Results of Adjuvant Chemotherapy Using Hydroxyurea after Stereotactic Radiosurgery for Atypical Meningiomas
Department of Neurosurgery, Division of Neuro-Oncology, School of Medicine, Samsung Changwon Hospital, Sungkyunkwan University, Changwon, South Korea
- *Corresponding Author:
- Young Zoon Kim
Department of Neurosurgery, Division of Neuro-Oncology
School of Medicine, Samsung Changwon Hospital
Sungkyunkwan University, Changwon, South Korea
Tel: ++82-55-2335241
Fax: +82-55-2338070
E-mail: yzkim@skku.edu
Received Date: March 02, 2018 Accepted Date: April 30, 2018 Published Date: May 07, 2018
Citation: Lee J, Park SH, Kim YZ (2018) Results of Adjuvant Chemotherapy Using Hydroxyurea after Stereotactic Radiosurgery for Atypical Meningiomas. J Med Oncol. Vol.1 No.2:7
Abstract
Objective: Stereotactic Radiosurgery for atypical meningiomas was reported with variable degrees of success. Hydroxyurea as radiation sensitizer and salvage chemotherapy was established. We investigated the effect of hydroxyurea after Gamma Knife Radiosurgery (GKS) for atypical meningiomas on tumor control and survival.
Materials and methods: Between November 2008 and December 2014, 35 patients with pathological atypical meningiomas were treated by GKS. The medical records were reviewed retrospectively, which included basic epidemiologic characteristics, radiologic features, and clinical courses.
Results: Among them, 19 patients with 20 tumors were treated by GKS and followed by adjuvant chemotherapy using hydroxyurea for 1 year or more and were included in this study. They were followed up for 2 years or more after hydroxyurea chemotherapy. Twenty tumors were treated with 26 GKSs. Five tumors had staged treatment. Median volume of tumor was 12.7 cc. Mean prescription dose/session was 14 Gy (10-16 Gy). Patients received hydroxyurea (1000 mg/day) for 1 year after GKS (range 7 to 20 months). Mean follow up duration was 43 months. Tumor control achieved in 18/20 tumors (90%), 15 shrank and 3 stable. Two tumors experienced the progression (14 and 15 months). Transient edema occurred in 6 tumors, but there was no grade 3 or 4 myelosuppression. Distant progression outside the field was observed in 5 patients. In 3 patients, new tumors developed at the edge of craniotomy and in 1 patient, the tumor progression occurred after GKS for another lesion after the adjuvant treatment. Actuarial progression free survival and overall survival at 3 years were 89.5% and 94%.
Conclusion: We suggested good tumor control of atypical meningiomas treated with GKS and a year of hydroxyurea. Randomized and double-arm studies are required to confirm the results.
Keywords
Hydroxyurea; Meningioma; Radiosurgery; Progression; Survival; Adverse effects
Introduction
Meningioma is an extra axial tumor which is a common primary tumor of the central nervous system (20-30%). Its incidence is between 4-6 per 100,000 per year [1,2]. World Health Organization (WHO) categorizes meningiomas into three grades; Grade 1 so called benign meningioma, Grade 2 atypical meningioma and Grade 3 anaplastic meningioma [3]. Anaplastic meningiomas carry a particularly poor prognosis, with a median survival of 1.5 years and a 5 year mortality of 68% [4,5]. Atypical meningioma is a meningioma of intermediate grade between benign and malignant forms, with increased mitotic activity, brain invasion, or at least three of the following features: increased cellularity, small cells with high nuclear-to cytoplasmic ratio, prominent nucleoli, sheeting and foci of necrosis [6].
Surgical treatment is the primary therapy but may be inadequate especially in tumors which are hard to remove completely because of their location [2,7]. The recurrence rate even in totally resected benign tumors is between 10-20% after 5 years, 20-30% after 10 years, and about 50% after 20 years of follow-up. In some patients, surgery may be impossible or associated with an unacceptable morbidity [7]. Long-term data using definitive external beam radiation have demonstrated prolonged tumor control comparable with that observed with surgery followed by adjuvant radiation [6]. However, conventional radiotherapy rarely reduces tumor size and may be complicated by longterm side effects [2]. Therefore, stereotactic radiotherapy (SRS) has become an alternative option to external-beam radiation for recurrent or partially resected meningiomas and for patients in whom surgery is not an option because of the tumor’s location or patient comorbidities [6]. Although many chemotherapeutic agents have been investigated, including adriamycin, temozolomide, dacarbazine, ifosfamide, irinotecan and so on, none showed conclusive results [8,9]. Hydroxyurea is an oral antimetabolite whose main use is in the treatment of chronic myeloid leukemia [10]. The mechanism of action appears to be inhibition of ribonucleotide reductase causing apoptosis in phase S of the cell cycle [2,5]. The drug is modestly active against meningioma, well tolerated and induces stable disease, however with no documented objective radiologic responses [5,7].
In the present study, we aimed to investigate the clinical results of adjuvant chemotherapy with hydroxyurea after GKS for atypical meningiomas, especially in terms of progression-free survival (PFS) and overall survival (OS). In addition, we estimated the side effect of adjuvant hydroxyurea chemotherapy for atypical meningiomas in the unique condition after GKS treatment.
Materials and Methods
Patient collections
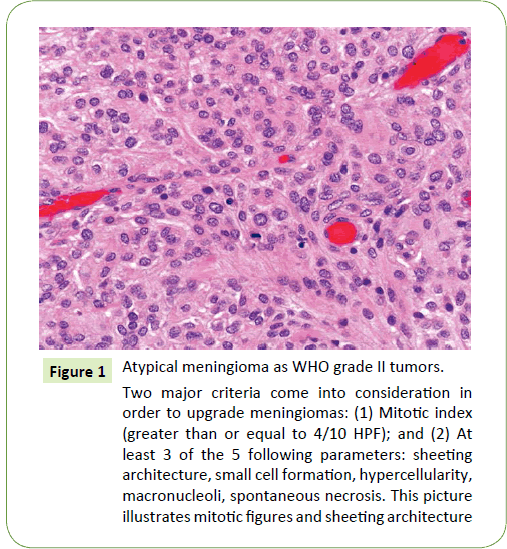
This retrospective study is a review of 35 patients with pathologically proven atypical meningiomas, diagnosed and reviewed according to WHO grading system, 2016 [6] brain invasion joins a mitotic count of 4 or more as a histological single criterion and additive criteria of 3 of the other 5 histological features such as spontaneous necrosis, sheeting (loss of whorling or fascicular architecture), prominent nucleoli, high cellularity and small cells (tumor clusters with high nuclear: cytoplasmic ratio) (Figure 1). Those patients were treated between November 2008 and December 2014 by GKS at our institute using Leksell Gamma Knife Unit Model C and Perfexion (Elekta AB, Stockholm, Sweden). All patients had undergone radical surgery and histopathologically diagnosed before GKS. The exclusion criteria were as follows: (1) those with recurrent atypical meningioma after treatment for a previous benign meningioma; (2) those with spinal cord meningioma; (3) those who had undergone previous radiotherapy for tumor; and (4) those with ≤ 12 months of followup due to follow-up loss.
Figure 1: Atypical meningioma as WHO grade II tumors.
Two major criteria come into consideration in order to upgrade meningiomas: (1) Mitotic index (greater than or equal to 4/10 HPF); and (2) At least 3 of the 5 following parameters: sheeting architecture, small cell formation, hypercellularity, macronucleoli, spontaneous necrosis. This picture illustrates mitotic figures and sheeting architecture
Patient sex, age at the time of surgery, symptoms at diagnosis, tumor location and size, extent of resection, histological grade, use of postoperative radiotherapy or chemotherapy, duration of follow-up and recurrence/progression were retrospectively reviewed for each patient by using the medical records.
The first goal of treatment was tumor control due to the progressive nature of the disease. Indications of GKS treatment included unoperated tumors, residual/recurrent tumors, patients refusing surgery and patients unfit for surgery. Inclusion criteria for the adjuvant chemotherapy with hydroxyurea in the patients with incompletely resected atypical meningioma were as follows: 1) those with histologically proven atypical meningioma, 2) chemotherapy and radiotherapy naïve patients, 3) those with a Karnofsky performance status better than or equal to 60, 4) those with a life expectancy greater than 3 months, 5) those with adequate hematologic, renal and hepatic functions, 6) those without serious concurrent medical illnesses or active infection.
Radiological findings
Tumor size was defined as the largest tumor diameter rounded to the nearest centimeter on gadolinium-enhanced T1 weighted MRI before the initial surgery. Peritumoral edema was estimated by the longest distance from the margin of the tumor on fluidattenuated inversion recovery (FLAIR) images. The locations of tumors were divided into convexity and non-convexity groups. Recurrence was defined as the presence of new tumor in patients with a completely resected tumor, as judged on the first postoperative MRI, and progression as evidence of new growth of an incompletely resected tumor on serial postoperative MRI compared with the immediate postoperative images. All patients had undergone preoperative MRI. Baseline postoperative MRI was performed immediately after surgery to evaluate the residual mass and then at 3 or 6 months intervals within the first 2 years. To assess tumor recurrence/progression, serial MRI was performed at 1 year interval in the patients, but if massrelated symptoms or focal neurological signs developed, MRI was performed immediately. Two neuroradiologists (Kim YM, M.D. and Sunwoo MP, M.D.) individually conducted radiological reviews to characterize tumors and to determine the presence of a recurrence/progression without any clinical or pathological information on the patients.
Applications of stereotactic radiosurgery
Before treatment all patients had a magnetic resonance image (MRI) brain with contrast. On the treatment day and after application of the stereotactic frame, an MRI was performed 1.6 mm thickness with no gap axial T1-weighted contrast enhanced images. On stereotactic fiducial definition, only images with a mean error of deviation of less than 1 mm were accepted. Gamma plan software, versions 9 and 10.1.1 (Elekta AB, Stockholm, Sweden) was used for dose planning. The intended prescription dose was between 10-16 Gy with a median of 14 Gy to the 50% isodose curve and with tumor coverage between 85-100 %.
Hydroxyurea chemotherapy
Hydroxyurea (Hydrin®; Korean United Pharm Inc. Seoul, Korea) was administered orally for 28-consecutive days (1,000 mg/m2/ day divided twice per day or rounded to the nearest dose level based on availability of hydroxyurea capsule size) every 4 weeks (operationally defined as a cycle of therapy). Hydroxyurea was prescribed in a routine practice at our institutes [11] and no pharmaceutical sponsorship was provided in the conduct of this study. No premedication was required with oral hydroxyurea. Administration of hydroxyurea was repeated every 28 days (4 weeks) provided that all toxicity from the previous cycle had resolved. If recovery had not occurred by day 28, the subsequent cycle of hydroxyurea was delayed until recovery. All toxicities including hematologic due to hydroxyurea chemotherapy were rated retrospectively according to the Common Terminology Criteria for Adverse Events version 4.0.
All patients underwent brain MRI for the base control images within 2 weeks of starting hydroxyurea administration. Blood counts were obtained on day 1 of each hydroxyurea cycle (or more often if clinically indicated), neurologic examination was performed every 4 weeks, and gadolinium-enhanced brain MRI was performed after every 3 cycles of hydroxyurea chemotherapy (i.e., every 12 weeks). Modified neuroradiographic response criteria as defined by Macdonald et al. [12] were used; complete response (CR), complete resolution of the enhancing tumor volume and an improved or normal neurological examination without steroid medication; partial response (PR), greater than 50% reduction of the enhancing tumor volume and a stable or improved neurological examination on stable or decreased doses of steroid; stable disease (SD), less than 50% reduction or 25% growth of enhancing tumor volume without a significant change in the neurological examination on stable or decreasing doses of steroids; and PD, greater than a 25% increase of the enhancing tumor volume. All neuroradiography was reviewed by 2 neuroradiologists (Kim and Sunwoo) blinded to treatment. In patients with radiographically SD, PR or CR, 3 additional cycles of hydroxyurea were administered, and repeat MR was performed. Patients were continued on hydroxyurea therapy until documentation of PD at which time patients discontinued hydroxyurea and were either monitored or offered alternative therapy. Alternative meningioma-directed therapy such as conventional radiotherapy and repeated surgery (or no additional therapy) was offered to patients that radiographically progressed.
Follow-up
Imaging follow-up examinations using contrast-enhanced MRI were done every 3 months for the 1st year, every 6 months for the 2nd year, and then annually. Additional imaging was obtained when a patient developed new symptoms or experienced the worsening of any preexisting symptoms. The patient’s history and examination findings were recorded and compared with those documented prior to treatment. A 20% or greater increase in the diameter measurements in at least 2 of the largest axes was considered tumor progression. In cases where the tumor was not sufficiently visible on regular imaging, the patient underwent MRI using the same protocol as the one used on the day of treatment. The images were then imported into Gamma Plan, fused and co-registered to the treatment images for comparison. A volume difference of more than 20% from the day of treatment was considered significant. Informed consent was obtained from all patients or their guardians.
Statistics
We used IBM SPSS statistics (version 20.0, IBM Corp.) for the data analysis. The following tests were performed: (1) a comparison between 2 independent groups for nonparametric data using the Wilcoxon rank sum test; and (2) a Chi-square test to study the association between 2 variables or a comparison between 2 independent groups as regards the categorized data. The probability of error at 0.05 was considered significant, whereas probabilities of error at 0.01 and 0.001 were considered highly significant. Kaplan-Meier curves were used to calculate actuarial tumor control rates and hearing preservation rates.
Results
Patients’ characteristics
Twenty-three patients were given hydroxyurea after GKS treatment, while 12 patients refused to take the treatment or were incompliant. These 12 patients were also excluded from the study. Sixty-five percent of patients were females and 35% were males. Age ranged from 30-60 years with a median of 52. All patients underwent surgical resection of meningiomas prior to GKS. Nineteen patients out of 23 were harboring 20 tumors and were available for radiological follow up for a minimum of 1 year and clinical follow up for a minimum of 2 years.
The mean follow-up period after ending 1 year of hydroxyurea intake was 43 months (14-76 months). Four patients were lost from follow up. Nineteen tumors underwent 26 gamma knife procedures. Five tumors underwent staged treatment. The tumor volume was 0.6-38.3 cc (median 12.7 cc). The prescription dose/session ranged from 10 to 16 Gy (mean 14 Gy).
Responses and outcomes
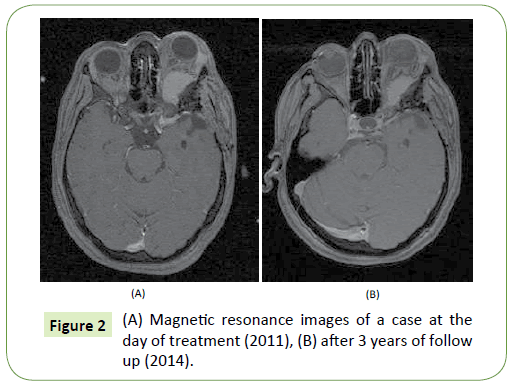
Tumor control was achieved in 18 out of 20 tumors where 15 tumors shrank (Figure 2) and 3 tumors remained stable with a tumor control rate of 90%; median volume of atypical meningioma before treatment was 12.2 cc (range from 8.5 to 19.4 cc) and after 9.2 cc (range from 4.0 to 14.1 cc) in patients with PR (p=0.047 in student’s t-test), and 19.3 cc (range from 14.7 to 24.3 cc) and 17.6 cc (range from 14.3 to 21.7 cc) in patients with SD (p=0.218 in student’s t-test). Tumor progression occurred in 2 patients (at 14 and 15 months). Transient edema was observed in the 6 tumors which were temporary, and no grade 3 or 4 myelosuppression were recorded during the hydroxyurea adjuvant chemotherapy. Two patients died from progression of other tumors not included in the study after 3 and 6 years. Distant tumor progression (in another intracranial location or outside the treatment field) was observed in 5patients. In 3 patients, new tumors developed at the edge of previous craniotomy, in one patient tumor progression occurred after receiving radiosurgery alone for another lesion after the end of adjuvant treatment and in one patient progression occurred in an untreated tumor that was under observation. The actuarial PFS and OS at 3 years were 89.5% and 94%, respectively.
Side effects
All patients received hydroxyurea, 13 patients received it for 12 months continuously and 1 patient received it for 12 months but interrupted due to side effects in the form of grade 2 anemia. Only 5patients received their treatment for less than 1 year (8- 10 months), one of those 5 patients had grade 2 epigastric pains and constipation. Such side effects were temporary and were medically well managed. One patient received hydroxyurea for more than 1 year (20 months). The results of the 19 studied patients are collected in Table 1, where patient number 8 had 2 lesions.
| Patient number | Gender | Age (year) | Period of hydroxyurea chemotherapy (month) | Radiological response | PFS (month) | Follow-up duration (month) |
|---|---|---|---|---|---|---|
| 1 | Female | 52 | 20 | SD | - | 84 |
| 2 | Female | 56 | 8 | SD | - | 72 |
| 3 | Male | 58 | 12 | PR | - | 33 |
| 4 | Female | 58 | 12 | PR | - | 36 |
| 5 | Male | 46 | 9 | PR | - | 72 |
| 6 | Male | 59 | 12 | PD | 14 | 24 |
| 7 | Female | 57 | 10 | PR | - | 26 |
| 8* | Female | 37 | 12 | PR | - | 28 |
| 9 | Female | 30 | 12 | PR | - | 72 |
| 10 | Female | 51 | 12 | PR | - | 72 |
| 11 | Male | 60 | 7 | PR | - | 60 |
| 12 | Male | 36 | 12 | PR | - | 60 |
| 13 | Female | 57 | 12 | PR | - | 48 |
| 14 | Female | 57 | 12 | PR | - | 32 |
| 15 | Male | 65 | 9 | PD | 15 | 30 |
| 16 | Male | 50 | 12 | PR | - | 60 |
| 17 | Female | 62 | 12 | PR | - | 60 |
| 18 | Female | 57 | 12 | PR | - | 48 |
| 19 | Female | 58 | 12 | PR | - | 26 |
* the patient had 2 meningiomas in the brain
PD: Progressive Disease; PFS: Progression Free Survival; PR: Partial Response; SD: Stable Disease
Table 1: Collective results of the 19 patients who had their data available.
Discussion
To the best of our knowledge and thorough research, this study is the first to use this combination treatment of gamma knife radiosurgery followed by hydroxyurea chemotherapy for the treatment of atypical meningioma. That being said, such work has many limitations especially being a retrospective one, the limited number of the studied patients and the lack of central pathological slides revision. In 2012, Adeberg et al. [13] published their data regarding the long-term outcome after radiotherapy in patients with atypical and malignant meningiomas. OS was 81% and 53% at 5 years for atypical or anaplastic meningiomas, respectively. PFS was 95% and 50% for atypical and 63% and 13% for anaplastic histology at 2 and 5 years [13].
GKS is considered the best radiation option for meningiomas and was reserved in the past years for the smaller volume lesions with more and more data published for treating larger meningiomas [14]. Wang et al. in 2016 published a retrospective analysis of 46 patients with atypical or anaplastic meningiomas treated with postoperative GKS [15]. The local tumor control at 1, 3 and 5 year was 91.1%, 59.3% and 39.5%, respectively, for WHO grade 2 meningiomas and 76.2%, 63.5% and 0% for WHO grade 3 meningiomas [15]. In terms of the efficacy of GKS treatment alone in this study, it is impossible for us to estimate the strict response to the GKS due to too short follow-up duration after GKS, because we started the hydroxyurea adjuvant chemotherapy within 4 weeks after GKS.
The management of atypical meningioma with chemotherapy had not been a standard of care even after many phase 2 trials have been published over the past 20 years [2,16-20]. One retrospective study suggested that adjuvant treatment after incomplete resection of atypical meningioma is associated with longer PFS than conservative treatment; however, there was no difference in PFS between hydroxyurea chemotherapy and radiotherapy after surgery [11].
A pilot study was published in 2005 which discussed the treatment of progressive or recurrent meningiomas with hydroxyurea and concurrent 3-dimensional conformal radiation and most of their patients (13/21) were WHO grade I meningioma, 4 cases were atypical and 4 were of unproven pathology. They showed tumor stabilization in 14/21 patients only and none of the cases showed tumor regression [21]. In 1997, Schrell et al. [17] were the first to report dramatic tumor reduction of meningioma following the use of hydroxyurea, and in the year 2000, Newton et al [18] reported that 14 out of 16 cases achieved stable disease with very little overall toxicity. Another more recent study that used hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma reported very limited activity of hydroxyurea and questioned its effectiveness [3]. Their retrospective case series of 35 patients with recurrent WHO Grade 2 (n=22) or 3 (n=13) meningioma treated with hydroxyurea following progression after surgery and radiotherapy was collated with primary study objectives of overall response rate, median and PFS at 6 months. Thirty-five patients with recurrent high-grade meningioma were treated with hydroxyurea (1,000 mg/m2 orally divided twice per day; one cycle operationally defined as 4 weeks of daily hydroxyurea). Their patients had progressed radiographically after prior therapy with surgery (35/35) and radiotherapy (35/35: external beam radiotherapy 35/35; stereotactic radiotherapy 35/35). Their patients experienced modest toxicity of hydroxyurea (28.5% all grades and 8.5% grade 3+ anemia or fatigue). There were no radiographic responses, 43% of patients had SD and 57% manifested PD at first evaluation. The overall PFS was 3.0% at 6 months and median PFS was 2.0 months. Most patients (80%) following progression on hydroxyurea were subsequently treated on an investigational trial. The results of some previous studies that used hydroxyurea for the treatment of meningioma are listed in Table 2 and are compared the data of the current study regarding the radiological response to treatment.
| Authors | Number of patients | Dropped or unavailable results | Response | Progression | Rate of tumor control (%) |
|---|---|---|---|---|---|
| Schrell et al. [17] | 4 | 0 | 4 | 0 | 100 |
| Newton et al. [18] | 16 | 0 | 14 | 2 | 88 |
| Rosenthal et al. [8] | 13 | 0 | 11 | 2 | 85 |
| Mason et al. [19] | 20 | 0 | 13 | 7 | 65 |
| Weston et al. [2] | 6 | 3 | 2 | 1 | 66 |
| Chamberlain [3] | 35 | 0 | 15 | 20 | 43 |
| Kim et al. [11] | 24 | 0 | 14 | 10 | 58 |
| Current study | 23 | 4 | 17 | 2 | 89 |
Table 2: Different studies that used hydroxyurea for the treatment of meningioma.
The protocol was well tolerated with no grade 3 or 4 toxicities and none of the studied cases were withdrawn from the study due to toxicity. Such toxicity profile was better than that reported by Weston et al. [2] and Loven et al. [22] with many sever hematologic toxicities that were the cause for treatment discontinuation. This could be attributed to the fixed low dose used in our study.
Conclusion
The results of the current study suggest a good tumor control with stabilization or shrinkage of atypical meningiomas treated with stereotactic radiosurgery followed by one year of adjuvant hydroxyurea. Larger randomized and double-arm studies are essential to confirm the possible role of hydroxyurea in the management of those tumors.
Disclosure & Acknowledgement
This paper was supported by Sungkyun Research Fund, Sungkyunkwan University, 2016. The authors have no financial conflicts of interest. I thank the following individuals: Young Min Kim, M.D. and Mi-Ok Sunwoo, M.D. (Department of Radiology, Samsung Changwon Hospital) for their review of the neuroradiological images; Eun Hee Lee, M.D. and Mee-Seon Kim, M.D. (Department of Pathology, Samsung Changwon Hospital) for their pathological review; and Young Wook Kim, M.D. (Department of Biostatistics, Samsung Changwon Hospital) for assistance with the statistical analysis.
References
- Chen TC, Chamberlain MC (2007) Adjuvant therapy for unresectable meningiomas: Benign and malignant. Neurosurg Focus 23: 1.
- Weston GJ, Martin AJ, Mufti GJ, Strong AJ, Gleeson MJ (2006) Hydroxyurea treatment of meningiomas: A pilot study. Skull Base 16: 157-160.
- Chamberlain MC (2012) Hydroxyurea for recurrent surgery and radiation refractory high-grade meningioma. J Neurooncol 107: 315-321.
- Gonzales M (2012) Classification and pathogenesis of brain tumors. In Kaye AH, Laws E Jr, editors. Brain Tumors, An Encyclopedic Approach. Third edition. Edinburgh, London, New York, Saunders, Elsevier, pp 36-58.
- Gurberg J, Bouganim N, Shenouda G, Zeitouni A (2014) A case of recurrent anaplastic meningioma of the skull base with radiologic response to hydroxyurea. J Neurol Surg Rep 75: e52-e55.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. (2016) The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 131: 803-820.
- Kim M, Yu D, Jung Y, Kim S, Chang C, et al. (2012) Long-term follow-up result of hydroxyurea chemotherapy for recurrent meningiomas. J Korean Neurosurg Soc 52: 517-522.
- Rosenthal MA, Ashley DL, Cher L (2002) Treatment of high risk or recurrent meningiomas with hydroxyurea. J Clin Neurosci 9: 156-158.
- Chamberlain MC, Blumenthal DT (2004) intracranial meningiomas: Diagnosis and treatment. Expert Rev Neurother 4: 641-648.
- Solary E, Itzykson R (2017) How I treat chronic myelomonocytic leukemia. Blood 130: 126-136.
- Kim J, Kim KH, Kim YZ (2017) The clinical outcome of hydroxyurea chemotherapy after incomplete resection of atypical meningiomas. Brain Tumor Res Treat 5: 77-86.
- 12.Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8: 1277-1280.
- Adeberg S, Hartmann C, Welzel T, Rieken S, Hormel D, et al. (2012) Long-term outcome after radiotherapy in patients with atypical and malignant meningioma clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys 83: 859-864.
- Ganz JC, Reda WA, Abdelkarim K (2009) Gamma knife surgery of large meningiomas: Early response to treatment. Acta Neurochir (Wien) 151: 1-8.
- Wang W, Lee C, Yang H, Liu K, Wu M, et al. (2016) Gamma knife radiosurgery for atypical and anaplastic meningiomas. World Neurosurg 87: 557-564.
- Saraf S, Mccarthy B J, Villano JL (2011) Update on meningiomas. Oncologist 16: 1604-1613.
- Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, et al. (1997) Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg 86: 840-844.
- Newton HB, Slivka MA, Stevens C (2000) Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neurooncol 49: 165-170.
- Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, et al. (2002) Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg 97: 341-346.
- Kyritsis AP (1996) Chemotherapy for meningiomas. J Neurooncol 29: 269-272.
- Hahn BM, Schrell UM, Sauer R, Fahlbusch R, Ganslandt O, et al. (2005) Prolonged oral hydroxyurea and concurrent 3D-conformal radiation in patients with progressive or recurrent meningioma: Results of a pilot study. J Neurooncol 74: 157-165.
- Loven D, Hardoff R, Sever ZB, Steinmetz AP, Gornish M, et al. (2004) Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol 67: 221-226.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences