Radiation Recall Pneumonitis in A Patient Treated by Nivolumab for Non-Small Cell Lung Cancer, No Relapse with Rechallenge
Pierre Thoré1*, Benoît Godbert2, Isabelle Petit3 and Ari Chaouat1,4
1Pôle des speÃŒÂÂcialiteÃŒÂÂs meÃŒÂÂdicales, deÃŒÂÂpartement de pneumologie, CHU de Nancy, rue du Morvan, Vandoeuvre-lès- Nancy, France
2Service de Pneumologie, Hôpitaux Privés de Metz, Parvis Robert Schuman, Rue du Champ Montoy, Vantoux, France
3Département de Radiologie, CHU de Nancy, rue du Morvan, Vandoeuvre-lès- Nancy, France
4Universite̠de lorraine, Vandoeuvre-lès- Nancy, France
- *Corresponding Author:
- Pierre Thoré
Emeritus Professor of Cell Pathology
University College London, United Kingdom
Tel: +33 387 3156 38
E-mail: thore.pierre@gmail.com
Received Date: September 19, 2018 Accepted Date: October 22, 2018 Published Date: October 29, 2018
Citation: Thoré P, Godbert B, Petit I, Chaouat A (2018) Radiation Recall Pneumonitis in A Patient Treated by Nivolumab for Non-Small Cell Lung Cancer, No Relapse with Rechallenge. J Med Oncol. Vol.1 No.3:12
Abstract
Introduction: Radiation Recall Pneumonitis (RRP) is an inflammatory phenomenon occurring in a previously irradiated area of the lungs in response to precipitating agents. The diagnosis is based on clinical features such fever, dyspnea, dry cough, asthenia, and radiologic abnormalities (ground-glass opacities and condensations) in the irradiated field and must occur after the administration of an inciting agent. We reported a case of RRP induced by nivolumab.
Report: A 58 year old woman previously treated by left mastectomy and adjuvant radiotherapy for a breast cancer (October 2016) was diagnosed with a right lung adenocarcinoma in December 2016. Concomitant radio-chemotherapy with irradiation of the upper part of the right hemithorax was performed from February to March 2017. Unfortunately, a new metastasis appeared in April 2017. A new line of treatment with nivolumab was started. Four weeks after the onset of the checkpoint inhibitor, she presented clinical and imaging criteria evoking the diagnosis of RRP induced by nivolumab. A high dose systemic steroid was introduced. As a result, general state, respiratory conditions and imaging features were improved.
Conclusion: Some drugs are known to be associated with RRP, mainly traditional chemotherapies. Patients generally improved their condition after a break of the precipitating agent and with steroids. RRP induced by nivolumab has only been described once in a Japanese case report. It constitutes the first report of such a case in Europe. In our case, nivolumab was reintroduced after the resolution of the RRP. After 22 new cycles of treatment we didn’t observe recurrence of RRP.
Keywords
Radiation pneumonitis; Immunotherapy; Radiation recall reactions
Introduction
Nivolumab is an anti-programmed cell death-1 antibody (immunotherapy). Since few years, nivolumab has been approved as second-line treatment of Non-small Cell Lung Cancers (NSCLC). The most common side effects, mainly dysimmune toxicities are now well-known [1]. Dysimmune toxicities can mainly affect the gastrointestinal tract (colitis, gastritis), the lung (pneumonitis, pleural effusion), the skin (psoriasis, maculopapular rash, DRESS (Drug Rash with Eosinophilia and Systemic Symptoms)), endocrine system (dysthyroidism, indrenal insufficiency, diabetes), the licer (hepatitis), musculo-articular system (arthritis, myopathies) [1].
Radiation Recall Reaction (RRR) is an inflammatory phenomenon occurring in a previously irradiated area in response to precipitating agents. Radiation Recall Pneumonitis (RRP) corresponds to a RRR occurring in a previously irradiated area of the lungs in response to precipitating agents. Main symptoms are fever, dyspnea, dry cough, chest pain and asthenia. The diagnosis is established by radiologic abnormalities (mainly ground-glass opacities) in the irradiated field and triggered after the administration of inciting agents [2]. The most commonly involved agents are chemotherapeutic drugs such as taxanes, anthracyclines, gemcitabine [3-5].
This side effect is almost unknown for immunotherapy. We describe here an RRP induced by nivolumab.
Case Report
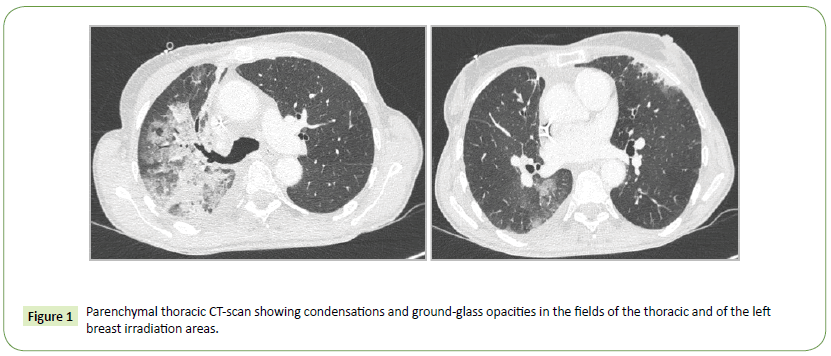
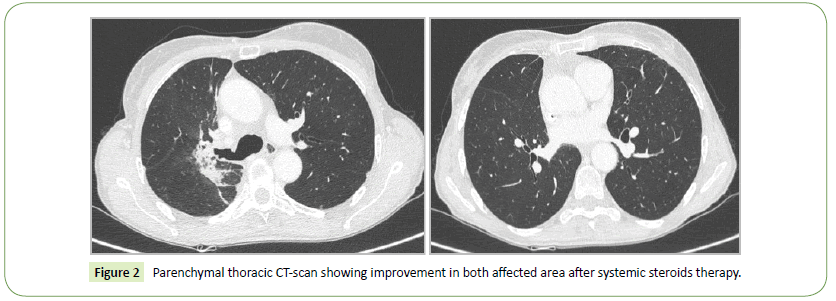
In December 2016, a 58 years old woman was diagnosed with a lung adenocarcinoma in the right upper lobe. She smoked about 10 packs a year, and stopped around 25 years ago. Her medical history was marked by a breast cancer treated by left mastectomy and adjuvant radiotherapy in October 2016 (55 Gy). Her nonsmall cell lung cancer was classified T3N2M1b: the extension work-up revealed mediastinal adenopathies and a single bone metastasis on the anterior superior iliac spine. First-line treatment consisted in neoadjuvant chemotherapy including carboplatin and pemetrexed plus the irradiation of the bone metastasis. In January 2017 she underwent surgery, sadly the primitive lung lesion appeared ineradicable unresecable. Thereafter concomitant radiochemotherapy (from February to March 2017-66 Gy) including carboplatine and paclitaxel was performed. In April 2017, a new metastasis appeared. Nivolumab was started. Four weeks after the onset of the checkpoint inhibitor, she presented dyspnea, fever, asthenia, cough and mucopurulent expectorations. Chest X-ray showed a right upper lobe interstitial pneumopathy. Thoracic CT scan showed ground-glass opacities and condensations in the field of the thoracic irradiation and in the area of the left breast irradiation (Figure 1). Bacterial examination of the sputum sampled before antibiotics was negative. At broncho-alveolar lavage, we found 15% of lymphocytes and 41% of eosinophils, the microbiologic exploration was negative. Nivolumab was stopped for six weeks and high dose systemic steroid was introduced. General and respiratory conditions resolved. The thoracic CT scan performed 6 weeks later showed dramatic improvement (Figure 2). The conclusion of the multidisciplinary meeting of our institution was RRP induced by nivolumab especially because of the special distribution of the lesions in fields of previous irradiations (right upper thorax and left breast) what differentiates it from classical immuno-induced pneumopathy. In addition, the multidisciplinary meeting considered that the mechanism of pneumopathy was due to previous irradiations, and that nivolumab was only a trigger, hence the diagnosis of RRP. Nivolumab has been therefore carefully rechallenged without relapse after 22 new cycles.
Discussion
Nivolumab is an anti-programmed cell death-1 antibody which is indicated as second-line treatment of non-small cell lung cancer [6,7]. Some drugs are known to be associated with RRP, mainly traditional chemotherapies. RRP has also been described in a patient treated by trastuzumab [8], and in two patients treated with vemurafenib after thoracic radiation of lung metastasis of melanomas [9].
Only one article reporting two cases of RRP induced by nivolumab [10] was found in PubMed. In general, patients with RRP improved significantly their condition after a break of the nivolumab and with steroids [2,10].
In our case, the patient presented with two areas of pneumonitis corresponding to the fields of irradiation of the right upper lobe lung cancer and of the left breast cancer while the two radiotherapy sequences were spaced several months apart.
Radiation recall phenomenon is hardly predictable. Some drugs are known to be associated with these reactions but it concerns mainly traditional chemotherapies. Increasing use of immunotherapies such as nivolumab in the treatment of neoplastic diseases explain the emergence of radiation recall reactions due to nivolumab. It mostly concerns dermatitis. RRP induced by nivolumab remains an unknown phenomenon. It must be suspected in patients previously treated by thoracic radiotherapy and developing signs of pneumonitis in the field of radiation while they are treated by nivolumab. The pathophysiology of this phenomenon is unknown but we can assume that its mechanism looks more like that of radiation-induced pneumopathy, because the lesions affect the irradiated parts of the lungs, than that of a pure immuno-induced pneumopathy. So RRP is probably not the same disease than immuno-induced pneumopathy. The treatment consists in systemic corticotherapy and generally allows an improvement or a resolution of the general and respiratory conditions and an improvement of the thoracic CT (Computed Tomography) scan.
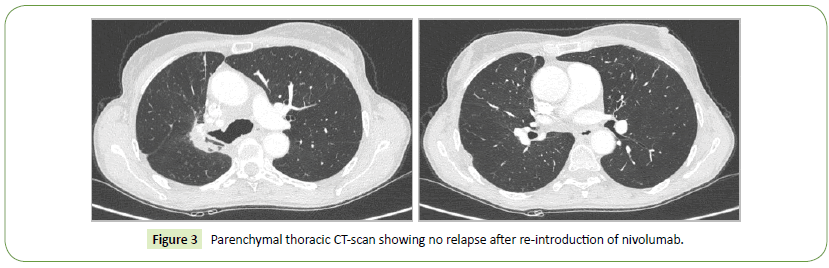
The case of our patient was presented to the multidisciplinary staff specifically dedicated to toxicities of immunotherapy at the Gustave Roussy institute. Given a toxicity inferior to a grade 2 CTCAE and the complete resolution of the toxicity (clinically rather than radiologically), we were allowed to reintroduce the treatment without steroids with a close respiratory monitoring. Nivolumab was resumed the 27th of July 2017. After 22 new cycles of treatment, we did not observe recurrence of RRP (Figure 3).
References
- Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, et al. (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27: 559-574.
- Ding X, Ji W, Li J, Zhang X, Wang L (2011) Radiation recall pneumonitis induced by chemotherapy after thoracic radiotherapy for lung cancer. Radiat Oncol 6: 24.
- Schweitzer VG, Juillard GJ, Bajada CL, Parker RG (1995) Radiation recall dermatitis and pneumonitis in a patient treated with paclitaxel. Cancer 76: 1069-1069.
- Schwarte S, Wagner K, Karstens JH, Bremer M (2007) Radiation Recall Pneumonitis Induced by Gemcitabine. Strahlenther Onkol 183: 215-217.
- Ma LD, Taylor GA, Wharam MD, Wiley JM (1993) “Recall” pneumonitis: adriamycin potentiation of radiation pneumonitis in two children. Radiology 187: 465-467.
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, et al. (2015) Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med 373: 1627-1639.
- Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, et al. (2015) Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med 373: 123-135.
- Lee HE, Jeong NJ, Lee Y, Seo YJ, Kim CD, et al. (2014) Radiation recall dermatitis and pneumonitis induced by trastuzumab (Herceptin®). Int J Dermatol 53: e159-e160.
- Forschner A, Zips D, Schraml C, Röcken M, Iordanou E, et al. (2014) Radiation recall dermatitis and radiation pneumonitis during treatment with vemurafenib. Melanoma Res 24: 512-516.
- Shibaki R, Akamatsu H, Fujimoto M, Koh Y, Yamamoto N (2017) Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann Oncol 28: 1404-1405.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences