Relationship between Circulating Plasma Galectin-3 Levels and T-Cell Activation during Cervical Cancer Chemotherapy
Folly Mawulolo Gaba1*, Maimouna Diop1, Doudou GM Niang1,2, Sidy Ka3, Doudou Diouf3, Moussa Ndour2, Comlan JG Montcho1, Moustapha Mbow1, Babacar Faye1, Rokhaya N Diallo4, Maguette S Niang1, Ahmadou Dem3, Babacar Mbengue1,2 and Alioune Dieye1
1Department of Immunology, University of Cheikh Anta Diop, Dakar, Senegal
2Department of Immunology, Aristide Ledantec Hospital (HALD), Dakar, Senegal
3Department of Oncology, University of Juliot Curie Institute, Dakar, Senegal
4Department of Human Genetic, University of Cheikh Anta Diop, Dakar, Senegal
- *Corresponding Author:
- Folly Mawulolo Gaba
Department of Immunology,
University of Cheikh Anta Diop,
Dakar,
Senegal,
Tel: 774910855;
E-mail: follymawulolo.gaba@ucad.edu.sn
Received date: October 03, 2022, Manuscript No. IPIIY-22-14659; Editor assigned date: October 06, 2022, PreQC No. IPIIY-22-14659 (PQ); Reviewed date: October 21, 2022, QC No. IPIIY-22-14659; Revised date: January 10, 2023, Manuscript No. IPIIY-22-14659 (R); Published date: January 17, 2023, DOI: 10.36648/IPIIY.7.1.001
Citation: Gaba FM, Diop M, Niang DGM, Ka S, Diouf D, et al. (2023) Relationship between Circulating Plasma Galectin-3 Levels and T-Cell Activation during Cervical Cancer Chemotherapy. J Immuno Immnother Vol:7 No:1
Abstract
Objective: Despite the existence of several therapeutic strategies, the management of cervical cancer remains challenging. In our region, there is very little data on the interaction between the immune system and the clinical response to chemotherapy.
This work examines plasma levels of Galectin-3 (Gal-3) and percentages of activated T-cells in patients with cervical cancer treated with chemotherapy and investigates if there is a relationship between the rates of these two elements.
Methods: We compared data from 37 patients with cervical cancer undergoing chemotherapy and 42 controls with normal cervical cytology. Plasma Gal-3 concentrations were assessed by ELISA and expression of activation markers by Tcells (CD69 and HLA-DR) was assessed by flow cytometry at three different time points during chemotherapy.
Results: Our results showed patients had a significantly higher concentration of Gal-3 compared to controls (4.025 vs. 1.340, pË?0.001), similarly, they had a significantly high percentage of activated lymphocytes (2.610 vs. 0.731; pË?0.0001). According to the response to treatment, patients with no response to treatment had a lower concentration of circulating Gal-3 but had approximately the same percentage of activated CD4 and CD8 lymphocytes as patients with a partial or total response. In addition, we found a positive correlation between the Gal-3 level and CD4 T-cells expressing the activation marker CD69 (pË?0.05; rho=0.44).
Conclusion: In conclusion, our results show that in cervical cancer there would be a relationship between circulating galectin-3 and the percentage of peripheral CD4+CD69+ cells.
Keywords
Uterine cervical neoplasm; Chemotherapy; Galectin 3; T-Lymphocytes activation
Abbreviations:
CC: Cervical Cancer; CT: Chemotherapy; ECM: Extracellular Matrix; FBS: Fetal Bovine Serum; FIGO: International Federation of Gynecology and Obstetrics; FU: Fluorouracil; Gal-3: Galectin-3; HC: Healthy Controls; HPV: Human Papillomavirus; S: Sample
Introduction
Cervical Cancer (CC) is the fourth most common cancer in women worldwide. According to a WHO report, around 500,000 cases were diagnosed annually and 300,000 deaths due to CC were observed. The highest incidences occur in developing countries, where incidences of Human Papilloma Virus (HPV) are often high [1]. Cervical cancer develops in the cervical epithelium and it results in 99% of cases from persistent HPV infection [2,3]. Based on current evidence, there are few reliable biomarkers for the treatment of cervical cancer. Therefore, the development of new therapeutic biomarkers is of paramount importance.
The galectins are a family of carbohydrate binding proteins with an affinity for β-galactosides, sharing a conserved Carbohydrate Recognition Domain (CRD) of about 130 amino acids [4,5]. Fifteen galectins have been identified so far and divided into 3 subgroups: Prototype, chimera and tandem. Galectin-3 (Gal-3) is the only galectin belonging to the chimera subgroup and it contains one CRD and an extended N-terminal domain [6,7]. It has a molecular mass ranging from 29 to 34 kDa. Gal-3, to an equal or greater extent than other galectins, plays an important function in cell-cell or cell-matrix interactions, cell migration, cell growth, differentiation and angiogenesis, cell activation and apoptosis [8-11]. It is localized not only in intracellular space such as cytoplasm or the nucleus but also in extracellular space such as the cell surface or the extracellular matrix [12]. It is often overexpressed in various human tumors such as colon [13,14], prostate, thyroid and breast cancer and is generally associated with poor prognosis [15].
Studies have shown that the extracellular binding of Gal-3 to the plasma membrane of T-cells alters membrane organization and immunological synapse formation. Its ability to interact specifically with different membrane proteins influences T-cell receptor signaling and thus alters T-cell activation and effector function [16-20]. Gal-3 may also suppress T-cell activation by binding to the α-subunit of the TCR, thus inhibiting its recruitment at immunological synapses [21]. Therefore, the ability of Gal-3 to inhibit T-cell responses may be a mechanism by which Gal-3 contributes to cancer progression.
Furthermore, since variations in Gal-3 are not associated with age, Body Mass Index (BMI) or gender, several studies were conducted to examine Gal-3 as a diagnostic marker and have suggested the use of Gal-3 in the diagnosis and prognosis of various types of diseases and thus may also serve as a therapeutic target for treatment [22-24].
The objective of this study was to analyze the plasma concentration of Gal-3, evaluate the percentage of activated Tcells in patients undergoing chemotherapy and search an eventual link between gal-3 and activated T-cells population [25].
Materials and Methods
Patients, samples and recruitment
This study included 37 patients with Cervical Cancer (CP) and 42 Healthy Controls (HC) with no history of cervical cancer and showed normal cervical cytology. The recruitment was carried out at the cancer department of the Juliot-Curie Institute (Aristide LeDantec Hospital, Dakar, Senegal) from July to October 2018 [26]. The patients enrolled in the study were those with a recent pathologically confirmed diagnosis of cervical cancer who had not received any cancer treatment, including chemotherapy and radiotherapy. Non-inclusion criteria included previous treatment with traditional herbal medicines, the existence of chronic conditions such as diabetes and bacterial or viral infections such as tuberculosis, HIV and hepatitis B. These infectious diseases were systematically investigated by the medical team in charge of follow-up. In addition, no participants with immunosuppression or cardiac pathologies or who had received blood transfusions were included in this study. All selected patients were managed by the same medical staff and the chemotherapy protocol was in accordance with Senegalese national recommendations specifying one cycle every 21 days [27]. Usually, three courses of chemotherapy are recommended before undertaking a thorough clinical examination to assess response to treatment. Each patient received a chemotherapy protocol based on individual clinical features of the disease and general health status. Overall, for the patients enrolled, cisplatin was used in combination with 5-Fluorouracil (5-FU). In terms of dose, cisplatin was administered between 50-75 mg/m2 of body surface area every 3 weeks and 5-FU was used up to 1000 mg/m2 every 3 weeks for 3 cycles.
The Healthy Controls (HC) selected were women free of cervical cancer with normal cervical cytology and no chronic inflammatory pathology. They also had no history of cancer or chronic pathology (diabetes, heart disease) [28].
Peripheral venous blood from all study participants (CP and HC) was collected on EDTA tube. For patients, sample collection was based on the chemotherapy protocol. A total of three blood samples were collected for each patient, corresponding to one sample before each cycle of chemotherapy as follows: Sample 1 (S1: day 0, before any treatment), Sample 2 (S2: day 21, 21 days after the first treatment corresponding to the second course of chemotherapy) and Sample 3 (S3: day 42, 42 days after the first treatment corresponding to the third course of chemotherapy). However, only one sample was collected from each Healthy control. All samples (patients and healthy controls) were immediately centrifuged. Supernatants are stored at -80°C. Peripheral Blood Mononuclear Cells (PBMCs) were immediately isolated from the pellet and used for phenotyping assay [29].
Definition of response to chemotherapy
The international classification defines nonresponders, partial responders and complete responders based on tumor size compared to baseline after three courses of chemotherapy [30]. In this study, a Complete Response (CR) is defined as the complete disappearance of all tumor lesions. A decrease in tumor size of 50% or more is defined as a Partial Response (PR). Patients with No Response (NR) include those with stability (tumor size decrease less than 50% or tumor size increase less than 25%) and those with progression (tumor size increase greater than or equal to 25% or an appearance of new lesions). Clinical response was assessed by vaginal pelvic examination and abdominal/pelvic CT scan before and after chemotherapy [31].
Ethics statement and procedure
This study was performed at Aristide LeDantec hospital in Dakar (Senegal) in the internal institute dedicated to cancer management. In this study, all the immunological assays were performed in the immunology laboratory of the faculty of medicine of Cheikh Anta Diop University in Dakar [32]. Sample collection and monitoring were done in collaboration with the immunology unit of HALD.
From each participant and/or relatives, informed consent was obtained before inclusion, after providing written or verbal information in their native language [33]. The protocol 0297/2018/CER/UCAD was approved by the institutional ethics committee of Cheikh Anta Diop University (Dakar, Senegal) and performed following the declaration of Helsinki.
Plasma Gal-3 measurements
Plasma Galectin-3 levels were determined using the Platinum Human Galectin-3 ELISA kit (eBioscience, Inc., San Diego, CA, USA) with Enzyme-Linked Immunosorbent Assay (ELISA) method. The 96-well plate (Corning Costar 9018) pre-coated with anti-human Gal-3 monoclonal antibody was washed twice (with wash buffer) before standards addition [34]. Standards reconstitution was used according to the manufacturer’s instructions and 100 μL were distributed to the appropriate wells. To establish a standard curve, we make double serial dilutions for a total of 8 points.
For each sample, 50 μL per well (duplicate) was added and the plate was incubated under agitation at 400 rpm for one hour. After washing 100 μL of HRP-conjugated detection antibodies were added to each well before 1 hour incubation with agitation at 400 rpm. Tetra-Methyl Benzidine (TMB) was used as a liquid substrate (100 μL added) and incubated for 30 minutes in the dark, at room temperature before the addition of 100 μL of stop solution (2N, H2SO ). Absorbance at 450 nm (with a reference wavelength of 620 nm) was measured on a microplate reader (Multiskan FC, Thermo Scientific). The results are expressed in pg/ml [35].
PBMCs isolation
Isolation of Peripheral Blood Mononuclear Cells (PBMC) was done by density gradient centrifugation using the ficoll 1077 method. Blood previously diluted to equal volume with RPMI was deposited in a thin layer on the surface of the ficoll in a 1:1 ratio and subjected to centrifugation at 1500 rpm for 15 minutes. The white layer representing the PBMC (between the ficoll and the dilution solution) was gently collected and transferred aseptically into sterile 15 ml centrifuge tubes. The cells were then washed with RPMI/Penicillin-Streptomycin by centrifugation at 1500 rpm for 30 minutes. The cell pellet was then suspended in 2 ml of PBS 5% FBS or 10% FBS/RPMI. Cell labeling was performed from this suspension.
Flow cytometric analysis
0.5.106 cells/ml was placed in each cytometer tube and decomplemented SAB was then added to block non-specific sites. Labelling was then performed by adding monoclonal antibodies (CD3-PE, CD4-APC, CD8-PercP, CD69-FITC and HLADR- PercP). The mixture is vortexed and incubated for 30 minutes at 4°C in the dark. A wash is then performed, before recovering the pellet and suspending it in the fixation solution followed by incubation for 20 minutes at room temperature [36,37]. Final centrifugation at 1800 rpm for 5 minutes was performed before suspending the cells in PBS 1X for cytometer reading. The acquisition was done using a FACS calibur® equipped with Cell Quest Pro software®. The data collected were analyzed with Flow Jo® 10.0.6 software.
Statistical analysis
Data were analyzed with Statview version 5.1 software. The non-parametric Mann-Whitney test was used for the different comparisons between patients and controls and the Kruskal- Wallis test was used to test the differences in concentrations between non-responders, partial responders and complete responders. Relationships between variables were assessed using the Spearman rank test. A value was considered statistically significant when p<0.05.
Results
Demographic, histological and clinical characteristics of patients
The present study involved 42 controls and 37 patients with cervical cancer. Controls and patients were age-matched. According to the FIGO classification, more than half (55.56%) of the patients had advanced disease at the time of diagnosis. In addition, approximately half of the patients (48.64%) were younger than 50 years of age at the time of diagnosis, with an average age of 53.7 years. As shown in Table 1, squamous cell carcinoma accounted for 81% of the tumor types diagnosed. Regarding the response to treatment, patients with a complete response to chemotherapy represented 35.1% of the patients and the remainder were 37.8% and 27% respectively for patients with a partial response and those with no response.
| Characteristics | n=37 (%) |
|---|---|
| Age at diagnosis (years) | |
| >=-< | |
| 35-50 | 18 (48.64) |
| 50-66 | 13 (35.14) |
| 66 | 6 (16.22) |
| Histology | |
| Squamous cell carcinoma | 30 (81.08) |
| Adenocarcinoma | 7 (18,92) |
| FIGO stages | |
| I-IIA | 11 (29.73) |
| IIB-IV | 26 (70.27) |
| Parity | |
| 0-5 | 9 (24.3) |
| 10 Jun | 22 (59.5) |
| >10 | 6 (16.2) |
| Gravidity | |
| 0-5 | 8 (21.6) |
| 10 Jun | 23 (62.2) |
| >10 | 6 (16.2) |
| Response to treatment | |
| Complete response | 13 (35.1) |
| Partial response | 14 (37.8) |
Table 1: Demographic, histological and clinical characteristics of patients.
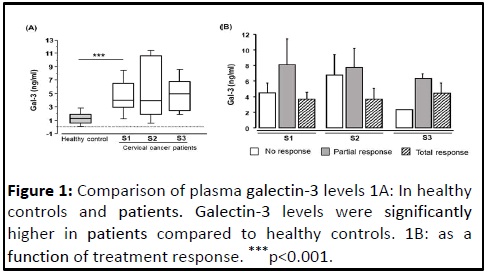
The comparison of Gal-3 levels between controls and patients before treatment reported in Figure 1 shows that Gal-3 levels were significantly higher in patients compared to controls (median 4.025 versus 1.340, pË?0.001). Also illustrates the levels of Gal-3 plasma levels before each of the three chemotherapy courses (S1, S2 and S3). No significant difference was observed between the different concentrations during treatment. Furthermore, analysis of the correlation between age and Gal-3 levels in patients and controls showed no significant relationship. This indicates that age would not influence the variation of Gal-3 levels. Depending on the FIGO stage, the analysis of the Gal-3 concentration showed no significant difference.
As previously described, an assessment of the response to chemotherapy was performed after treatment. Thus, Gal-3 levels were studied as a function of treatment response. The results of this analysis showed no significant difference between the three groups of patients classified based on response to treatment (2.2 ng/ml vs. 6.1 ng/ml vs. 4.3 ng/ml).
T-cell activation kinetics
We evaluated the expression of early (CD69+) and late (HLADR+) activation markers on T-cells (CD3+), but also on T-cell subpopulations (CD3+CD4+ and CD3+CD8+).
Early (CD69) and late (HLA-DR) activation marker expression by CD3+ T-cells
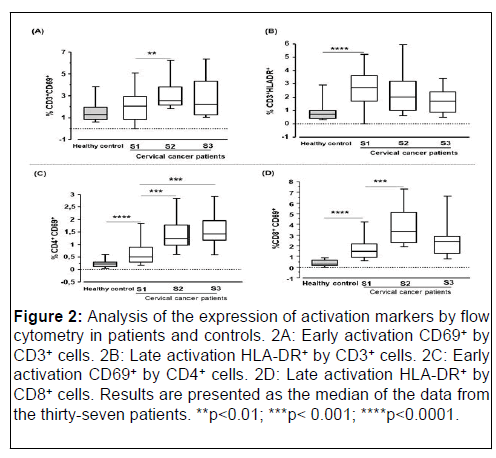
The median percentage of CD3+ T-cells expressing the HLA-DR+ marker was significantly higher in patients compared to controls (median 2.610 versus 0.731; pË?0.0001; Mann-Whitney test). In contrast, no significant difference was noted for CD3+ Tcells expressing the early activation marker CD69+ between patients and controls (median 2.047 versus 1.283; p=0.27; Mann- Whitney test).
In patients during treatment, for CD69+ marker expression by CD3+ cells, we found S1 (2.047), S2 (2.464) and S3 (2.205). A comparison the percentages showed a significant difference between the median percentage of CD3+CD69+ cells at S1 (2.047) versus S2 (2.464) (p=0.01). In parallel to the analysis of cells expressing the early activation marker, an analysis of CD3+HLADR+ cells was performed. Before treatment initiation, the median percentage of CD3+HLA-DR+ cells was 2.610 (S1). At 21 days after treatment initiation, it was 1.789 (S2) and 1.688 at 42 days after treatment initiation (S3). Although no difference was noted in the patients, a decrease in the expression of this marker was observed during treatment.
Kinetics of expression of activation markers by CD4+ and CD8+ T-cell sub-populations
The median percentage of CD4+ and CD8+ T-cell subpopulations expressing the activation marker (CD69+) was assessed. The expression of the early activation marker (CD69) by the CD4 cells (median 0.490 versus 0.230 respectively; pË? 0.0001) and CD8 cells (median 1.480 versus 0.325 respectively; pË?0.0001) subpopulations were significantly higher in patients than in controls (Figure 2).
Figure 2: Analysis of the expression of activation markers by flow cytometry in patients and controls. 2A: Early activation CD69+ by CD3+ cells. 2B: Late activation HLA-DR+ by CD3+ cells. 2C: Early activation CD69+ by CD4+ cells. 2D: Late activation HLA-DR+ by CD8+ cells. Results are presented as the median of the data from the thirty-seven patients. **p<0.01; ***p<0.001; ****p<0.0001.
Regarding the expression kinetics of the different markers during treatment, the percentage of CD4+ and CD8+ T-cells expressing the CD69+ marker increased significantly 21 days after initiation of chemotherapy, respectively (0.490 at S1 versus 1.230 at S2 with p=0.0007) for CD4+CD69+ cells and CD8+CD69+ cells (1.480 at S1 versus 3.285 at S2 with p=0.0003). Similarly, a significant increase was also noted 42 days after treatment initiation (S3) for CD4+CD69+ cells (median 0.490 (S1) versus 1.440 (S3); p=0.0099). In contrast, a non-significant decrease was observed for CD8+CD69+ cells 42 days after initiation of treatment.
Additionally, the search for a possible relationship summarized in Table 2, showed a positive correlation between CD4+CD69+ T-cells and CD8+CD69+ T-cells in controls (rho=0.539, p=0.0006). Similarly, in patients, a positive correlation was noted before initiation of treatment and at 21 days after initiation of treatment, respectively (S1: rho 0.499; p=0.0036) (S2: rho 0.610; p=0.012). In contrast, no relationship was noted at d42 after treatment initiation.
|
|
CD4+CD69+ |
|||
|---|---|---|---|---|
|
CD8+CD69+ |
|
rhoa |
p-value |
|
|
Controls (n=42) |
|
0.539 |
0.0006 |
|
|
Patients (n=37) |
S1 |
0.499 |
0.0036 |
|
|
S2 |
0.61 |
0.012 |
||
|
S3 |
0.418 |
0.186 |
||
Note: aSpearman’s Rank Test; Bold text indicates a significant p-value.
Table 2: Correlation between CD4+CD69+ and CD8+CD69+ cells.
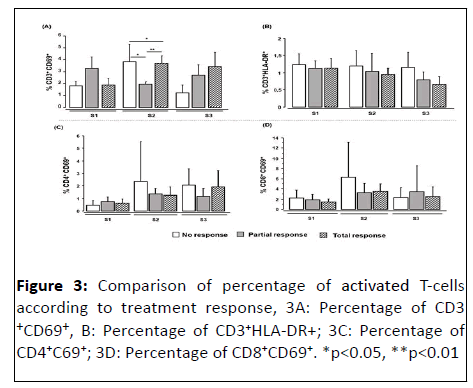
Activated T-cells according to the response to chemotherapy
We also analyzed the percentage of activated lymphocytes according to the response to treatment (Figure 3). Our results show that in patients at S1 (before treatment) and at S3 (42 days after treatment) no significant difference was observed between the groups of patients as defined above (no response, partial response and total response). In contrast, at 21 days after the start of treatment (S2), a significant difference was noted in the percentage of CD3+ T-cells expressing the activation marker CD69+ between the three patient groups (n=10, median 1.06), (n=14, median 0.89) and (n=13, median 1.355) in patients classified as no response, partial response and complete response, respectively (p<0.01). Additionally, the median percentage of CD3+CD69+ T-cells was significantly lower in patients classified as no response compared with partial response patients (p<0.05 and total responders (p<0.001).
Relationship between galectin-3 levels and cells expressing the early and late activation markers.
The search for a possible relationship between the level of Gal-3 and the expression of activation markers by T-cells showed no correlation in the controls. However, in the patients before treatment, a significant correlation was observed between the level of plasma Gal-3 and the expression of the early activation marker by CD4 T-cells. In contrast, no significant relationship was observed during treatment in patients. Table 3 summarizes all the data obtained [38].
|
|
|
CD3+CD69+ |
CD4+CD69+ |
CD8+CD69+ |
CD3+HLA-DR+ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Gal-3 |
|
rhoa |
p |
rhoa |
p |
rhoa |
p |
rhoa |
p |
|
|
Controls (n=42) |
0.107 |
0.592 |
0.088 |
0.65 |
-0.228 |
0.25 |
0.008 |
0.96 |
||
|
Patients (n=37) |
S1 |
0.268 |
0.279 |
0.443 |
Ë?0.05 |
-0.196 |
0.39 |
0.364 |
0.11 |
|
|
S2 |
-0.172 |
0.479 |
0.149 |
0.53 |
0.26 |
0.28 |
-0.377 |
0.12 |
||
|
S3 |
0.318 |
0.314 |
-0.291 |
0.35 |
0.291 |
0.35 |
0.036 |
0.9 |
||
Note: a Spearman’s rank test, Bold text indicates a significant p-value.
Table 3: Relationship between galectin-3 levels and early and late activation markers.
Discussion
In Senegal, the early emergence of cervical cancer, the difficulties in its management and its aggressiveness are compounded by the lack of diagnostic and prognostic biomarkers. Therefore, the identification of reliable biomarkers is essential for early diagnosis and assessment of the therapeutic prognosis, but also for the development of potential therapeutic targets for cervical cancer. In this study, we determined the plasma Gal-3 concentrations and assessed the proportions of activated T-cells in patients with cervical cancer. We then investigated a possible relationship between galectin-3 and activated lymphocytes during chemotherapy.
As a multifunctional protein widely expressed by many human cell types, overexpression of Gal-3 is commonly observed in various cancer types. The significant increase in plasma levels of circulating Gal-3 in cancer patients has been observed in many cancers, including breast cancer, colorectal cancer, lung cancer, pancreatic carcinoma, bladder cancer, thyroid cancer, gastrointestinal cancer, prostate cancer and squamous cell carcinoma of the head and neck [38]. Despite this, studies on the role of Gal-3 in human cervical carcinogenesis remain few, especially in Senegal, where such studies are almost absent [39].
In this study, plasma Gal-3 levels were significantly higher in cervical cancer patients compared to controls. This is in line with the results of the previously mentioned studies, in which patients with cancer had higher Gal-3 concentrations than healthy controls [40]. However, the origin of the increase in plasma Gal-3 in cancer patients remains unclear, especially as the sources of circulating Gal-3 are not only tumor cells, but also macrophages, mast cells, eosinophils and stromal cells in the vicinity of cancer nests [41]. Furthermore, the study of Castronovo et al. showed that Gal-3 is down regulated in breast cancer and suggests that the decrease in the expression of this galactoside binding lectin is associated with the acquisition of the invasive and metastatic phenotype [42]. Our results also show a lack of correlation between patient age, age of healthy controls and Gal-3, indicating that in this case, age does not influence the variation in Gal-3 levels [43]. A study in heart failure patients also showed that Gal-3 was not associated with age, Body Mass Index (BMI) or gender [44].
Additionally, in patients classified according to treatment response no response, partial response and total response), no significant differences were observed in the different chemotherapy courses. This lack of significance may be explained by the small number of patients in each group and by the type of sample used. Nevertheless, we noted that among the three groups of patients, patients with a complete response had a consistently stable level of Gal-3 during treatment. In contrast, in patients with no response, Gal-3 levels were variable and lower than in patients with partial and complete responses. This may be because circulating Gal-3 binds to elements normally present in the tumor micro environment and these elements would be absent in partial and complete response patients. Therefore, complete and partial response patients have high concentrations of Gal-3 and no response patients have low concentrations. Some studies have shown that soluble Gal-3 interacts with more than 30 ligands on the cell surface, some of which are well characterized and include several Extracellular Matrices (ECM) components such as collagen IV, elastin and fibronectin, as well as signaling receptors such as CD4, CD66 and CD98 [45].
Similarly, in a study of pancreatic ductal adenocarcinoma, Gal-3 was shown to bind to T CD8+ cells in the tumor microenvironment expressing certain inhibitory co-receptors such as PD-1 and LAG-3, resulting in a "depleted" TCD8 cell phenotype [46]. In addition, previously published studies have shown that a decrease in Gal-3 is associated with the progression of cervical neoplasia in cervical carcinoma tissues and suggested the use of plasma Gal-3 levels as a marker of metastatic potential [47]. The low concentrations of Gal-3 observed in patients with no response to treatment would be due to its storage in tumor cells leading to the acquisition of an invasive and metastatic phenotype intracellular Gal-3 being antiapoptotic or to binding to ligands of the extracellular matrix which would affect the plasma bioavailability of circulating Gal-3.
Additionally, for the study of lymphocyte activation, we used the markers CD69 and HLA-DR. The presence of activated lymphocytes in healthy subjects suggests the existence of a small immune activation background allowing for immune surveillance and that this immune surveillance background becomes more pronounced in an infectious or tumor context. Thus, we observed that the percentage of CD3HLA-DR+ T-cells was significantly higher in patients compared to controls. Numerous studies have reported an increase in HLA-DR+ T-cells in various disease states characterized by immune dysfunction. For example, abnormally high percentages of CD3HLA-DR+ cells have been observed in asymptomatic HIV patients and patients with active tuberculosis [48]. This increase in the percentage of CD3HLA-DR+ cells in cervical cancer and other cancerous conditions is thought to be due to lymphocyte hyper stimulation, which is itself caused by the release of large amounts of tumor antigens into the bloodstream. Indeed, HLA II receptors normally present exogenous antigens from antigen-presenting cells to CD4 helper T-cells and exposure to foreign antigens seems to be the most logical explanation for the increased HLA-DR in patients. It is also possible that other activated immune cells themselves produce cytokines that continue to amplify and perpetuate immune activation. Furthermore, in a study assessing the expression of HLA-DR on systemic cytotoxic T-cells by cytometry during neoadjuvant chemotherapy in breast cancer, the results showed that patients had higher levels of HLA-DR than healthy controls. In addition, HLA-DR expression was higher in responders compared to non-responders. However, in our study, no difference was noted for HLA-DR expression in CD3+ lymphocytes between patient groups.
In the CD69 marker assessment, no statistical difference was observed for CD3+ lymphocytes between patients and controls, although the percentage of CD3+CD69+ cells was higher in patients; evidence of stimulation of the immune system due to the release of tumor antigens in the bloodstream during the disease [49].
In contrast, if we consider the expression of CD69 by CD4+ and CD8+ T-cell subpopulations, it was significantly higher in the pretreatment patients compared to the control groups. Our results are consistent with the findings of previous reports showing higher CD4+CD69+ and CD8+CD69+ cell percentages in patients with cervical cancer. These elevated proportions of CD4 and CD8 subpopulations at the systemic level are thought to reflect immunological changes in the cervical cancer tumor micro environment. However, it is important to note that local immunological changes are not fully reflected in the analysis of peripheral blood lymphocytes. The percentage of activated CD4+CD69+ T-cells increased continuously during treatment and CD8 T-cells expressing the CD69 marker peaked at week three. Although the specific mechanism involved in this phenomenon is not understood in cervical cancer, the most plausible explanation would be that the treatment resulted in the release of tumor antigens, thereby enhancing antigen presentation by antigen-presenting cells and further inducing T-cell activation and recruitment to the tumor. Therefore, analysis of the cervical microenvironment may provide more information on the phenotype and functionality of immune cells in this region during chemotherapy and be useful for designing future cancer immunotherapy strategies. We also observed a positive correlation between the percentage of CD4+CD69+ and CD8+CD69+ cells in patients, suggesting a shift in the anti-tumor response towards a cell-mediated immunity, i.e. a type 1 T helper response, which would allow the differentiation of CD8+ T-cells into cytotoxic T-cells and promote the elimination of cervical cancer cells rather than a type 2 T helper response predictive of recurrence and poor prognosis.
Depending on the treatment response, the difference between the percentages of CD4+CD69+ and CD8+CD69+ cells was not significant between the different responder groups. The explanation would be that the CD4 and/or CD8 cells present in no response patients are likely to be "suppressive" or express inhibitory co-receptors giving them a "depleted" phenotype, which consequently may lead to an inhibition of the anti-tumor response, whereas those present in the total response patients are "effector" cells promoting the anti-tumor response to eliminate the tumor cells. This would explain the difference in clinical response between the different responder groups, despite the presence of CD4+ and CD8+ T-cells. These observations are in agreement with our previous results in which we noted a low concentration of Gal-3 in none responders and suggested that this low concentration would be due to the binding of Gal-3 to inhibitory co-receptors expressed by T-cells. In addition, studies of anti-cancer drugs, including cisplatin, have reported that these antineoplastic agents not only activate the immune system by inducing Immunogenic Cell death (ICM) of tumor cells but also increase PD-L1 expression on tumor cells, leading to suppression of cytotoxic T-cell function. Therefore, the presence of CD4+ and/or CD8+ T-cell subpopulations does not necessarily confer an effective antitumor response that can lead to tumor cell elimination.
Regarding the probable link between Gal-3 and activated Tcells, in our study, this link was only observed between Gal-3 and CD4 T-cells expressing the CD69 marker (CD4+CD69+), this before treatment. However, this observation does not explain the difference in clinical response between the groups of patients and does not allow us to know if Gal-3 was the cause of the inhibition of CD4 T-cells in some patients as has been shown in other studies. Therefore, it would be relevant to perform an analysis of the tumor microenvironment to identify the role of Gal-3 in CD4 T-cell function.
Conclusion
In summary, our results show that cervical cancer patients had significantly elevated levels of Gal-3. These patients also had proportions of activated lymphocytes. Additionally, depending on the response to chemotherapy, Gal-3 levels were lower in patients with no response. However, our current study does not allow us to understand the causes of the decrease in Gal-3 levels in these patients. We also observed a correlation between the concentration of Gal-3 and the percentage of CD4+ T-cells expressing the CD69 marker. Our results, therefore, show the existence of a relationship between circulating galectin-3 and the percentage of early activated CD4+ cells in the periphery.
Therefore, further studies with comparative biopsy analyses and more detailed immune analyses may provide information about the effects of chemotherapy at the tumor site and identify immune phenotypes that correlate with chemotherapy efficacy.
Limitation
This study would have provided much more information regarding the involvement of the immune system in the response to chemotherapy, if functional analyses of CD4 and CD8 cells were performed, including the determination of different cytokines produced by these cells with or without stimulation by cervical carcinoma antigens. Furthermore, a comparison of the results of this study with data from the analysis of the tumor microenvironment would provide information on the likely immune changes at the blood level and the level of the different regions of the tumor.
Authors’ Contribution
Contributed to data collection: FMG, MD, BM. Contributed to participant recruitment and evaluation: FMG, MD, BM, SK and DD. Contributed to study design: FMG, MD, BM and AD. Contributed to data analysis: FMG, BM. Wrote the paper: FMG. Contributed to editing the paper: All authors have read and agreed to the published version of the manuscript.
Informed consent statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The author would like to thank the technical staff, in particular Mrs. Aissata Soumah of the immunology department of the faculty of medicine at Cheikh Anta Diop university for her logistical support. We would also like to thank all the participants in this study.
References
- Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, et al. (2020) Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health 8:191-203
[Crossref] [Googlescholar] [Indexed]
- De Martel C, Georges D, Bray F, Ferlay J, Clifford GM (2020) Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob Health 8:180-190
[Crossref] [Googlescholar] [Indexed]
- Parkin DM, Hammerl L, Ferlay J, Kantelhardt EJ (2020) Cancer in Africa 2018 The role of infections. Int J Cancer 146:2089-2103
[Crossref] [Googlescholar] [Indexed]
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, et al. (1994) Galectins: A family of animal beta-galactoside-binding lectins. Cell 76:597-598
[Crossref] [Googlescholar] [Indexed]
- Funasaka T, Raz A, Nangia-Makker P (2014) Nuclear transport of galectin-3 and its therapeutic implications. Semin Cancer Biol 27:30-38
[Crossref] [Googlescholar] [Indexed]
- Dumic J, Dabelic S, Flogel M (2006) Galectin-3: An open-ended story. Biochim Biophys Acta 1760:616-35
[Crossref] [Googlescholar] [Indexed]
- Wang L, Zhao Y, Wang Y, Wu X (2018) The role of galectins in cervical cancer biology and progression. Biomed Res Int 2018:2175927
[Crossref] [Googlescholar] [Indexed]
- Gao X, Balan V, Tai G, Raz A (2014) Galectin-3 induces cell migration via a calcium-sensitive MAPK/ERK1/2 pathway. Oncotarget 5:2077-2084
[Crossref] [Googlescholar] [Indexed]
- Inohara H, Akahani S, Koths K, Raz A (1996) Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res 56:4530-4534
[Googlescholar] [Indexed]
- Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, et al. (2002) Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem 277:6852-6857
[Crossref] [Googlescholar] [Indexed]
- Markowska AI, Jefferies KC, Panjwani N (2011) Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J Biol Chem 286:29913-29921
[Crossref] [Googlescholar] [Indexed]
- Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, et al. (2009) Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci USA 106:14496-14501
[Crossref] [Googlescholar] [Indexed]
- Yang RY, Liu FT (2003) Galectins in cell growth and apoptosis. Cell Mol Life Sci 60:267-276
[Crossref] [Googlescholar] [Indexed]
- Nangia-Makker P, Nakahara S, Hogan V, Raz A (2007) Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr 39:79-84
[Crossref] [Googlescholar] [Indexed]
- Sakaki M, Oka N, Nakanishi R, Yamaguchi K, Fukumori T, et al. (2008) Serum level of galectin-3 in human bladder cancer. J Med Invest 55:127-132
[Crossref] [Googlescholar] [Indexed]
- Schoeppner HL, Raz A, Ho SB, Bresalier RS (1995) Expression of an endogenous galactoseâ?ÂÂbinding lectin correlates with neoplastic progression in the colon. Cancer 75:2818-2826
[Crossref] [Googlescholar] [Indexed]
- Berberat PO, Friess H, Wang L, Zhu Z, Bley T, et al. (2001) Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem 49:539-549
[Crossref] [Googlescholar] [Indexed]
- Orlandi F, Saggiorato E, Pivano G, Puligheddu B, Termine A, et al. (1998) Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res 58:3015-3020
[Googlescholar] [Indexed]
- Yamaki S, Fujii T, Yajima R, Hirakata T, Yamaguchi S, et al. (2013) Clinicopathological significance of decreased galectin-3 expression and the long-term prognosis in patients with breast cancer. Surg Today 43:901-905
[Crossref] [Googlescholar] [Indexed]
- Gilson RC, Gunasinghe SD, Johannes L, Gaus K (2019) Galectin-3 modulation of T-cell activation: Mechanisms of membrane remodelling. Prog Lipid Res 76:101010
[Crossref] [Googlescholar] [Indexed]
- Demetriou M, Granovsky M, Quaggin S, Dennis JW (2001) Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409:733-739
[Crossref] [Googlescholar] [Indexed]
- Schindler EI, Szymanski JJ, Hock KG, Geltman EM, Scott MG (2016) Short and long-term biologic variability of galectin-3 and other cardiac biomarkers in patients with stable heart failure and healthy adults. Clin Chem 62:360-366
[Crossref] [Googlescholar] [Indexed]
- Balan V, Wang Y, Nangia-Makker P, Kho D, Bajaj M, et al. (2013) Galectin-3: A possible complementary marker to the PSA blood test. Oncotarget 4:542-549
[Crossref] [Googlescholar] [Indexed]
- Saussez S, Lorfevre F, Lequeux T, Laurent G, Chantrain G, et al. (2008) The determination of the levels of circulating galectin-1 and-3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol 44:86-93
[Crossref] [Googlescholar] [Indexed]
- Iacovazzi PA, Notarnicola M, Caruso MG, Guerra V, Frisullo S, et al. (2010) Serum levels of galectin-3 and its ligand 90k/mac-2bp in colorectal cancer patients. Immunopharmacol Immunotoxicol 32:160-164
[Crossref] [Googlescholar] [Indexed]
- Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, et al. (2000) Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res 6:1389-1393
[Googlescholar] [Indexed]
- Shimura T, Shibata M, Gonda K, Nakajima T, Chida S, et al. (2016) Circulating galectin-3 correlates with angiogenetic factors and indicators of systemic inflammation in patients with stage IV esophageal cancer. Ann Cancer Res Ther 24:30-34
[Crossref] [Googlescholar] [Indexed]
- Xie L, Ni WK, Chen XD, Xiao MB, Chen BY, et al. (2012) The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol 138:1035-1043
[Crossref] [Googlescholar] [Indexed]
- Isic T, Savin S, Cvejic D, Marecko I, Tatic S, et al. (2010) Serum Cyfra 21.1 and galectin-3 protein levels in relation to immuno histochemical cytokeratin 19 and galectin-3 expression in patients with thyroid tumors. J Cancer Res Clin Oncol 136:1805-1812
[Crossref] [Googlescholar] [Indexed]
- Cheng D, Liang B, Li Y (2015) Serum galectin-3 as a potential marker for gastric cancer. Med Sci Monit 21:755-760
[Crossref] [Googlescholar] [Indexed]
- Balasubramanian K, Vasudevamurthy R, Venkateshaiah SU, Thomas A, Vishweshwara A, et al. (2009) Galectin-3 in urine of cancer patients: Stage and tissue specificity. J Cancer Res Clin Oncol 135:355-363
[Crossref] [Googlescholar] [Indexed]
- Liu J, Cheng Y, He M, Yao S (2014) Vascular endothelial growth factor C enhances cervical cancer cell invasiveness via upregulation of galectin-3 protein. Gynecol Endocrinol 30:461-465
[Crossref] [Googlescholar] [Indexed]
- De Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, et al. (2012) The fibrosis marker galectin-3 and outcome in the general population. J Intern Med 272:55-64
[Crossref] [Googlescholar] [Indexed]
- Punt S, Thijssen VL, Vrolijk J, de Kroon CD, Gorter A, et al. (2015) Galectin-1, -3 and -9 Expression and Clinical Significance in Squamous Cervical Cancer. PloS one 10:e0129119
[Crossref] [Googlescholar] [Indexed]
- Castronovo V, Van Den Brûle FA, Jackers P, Clausse N, Liu FT, et al. (1996) Decreased expression of galectin-3 is associated with progression of human breast cancer. J Pathol 179:43-48
[Crossref] [Googlescholar] [Indexed]
- Meeusen JW, Johnson JN, Gray A, Wendt P, Jefferies JL, et al. (2015) Soluble ST2 and galectin-3 in pediatric patients without heart failure. Clin Biochem 48:1337-1340
[Crossref] [Googlescholar] [Indexed]
- Song L, Tang JW, Owusu L, Sun MZ, Wu J, et al. (2014) Galectin-3 in cancer. Clin Chim Acta 431:185-191
[Crossref] [Googlescholar] [Indexed]
- Kouo T, Huang L, Pucsek AB, Cao M, Solt S, et al. (2015) Galectin-3 shapes antitumor immune responses by suppressing CD8+ T-cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res 3:412-423
[Crossref] [Googlescholar] [Indexed]
- Lee J-W, Song SY, Choi J-J, Choi CH, Kim TJ, et al. (2006) Decreased galectin-3 expression during the progression of cervical neoplasia. J Cancer Res Clin Oncol 132:241-247
[Crossref] [Googlescholar] [Indexed]
- Wang Y, Liu S, Tian Y, Wang Y, Zhang Q, et al. (2018) Prognostic role of galectin-3 expression in patients with solid tumors: A meta-analysis of 36 eligible studies. Cancer Cell Int 18:172
[Crossref] [Googlescholar] [Indexed]
- Ziegler-Heitbrock HW, Stachel D, Schlunk T, Gurtler L, Schramm W, et al. (1988) Class II (DR) antigen expression on CD8+ lymphocyte subsets in Acquired Immune Deficiency Syndrome (AIDS). J Clin Immunol 8:473-478
[Crossref] [Googlescholar] [Indexed]
- Tippalagama R, Singhania A, Dubelko P, Lindestam Arlehamn CS, Crinklaw A, et al. (2021) HLA-DR marks recently divided antigen-specific effector CD4 T-cells in active tuberculosis patients. J Immunol 207:523-533
[Crossref] [Googlescholar] [Indexed]
- Saraiva DP, Jacinto A, Borralho P, Braga S, Cabral MG (2018) HLA-DR in cytotoxic t lymphocytes predicts breast cancer patients' response to neoadjuvant chemotherapy. Front Immunol 9:2605
[Crossref] [Googlescholar] [Indexed]
- Tsuyama S, Hashimoto K, Nakamura K, Tamura H, Sasaki K, et al. (1991) Different behaviors in the production and release of SCC antigen in squamous-cell carcinoma. Tumour Biol 12:28-34
[Crossref] [Googlescholar] [Indexed]
- Sheu BC, Lin RH, Ho HN, Huang SC (1997) Down-regulation of CD25 expression on the surface of activated tumor-infiltrating lymphocytes in human cervical carcinoma. Hum Immunol 56:39-48
[Crossref] [Googlescholar] [Indexed]
- Castello G, Esposito G, Stellato G, Dalla Mora L, Abate G, et al. (1986) Immunological abnormalities in patients with cervical carcinoma. Gynecol Oncol 25:61-64
[Crossref] [Googlescholar] [Indexed]
- Delgado FG, Martínez E, Céspedes MA, Bravo MM, Navas MC, et al. (2009) Increase of human papillomavirus-16 E7-specific T helper type 1 response in peripheral blood of cervical cancer patients after radiotherapy. Immunology 126:523-534
[Crossref] [Googlescholar] [Indexed]
- Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, et al. (2015) Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 521:94-98
[Crossref] [Googlescholar] [Indexed]
- Wakita D, Iwai T, Harada S, Suzuki M, Yamamoto K, et al. (2019) Cisplatin augments antitumor T-cell responses leading to a potent therapeutic effect in combination with PD-L1 blockade. Anticancer Res 39:1749-1760
[Crossref] [Googlescholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences