusion while hospitalized. On discharge, only warfarin was prescribed after hemoglobin normalized. For the two bleeding events that occurred during surgery, patient number 5 had an EBL of about 700 mL and bleeding was controlled by applying pressure to the site between the spleen and stomach where an incision was made intra-operatively. The second patient had a lower EBL of 250-300 mL during surgery, but no blood products were given and hemoglobin stabilized throughout admission.
VTE events
The rate of VTE was 0.5% (4 patients) with events occurring between POD 5 to 10. Two patients developed portal vein thrombosis, one patient developed a submassive PE and the other portal and splenic vein DVTs. None of these 4 patients were discharged with DVT chemoprophylaxis. Patient number 1 was continued an estrogen/progesterone oral contraception prior to and post-surgery. Given that estrogen is known risk factor for development of VTE, the patient was switched to a progesterone only oral contraceptive on discharge. Patient number 2 presented with persistent nausea and emesis and was found to have mesenteric and portal vein thrombosis and was diagnosed with a family inherited thrombophilia. She was discharged home on therapeutic enoxaparin regimen 1 mg/kg SC every 12 hours and was referred for outpatient management of inherited thrombophilia. The patient who developed submassive PE had a complex cardiac history with right ventricular (RV) strain. Patient underwent catheter directed thrombolysis with systemic heparin infusion and was transitioned to apixaban 10 mg by mouth twice daily for 7 days then followed by 5 mg by mouth twice daily on discharge. The final patient was found to have acute portal and splenic vein DVTs with splenic infarction. The patient was transitioned from a heparin infusion to therapeutic enoxaparin 1mg/kg every SC 12 hours and a warfarin bridge (INR goal 2-3) on discharge.
Discussion
In the current era of bariatric surgery, the incidence of symptomatic DVT and PE ranges from 0%–5.4% and 0%–6.4%, respectively, with more recent data from the Michigan Bariatric Surgery Collaborative demonstrating a VTE rate less than 0.5% in average risk bariatric patients, which are comparable to rates of other elective low-risk procedures [4-10]. However, bariatric surgical patients have an increased risk of a VTE event after bariatric surgery such as the LSG.
The utilization of VTE prophylaxis in this patient population continues to be controversial. In 2007 The American Society for Metabolic and Bariatric Surgery (ASMBS) issued a statement on VTE prophylaxis recommending early post-operative ambulation, the use of low extremity sequential compression devices, and unless contraindicated, the use of chemoprophylaxis. However, the type, dose, and duration of chemoprophylaxis were not clearly defined [11]. Since then, several analyses and systematic reviews have emerged supporting the use of VTE prophylaxis in certain bariatric surgery patients. Raftopoulos and colleagues demonstrated that patients who received in-hospital VTE prophylaxis with enoxaparin 30 mg SC twice daily until discharge versus enoxaparin 40 mg SC once daily for 10 days following discharge, had higher rates of a VTE event within 30 days of surgery compared to none in the discharge prophylaxis group. They found no differences in bleeding events [8]. Magee et al. demonstrated a trend towards fewer PE events in the extended duration group with UFH SC compared to no prophylaxis for 15 days, although the bleeding rate was higher [12]. In 2019, a systemic review by Clark et al. demonstrated that approximately 11% of bariatric surgery patients are prescribed prophylaxis on discharge and that prescribing practices are still variable [13].
Accurate evidence-based risk assessment tools for VTE in bariatric patients are not currently available, but the literature highlights several risk factors that must be taken into consideration when determining a prophylaxis strategy. These risk factors include prior VTE, higher BMI, age, gender, immobility, use of hormone therapy, obesity, hypoventilation syndrome, pulmonary hypertension, venous stasis disease, operative time, and procedure type and approach [13-15].
This analysis demonstrated that in a large cohort of LSG patients, the use of VTE prophylaxis with enoxaparin 40 mg SC once daily for 28 days in patients deemed high risk per surgeon may be a safe and effective strategy to decrease the rates of VTE and mitigate bleeding events. Evidence supporting shorter durations less than 28 days has been studied and published, however the risk of VTE has been shown to extend beyond 30 days [14]. Although we did not monitor for anticoagulant effects to correlate with events in these patients, we identified patient populations to consider using discharge chemoprophylaxis in, such as those with BMI greater than or equal to 50 units and those with a history of VTE prior to surgery who are not currently receiving therapeutic anticoagulation. Additionally, this study provides insight into prescribing practices prior to surgery, during hospitalization and post-discharge which have not been demonstrated prior to our analysis. Although Clark et al. described the use of chemoprophylaxis at discharge in a large patient cohort, they did not analyze risk factors that may warrant chemoprophylaxis, such as BMI greater than or equal to 50, patients with a previous VTE, or those who are taking oral contraceptives [16].
The ideal agent and dosing strategy are not known as data supporting enoxaparin and heparin had variable dosing strategies and lengths of treatment, as well as differing definitions for follow up and major outcomes. Direct acting oral anticoagulants (DOACs) have been considered and utilized in some centers are an attractive option for patients as they do not require injection or routine laboratory monitoring. However, due to alternations in the anatomy of the gastrointestinal tract post bariatric surgery and the unknown impact of the surgery on pharmacokinetic properties of these agents, neither safety nor efficacy of DOACs in this population is known. At this time, available evidence in comparable surgical patients supports the use of chemoprophylaxis in high risk groups, such as those we identified in this analysis. The lack of randomized, controlled analyses limits the ability to provide specific recommendations.
Limitations
There are many limitations to the study. The retrospective chart review was not able to control for confounding variables, such as provider and surgeon technique, as well as adherence of chemoprophylaxis post discharge. This study only looked at a small subset of patients and was also not powered to detect a statistically significant difference for the designated endpoints, but a trend was identified with regards to prescribing practices. Rates of VTE were assessed 30 days post discharge thus there may be events that happened outside this time frame that were not analyzed. Patients may have also sought care at an outside institution and thus an event may have been missed. Given the retrospective nature of the study, adherence could also not be assessed with respect administrations to medication use prior to and post-surgery. There were a portion of patients that were reported to be taking oral contraception prior to surgery due to documentation in the patient’s medical chart. However, patients were likely told verbally to stop the medication prior to surgery as that is the routine recommendation given increased risk of thrombosis associated with estrogen containing agents, thus the number of those receiving OCP reported in this analysis is not reflective of true administrations.
Conclusion
Despite the advances in surgical techniques for bariatric patients, the risk of developing a VTE post-surgery still exists. Chemoprophylaxis for high risk patients, such as those with BMI ≥ 50 kg/m2 or a history of VTE event, may be considered for a 28-day regimen of therapy.
Disclosures
The authors of this manuscript have no disclosures concerning possible financial or personal relationships with commercial entities that may have a direct or indirect interest in the subject matter of this manuscript.
Acknowledgement
Gretchen Stern, PharmD and Leo Buckely, PharmD for their contributions to statistical analysis for the manuscript. Department: Brigham and Women ’ s Hospital Department of Pharmacy.
References
- Centers for Disease Control and Prevention. Overweight and obesity. Accessed May 2019. https://www.cdc.gov/obesity/data/adult.html.
- Thorell A, MacCormick A, Awad S, N Reynolds, D Roulin, et al. (2016) Guidelines for perioperative care in bariatric surgery: Enhanced recovery after surgery (ERAS) Society Recommendations. World J Surg 40 :2065-2083.
- Halliday TA, Sundqvist J, Hultin M, Wallden J (2017) Post-operative nausea and vomiting in bariatric surgery patients: An observational study. Acta Anaesthesiol Scand 61:471-479.
- American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS updated positions stsatement on prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Rel Dis 2013;9:493-497.
- Steele KE, Schweitzer MA, Prokopowicz G, Shore AD, Eaton LC, et al. (2011) The long-term risk of venous thromboembolism following bariatric surgery. Obesity Surgery 21:1371-1376.
- Hamad GG, Choban PS (2005) Enoxaparin for thromboprophylaxis in morbidly obese patients undergoing bariatric surgery: Findings of the prophylaxis against VTE outcomes in bariatric surgery patients receiving enoxaparin (PROBE) study. Obesity Surgery 15:1368-1374.
- Ojo P, Asiyanbola B, Valin E, Reinhold R (2008) Post discharge prophylactic anticoagulation in gastric bypass patient—how safe? Obesity Surgery 18:791-6.
- Raftopoulos I, Martindale C, Cronin A, Steinberg J (2008) The effect of extended post-discharge chemical thromboprophylaxis on venous thromboembolism rates after bariatric surgery: A prospective comparison trial. Surgical Endoscopy 22:2384-2391.
- Schulman S, Angeras U, Berggvist D, B Eriksson, M R Lassen, et al. (2010) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Throm Haemost 8:202–204.
- Finks JF, English WJ, Carlin AM, Krause KR, Share DA, et al. (2012) Predicting risk for venous thromboembolism with bariatric surgery: Results from the Michigan Bariatric Surgery Collaborative. Annals of Surgery 255:1100-1104.
- American Society for Metabolic and Bariatric Surgery Clinical Issues Committee (2007) Prophylactic measures to reduce the risk of venous thromboembolism in bariatric surgery patients. Surg Obes Relat Dis 3:494-495.
- Magee CJ, Barry J, Javed S, Macadam R, Kerrigan D (2010) Extended thromboprophylaxis reduces incidence of postoperative venous thromboembolism in laparoscopic bariatric surgery. Surg Obes Relat Dis 6:322-325.
- Clark L, Helm M, Gould J (2019) Practice patterns regarding post-discharge chemoprophylaxis for venous thromboembolism following bariatric surgery in the United States. Surg Obes Relat Dis 703-707.
- Caprini JA (2005) Thrombosis risk assessment as a guide to quality patient care. Disease-a-Month 51:70-78.
- Caprini JA. (2010) Risk assessment as a guide for the prevention of the many faces of venous thromboembolism. Am J Surg 199:3-10.
- Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, et al. (2016) Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 375:534-44.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences