ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Prostatic Therapeutic Efficacy of Prostanox® : A Novel and Multi-active Principle Formulation
1ECSIN-European Centre for the Sustainable Impact of Nanotechnology, ECAMRICERT SRL, Corso Stati Uniti 4, 35127 Padova, Italy
2Department of Translational Medicine, Laboratory of Physiology, University of Eastern Piedmont, via Solaroli 17, 28100 Novara, Italy
3Department of Health, Animal Science and Food Safety, University of Milan, Via Celoria 10, Milano, Italy
- *Corresponding Author:
- Federico Benetti
ECSIN-European Centre for the Sustainable Impact of Nanotechnology
ECAMRICERT SRL
Corso Stati Uniti 4, 35127 Padova, Italy
E-mail: f.benetti@ecamricert.com
Received Date: November 18, 2021; Accepted Date: December 01, 2021; Published Date: December 07, 2021
Citation: Tedesco E, Uberti F, Morsanuto V, Rossi L, Benetti F (2021) Prostatic Therapeutic Efficacy of Prostanox®: A Novel and Multi-active Principle Formulation. Am J Phytomed Clin Ther Vol.9 No.11:55.
Abstract
Objective: To demonstrate, with an in vitro approach, the efficacy and safety of a novel, multi-active principle formulation, Prostanox®, for the prevention and treatment of prostatic diseases, such as benign prostatic hyperplasia and correlated symptoms, with the aim of improving patients well-being.
Methodology: In the present work, we compared, with an in vitro approach based on a prostate and smooth muscle in vitro models, the prostatic safety and efficacy of Prostanox® with a commercial formulation, based only on Serenoa repens, and a 5αR inhibitor, Finasteride.
Results: Our results showed a better prostatic safety and therapeutic efficacy of Prostanox®, compared to the commercial formulation and Finasteride, with an increased anti-inflammatory, pro-apoptotic and myorelaxing activity, and a stronger inhibitory effect on the key enzyme 5αR and Prostatic-Specific Antigen (PSA) release.
Conclusion: Based Considering the obtained results, thanks to its unique blend of active principles, Prostanox® formulation is more promising for BPH and LUTs therapy compared to Serenoa repens only-based formulations.
Keywords
Urology; BPH; LUTs; LNCaP; Prostate; Dietary supplements; Inflammation
Introduction
BPH is a benign enlargement of the prostate gland, due to the proliferation of stromal and epithelial glandular cells, leading to transitional and periurethral area overgrowth [1]. Among aging men, BPH is considered the most common prostatic nonmalignant condition and its incidence increases by 30% from 50 to 80 years of age [2]. These conditions represent a significant challenge for the health care system of many countries, and their impact is likely to increase in the future due to the progressive consistency of worldwide population [3,4]. Furthermore, BPH may lead to the development of specific symptoms, commonly known as Low Urinary Tract Symptoms (LUTs), which negatively affect aging men lifestyle [5]. However, despite the scientific community effort, BPH etiology is not fully understood yet and, as such, the identification of an effective therapy is difficult [6-9]. At present, it is generally agreed that BPH is a consequence of stromal and epithelial prostatic cell proliferation [10], in particular of its smooth muscle component mediated, among others, by inflammatory, oxidative, hormonal and genetic factors [11-13]. The contraction of the hypertrophic prostatic smooth muscle component is responsible for many BPH-related symptoms [14,15]. However, as pointed out by the MTOPS (Medical Therapy of Prostatic Symptoms). the extension of the acronym was requested by the reviewer. and REDUCE (REduction by DUtasteride of prostate Cancer Events). The extension of the acronym was requested by the reviewer study [16,17], a prominent role for inflammation in BPH insurgence and development was proposed [12,13,18,19]. Taken together, this information indicates to a complex etiology for BPH, which treatment should require a multidrugbased approach. Accordingly, BPH is presently treated with a combination of 5αR inhibitors, which block testosterone to di dihydrotestosterone conversion, α-adrenergic receptor agonists that favour smooth muscle relaxation, and plant extracts, or phytotherapic agents [20,21]. Indeed, formulation based on plant extracts-based formulations are among the most popular drugs used in the medical management of BPH-induced LUTS [22,23]. Serenoa repens, extracted from saw palmetto tree berries, is the most popular phytotherapic medication for BPH since it is endowed with 5αR inhibitory activity and favour smooth muscle relaxation [24-26]. However, a formulation based on multiple active principles, other than Serenoa repens, would be more effective in BPH treatment, considering its multi-faceted origin [27,28]. To test this hypothesis, a novel formulation, Prostanox®, in which Serenoa repens therapeutic activity is complemented with other active principles (Boswellia carteri, Berberis vulgaris, Urtica dioica, Curcuma longa, Vaccinium spp. and magnesium), was compared to a commercially available, Serenoa repens onlybased formulation. Each phytoextract added to Serenoa repens in Prostanox® is endowed with specific activities in preventing BPH endpoints. Barberry (Berberis vulgaris) [29], curcuma (Curcuma longa) [30] and frankincense (Boswellia carterii) [31] extracts are known to be strong anti-inflammatory, anti-oxidant and antibacterial substances. Similarly, the black pepper (Piper nigrum) [32] and the stinging nettle (Urtica dioica) [33] are antiinflammatory substances, while the cranberry (Vaccinium spp.) [34] is endowed with antibacterial activity. Finally, magnesium has a myorelaxing effect on the smooth muscles. The effect of Prostanox® and the commercial formulation on different prostatic parameters, such as inflammation, 5αR inhibitory activity, prostate-specific antigen (PSA) release and smooth muscle activity were investigated with an in vitro approach, based on prostate and smooth muscle in vitro models.
Materials and Methods
LNCaP androgen-sensitive human prostate adenocarcinoma cell line (ATCC® CRL-1740™), WPMY-1 human myofibroblast stromal cell line (ATCC® CRL-2854™) and THP-1 human monocytic cell line (ATCC® TIB-202™) were purchased from ATCC (Manassas, VA, USA). High glucose Dulbecco’s Modified Eagle Medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 Medium, Hanks’ Balanced Salt Saline (HBSS), non-essential amino acids (NEAA), L-glutamine, Penicillin-Streptomycin mix, lipolysaccharide (LPS), Diclofenac, dihydrotestosterone (DHT) and phorbol 12-myristate 13-acetate (PMA) were purchased from Sigma-Aldrich (St Louis, MO, USA). Foetal bovine serum (FBS) was purchased from Euroclone (Milan, IT). Interleukin 1β (IL-1β), Tumor Necrosis Factor α (TNF-α), PSA and DHT ELISA kit were purchased from R&D Systems, PeproTech (London, UK), Abcam (Cambridge, UK) and Cloud Clone (Katy, TX, USA) respectively. Cell contraction assay was purchased from Cell Biolabs (San Diego, CA, USA). Transwell® insert were purchased from Millipore (Burlington, MA, USA). CellTiter 96® AQueous One Solution Cell Proliferation Assay (MTS) and Apo-ONE® Homogeneous Caspase-3/7 Assay were purchased from Promega (Madison, WI, USA).
Formulation composition
The comparative efficacy evaluation on prostate and smooth muscle in vitro models was performed between Prostanox® and commercially available formulation (CF; Permixon®), whose compositions are detailed in Supplementary Materials 1 (SM1), and Finasteride.
LNCaP cell culture
The human prostate cancer cell line LNCaP cells (passage 25 to 40) were maintained in RPMI-1640 medium supplemented with 10% FBS and 1% Penicillin-Streptomycin mix. The cells were grown at 37°C in a humidified atmosphere with 5% CO2. Cells were seeded at 1 × 104 cell/cm2 and medium changed every other day. Cells were sub cultivated by tryspinization every 7 d when 80–90% confluent. For viability and anti-inflammatory experiments, LNCaP were seeded 96-well plates and 6-well plates respectively at a density of 1 × 105 cell/cm2 and allowed to adhere for 2 days prior to experiments experiment cells were seeded at a density of 5 x 104 cells/cm2 in 24-well plates.
THP-1 cell culture
Human THP-1 monocytes (passage 22 to 32) was maintained in RPMI-1640 medium with glutamate supplemented with 10% FBS, 100 U/ mL penicillin and 100 μg/mL streptomycin (GIBCO, UK). Cells were cultured at a density of 5 × 105 cells/mL in 5% CO2 humidified atmosphere at 37°C and sub cultured twice a week. Macrophage differentiation was induced by incubation with 500 nM phorbol myristate acetate (PMA; Sigma-Aldrich, MO, USA) for 24 h. Culture medium was then replaced and cells cultured for an additional 24 h. For medium conditioning, 6 × 106 cell were seeded in 75 cm2 flask, differentiate into macrophages as described before and treated with 1 ng/mL for 6 h. At the end of the LPS treatment, medium was recovered and stored at -80°C until use.
WPMY-1 cell culture
Human prostate stromal cells (myofibroblast) (WPMY-1) (passage 40 to 50) were cultured in DMEM, supplemented with 10% foetal bovine serum (FBS) and 1% Penicillin-Streptomycin mix. Cells were seeded at 2 x10^3, always for consistency and sub cultivated by tryspinization every 7 d when 80–90% confluent. The medium was refreshed every other day. For cell contraction experiments, 1 × 106 cells were loaded into each collagen gel.
Evaluation of the impact of tested formulations, Finasteride and Diclofenac on LNCaP and WPMY-1 cells
To evaluate Prostanox®, CF, Finasteride and anti-inflammatory drug Diclofenac impact on human prostatic cellular model, and to determine their higher, non-toxic concentration, a doseresponse curve experiment on LNCaP cells was performed. Briefly, LNCaP cells were treated with increasing concentrations of Prostanox® (from 0 to 2000 μg/mL), CF (from 0 to 4000 μg/ ml) and Finasteride (from 0 to 372.5 μg/mL) for 6 and 24 h. Diclofenac treatment (from 0 to 1591 μg/mL) was limited to 6 h, corresponding to the duration of anti-inflammatory activity experiments. At the end of incubation time, LNCaP cells viability was determined by MTS assay, according to the manufacturer’s instruction. Obtained dose-response curves were fitted with OriginLab software and half-maximal effective concentration (EC50) calculated. Dose-response curve were also determined for human prostate stromal cells (WPMY-1), the in vitro model of smooth muscle, considering the same concentration range tested for Prostanox®, CF and Finasteride on LNCaP cells, but limiting the exposure to 24 h.
Prostate-specific anti-inflammatory activity
The prostate-specific anti-inflammatory activity of tested formulations and drugs was evaluated in a LNCaP cell-based prostatic epithelium in vitro model, with a two-step protocol:
i) 2 h pre-treatment of the prostatic epithelium in vitro model with the highest, non-toxic concentrations of formulations and drugs;
ii) 4 h exposure to inflammatory stimulus (LPS-stimulated macrophage conditioned medium) in presence of formulations and drugs.
LNCaP cells inflammation was achieved with the method described by Wong CP, et al. [35]. At the end of the treatment, LNCaP cells were washed with DPBS, scraped in ice-cold PBS, centrifuged and lysed by sonication in lysis buffer. Following centrifugation at 10000 g for 15 min, the level of interleukin- 1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) cytokines in obtained surnatants were quantified by commercial ELISA (Enzyme-Linked Immuno sorbent Assay) kits, following the manufacturer’s instructions.
Prostanox®, CF and Finasteride pro-apoptotic activity
To evaluate the pro-apoptotic activity of Prostanox®, CF and Finasteride on the prostatic epithelium in vitro model, LNCaP cells were exposed for 6 h at the highest, non-toxic concentration of formulations and Finasteride. Staurosporine (STS), a well-known pro-apoptotic agent, was used as positive control. To correlate anti-inflammatory activity with pro-apoptotic activity, the same experimental setup described above for the anti-inflammatory activity was applied. At the end of the incubation, pro-apoptotic activity was measured with a commercial fluorometric assay, based on caspases 3/7 activation of a fluorescent probe, following the manufacturer’s instruction. Fluorescence measurements were acquired with a multiwall-plate reader setting the excitation wavelength at 499 nm and emission wavelength at 521 nm.
5αR activity
The formulations impact on 5αR activity was assessed following the protocol described by Assinder SJ [36]. Briefly, equal amounts of the same LNCaP cell lysate (i.e. same total protein content) were incubated for 16 h at 37°C under agitation with Prostanox® (750 μg/mL) and CF (100 μg/mL), in presence of 5αR cofactor NADPH (100 μM) and the substrate testosterone (100 μM). Finasteride was used as a positive control (93.1 μg/mL) while no testosteroneincubated cell lysate was considered as negative control. At the end of the incubation period, the reactions were blocked with ice and the DHT content of the different lysate measured by ELISA assay, according to the manufacturer’s indications.
Measurement of PSA secretion by LNCaP prostatic cells
The effect of Prostanox®, CF and Finasteride on PSA secretion was evaluated in LNCaP prostatic cells, following the protocol described by Kampa M, et al. [37], in presence and absence of DHT (10 nM), an androgen known to increase PSA release. Secreted PSA levels were measured with a commercial ELISA kit, following the manufacturer’s instructions. Results were expressed as percentage of secreted PSA in cells treated with different formulations compared to control.
Smooth muscle myorelaxing activity
The myorelaxing activity of tested formulations bioavailable fraction was evaluated in an in vitro model of smooth muscles, based on WPMY-1 human myofibroblast stromal cell. The myorelaxing effect was analysed by means of a two-step gel-contraction assay, following the manufacturer's instructions. Fibroblast-containing gels were exposed to the bioavailable fraction of the formulations for the duration of the experiment (24 h). The myorelaxing activity was calculated with the following formula:
Myorelaxing Activity (%) = 100 - (CFibroblast /Ccontrol) X 100
where Cfibroblast is the contraction of the fibroblast-containing gels exposed to the bioavailable fraction of the different formulations and Ccontrol the contraction of the untreated fibroblast-containing gel (positive control of contraction).
Statistical analysis
Results were statistically analysed by t-test, using Origin Lab software (Origin Lab Corporation, MA, US). Experiments were performed in triplicate and results presented as average ± standard deviation. A p value of ≤ 0.05 was considered significant.
Results
Impact of Prostanox®, CF, Finasteride and Diclofenac on the in vitro prostatic model
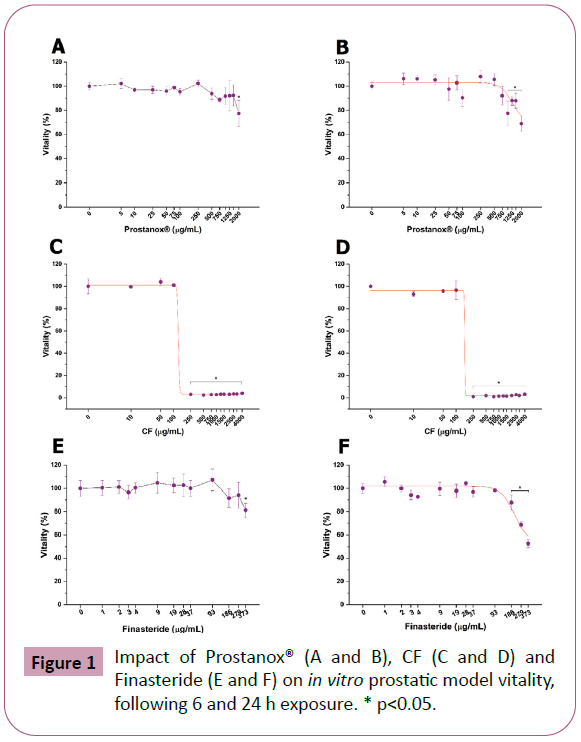
Before comparing the efficacy of Prostanox® with a commercial formulation (CF) and Finasteride, we investigated their safety on LNCaP cells through dose-response toxicological analysis, considering 6 and 24 h as relevant exposure times. LNCaP cells viability is significantly reduced, following 6 and 24 h treatment, at 2000 μg/mL and 1000 μg/mL of Prostanox® respectively (Figure 1E and Figure 1F) while CF completely abrogates LNCaP viability at 250 μg/mL, independently of exposure time. Concerning Finasteride, an adverse effect on LNCaP cells was observed at 373.0 μg/mL and 186.3 for a 6 and 24h treatment respectively. Finally, since Diclofenac is used as a positive control in inflammation experiment, its impact on LNCaP cells were also evaluated (SM2). EC50 value is reported in SM3. Based on cytotoxicity results, 6 h exposure-efficacy tests were performed with 1500 μg/mL of Prostanox®, 100 μg/mL of CF, 279 μg/mL of Finasteride and 32 μg/mL of Diclofenac, while for 24 h exposureefficacy a concentration of 750 μg/mL, 100 μg/mL and 93 μg/mL was applied for Prostanox®, CF and Finasteride respectively.
Figure 1: Impact of Prostanox® (A and B), CF (C and D) and Finasteride (E and F) on in vitro prostatic model vitality, following 6 and 24 h exposure. * p<0.05.
Effect of Prostanox®, CF and Finasteride treatment on pro-inflammatory cytokine release from the in vitro prostatic model
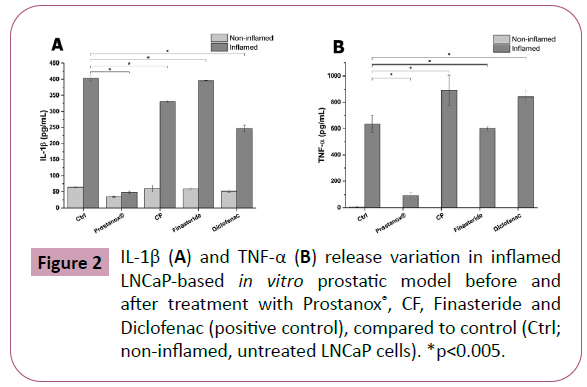
In recent years, a direct correlation between inflammation and BPH development was highlighted [17–19]. Prostanox®, CF and Finasteride anti-inflammatory activity was assessed by measuring pro-inflammatory cytokine (IL-1β and TNF-α) release. Diclofenac, an anti-inflammatory drug, was used as positive control. As shown in Figure 2A and SM4, a significant reduction in IL-1β release, compared to inflamed and untreated LNCaP cells (403 ± 8 pg/mL), was observed for Prostanox® (48 ± 4 pg/mL), CF (331 ± 3 pg/ml) and Diclofenac (247 ± 11 pg/mL) following 6 h treatment. Prostanox®-induced IL-1β release reduction is significantly higher compared to CF, Finasteride and Diclofenac, with the latter being more effective of CF and Finasteride. CF is more effective than Finasteride in reducing IL-1β release. Prostanox® anti-inflammatory activity was further confirmed by TNF-α release (Tables 6 and 7). Indeed, conversely to CF and Diclofenac, Prostanox®, compared to inflamed, untreated control, reduced TNF-α release by 85% (93 ± 19 pg/mL compared to 635 ± 66 pg/mL of the inflamed, untreated LNCaP cells). No effect on both cytokine releases was observed following treatment with Finasteride.
Figure 2: IL-1β (A) and TNF-α (B) release variation in inflamed LNCaP-based in vitro prostatic model before and after treatment with Prostanox®, CF, Finasteride and Diclofenac (positive control), compared to control (Ctrl; non-inflamed, untreated LNCaP cells). *p<0.005.
Pro-apoptotic activity of Prostanox®, CF and Finasteride
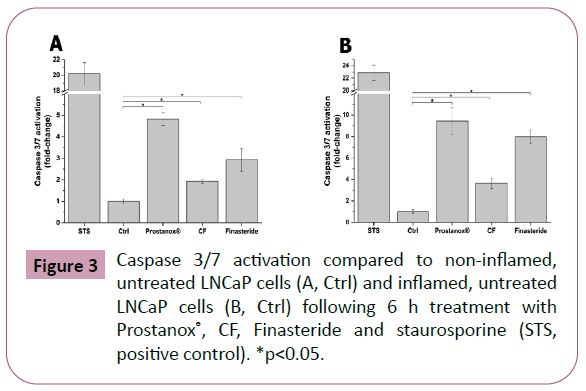
This prostate enlargement is mainly due to the uncontrolled proliferation of prostatic cells. As a consequence, a formulation able to contain such proliferation, via cell death mechanisms like apoptosis, may slow down the BPH onset and development. Considering the underlying inflammatory processes, it is also fundamental for such formulation to affect cell proliferation in an inflamed environment. To this aim, we investigated the potential pro-apoptotic activity of Prostanox®, CF and Finasteride in both non-inflamed and inflamed LNCaP cells. The activation of key enzymes in the apoptotic signaling cascade (caspase 3 and 7) was used as an indicator of apoptosis activation. In non-inflamed conditions, Prostanox® treatment showed a caspase 3/7 activity increase of 4.8 ± 0.3 fold-change times compared to control. CF and Finasteride also increased caspase 3/7 activity but to a lower extent (1.9 ± 0.1 and 2.9 ± 0.5 fold-change compared to control respectively) (Figure 3 and SM5). The same trend was maintained in inflamed condition (9.4 ± 1.2, 3.6 ± 0.5 and 7.9 ± 0.6 fold-change compared to inflamed, untreated LNCaP cells for Prostanox®, CF and Finasteride respectively) (SM5).
Impact of Prostanox®, CF and Finasteride treatment on in vitro prostatic model 5αR activity
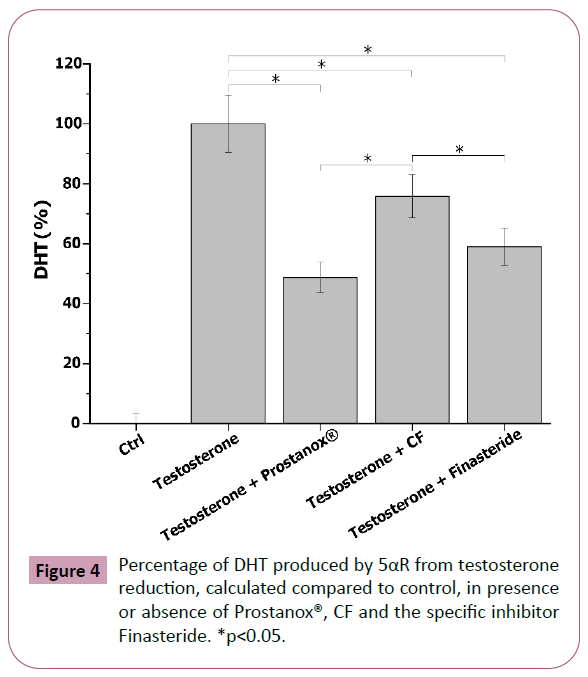
The 5αR is fundamental in the insurgence and development of some prostatic pathologies (i.e. BPH). Indeed, it stimulates PSA production through dihydrotestosterone, a hormone characterized by a higher androgenic activity compared to testosterone itself [43]. As such, 5αR activity inhibition may be indicative of a positive effect at prostatic level. To evaluate the impact on 5αR activity of tested formulations and Finasteride, a specific 5αR inhibitor, LNCaP cell lysates were incubated with Prostanox®, CF and Finasteride for 16 h, in presence of with 5αR cofactor (NADPH) and substrate (testosterone). DHT, the product of 5αR-catalyzed reaction, was measured at the end of the incubation period. Cell lysates incubated in absence of testosterone were considered as controls. As shown in Figure 4 and SM6, the addition of testosterone to 5αR, in presence of NADPH, stimulated the enzyme activity, leading to an increase in DHT synthesis compared to control. Prostanox® showed to be more effective in reducing DHT production than CF, with CF being the less effective, while Finasteride showed a 5αR inhibitory efficacy comparable to Prostanox® (51.0, 24.2 and 41.3% reduction for Prostanox®, CF and Finasteride respectively).
Figure 4: Percentage of DHT produced by 5αR from testosterone reduction, calculated compared to control, in presence or absence of Prostanox®, CF and the specific inhibitor Finasteride. *p<0.05.
Effect of Prostanox®, CF and Finasteride on the release of PSA
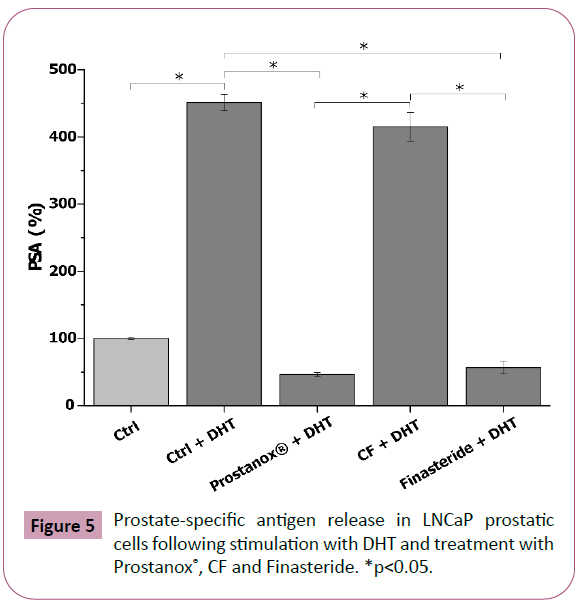
PSA is a protein released from the prostatic epithelium and its increase in the bloodstream is associated with the development of prostatic pathologies, like the BPH. As such, PSA is a useful marker to assess the potentially positive effect of a formulation at the prostatic level. Following stimulation with androgenic hormone DHT, a significative increase in PSA release (361.2 ± 11.9% increase compared to untreated control) was observed in LNCaP cells (Figure 5 and SM7). Both Prostanox® (750 μg/mL) and Finasteride (93 μg/mL) significantly reduced PSA release, with Prostanox® being the most effective (46.1 ± 3.3% and 56.4 ± 9.5% PSA release respectively, compared to 461.2 ± 11.9% PSA release of the DHT-stimulated control) (Figure 5 and SM7). Conversely, no decrease in PSA release from DHT-stimulated LNCaP cells was observed for CF, indicating that both Prostanox® and Finasteride are more effective than CF. Effect of Prostanox®, CF and Finasteride on non DHT-stimulated LNCaP cells PSA release is reported in SM8 and SM9.
Figure 5: Prostate-specific antigen release in LNCaP prostatic cells following stimulation with DHT and treatment with Prostanox®, CF and Finasteride. *p<0.05.
Smooth muscle myorelaxing activity of Prostanox®, CF and Finasteride
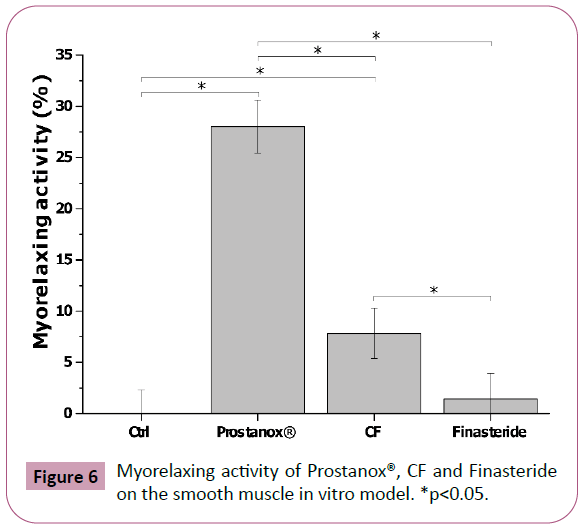
Some of LUTS, like voiding or obstructive symptoms, are due to the progressive increase and contraction of the stromatic portion of the prostate, composed mainly of smooth muscle. For this reason, a therapy based on α-blocker drugs (i.e. doxazosin, terazosin, etc.) are effective in LUTS mitigation [44]. To explore their potential myorelaxing ability, WPMY-1 cells (smooth muscle cell line) were treated with Prostanox®, CF and Finasteride. As for the LNCaP cells, a toxicological, dose-response analysis was performed (SM10 and SM11). To test their effect on smooth muscle contraction, WMPY-1 cells were exposed to 750 μg/mL of Prostanox®, 100 μg/mL of CF and 93.1 μg/mL of Finasteride for 24 h. No effect was observed for Finasteride (Table 6), while Prostanox® and CF significantly increased smooth muscle relaxation, with Prostanox® being endowed with the highest myorelaxing activity (28.0 ± 2.6% and 7.8 ± 2.5% of myorelaxation) (Figure 6 and SM12). Contraction data are reported in SM12.
Discussion
BPH represents a significant burden in aging men frequently associated with LUTS, which may impair quality of life [1,5]. BPH etiology is complex, underlying different mechanisms, such as steroid-mediated cell proliferation and inflammation [10,17-19]. However, Serenoa repens, the most widely used phytotherapic agent for the treatment of BPH, is able to mitigate only parts of BPH-related symptoms [24-26]. To enhance and complement Serenoa repens therapeutic activity, a new multi active ingredientbased formulation, Prostanox®, have been proposed. Our in vitro approach, demonstrated, that the presence of multiple active improved the overall efficacy of Prostanox® by enhancing its anti-inflammatory, anti-androgenic, pro-apoptotic and smooth muscle relaxing activity. Indeed, our results clearly show that the association of multiple active principle decreases more efficiently the release of pro-inflammatory cytokine IL-1β and TNF-α, compared to Serenoa repens only based formulations. Such behavior is probably due to the synergic effect of the phytotherapic agents contained in the formulation, such as Boswellia carteri [40], Curcuma longa [30,41] and Berberis vulgaris [42]. Similarly, the higher pro-apoptotic activity of Prostanox® on inflamed or non-inflamed LNCaP cells, could be explained by the presence of curcumin among formulation’s active principles. Indeed, curcumin and its active principles (i.e. curcuminoids), are wellknown to induce apoptosis in tumoral cells [43]. Furthermore, compared to the Serenoa repens-based commercial formulation, Prostanox® is more effective in reducing some BPH-connected symptoms, such as PSA release and smooth muscle contraction, while retaining a better safety towards the prostate.
Conclusion
Prostanox® formulation, thanks to its unique blend of active principles, might be useful in BPH and LUTs treatment, in particular compared to Serenoa repens only-based formulations.
Acknowledgement
Uriach Italy donated the substances. Uriach Italy had no role in study design, data collection and analysis, or manuscript.
References
- Untergasser G , Madersbacher S, Berger P (2005) Benign prostatic hyperplasia: Age-related tissue-remodeling. Exp Gerontol 40: 121–128.
- Fitzpatrick JM (2006) The natural history of benign prostatic hyperplasia. BJU Int 97: 3–6.
- Roehrborn CG, Black LK (2011) The economic burden of prostate cancer. BJU Int 108: 806–813.
- Siegel RL, Miller K D, Jemal A (2019) Cancer statistics. Cancer J Clin. 69:7 –34.
- Ficarra V , Rossanese M, Zazzara M, Giannarini G, Abbinante , et al . (2014) The Role of Inflammation in lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia (BPH) and its potential impact on medical therapy. Curr Urol Rep 15: 463.
- Lee KL, Peehl DM (2004) Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol Balt. 172: 1784–1791.
- Aaron L, Franco O, Hayward SW (2016) Review of prostate anatomy and embryology and the etiology of BPH. Urol Clin North Am 43: 279–288.
- Minciullo PL, Inferrera A, Navarra M, Calapai G, Magno, et al. Oxidative stress in benign prostatic hyperplasia: A systematic review. Urol Int 94: 249–254.
- Vickman RE, Franco OE, Moline DC, Vander Griend DJ, et al. (2020) The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A Review. Asian J Urol 1;7:191-202.
- Lucia MS, Lambert JR (2008) Growth factors in benign prostatic hyperplasia: Basic science implications. Curr Urol Rep 9:272-278.
- Patel ND, Parsons JK (2014) Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol 30:170-176.
- Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, et al. (2014) The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int 112:432-441.
- Ørsted DD, Bojesen SE. (2013) The link between benign prostatic hyperplasia and prostate cancer. Nat Rev Urol 10:49-54.
- Wang Y, Kunit T, Ciotkowska A, Rutz B, Schreiber A, et al. (2015) Inhibition of prostate smooth muscle contraction and prostate stromal cell growth by the inhibitors of R ac, NSC 23766 and EHT 1864. Br J Pharmacol172:2905-2917.
- Hennenberg M, Miljak M, Herrmann D, Strittmatter F, Walther S, et al. (2013) The receptor antagonist picot amide inhibits adrenergic and thromboxane induced contraction of hyperplastic human prostate smooth muscle. Am J Physiol 305: 1383–1391.
- Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, et al. (2008) The relationship between prostate inflammation and lower urinary tract symptoms: Examination of baseline data from the REDUCE trial. Eur Urol 54:1379-1384.
- Torkko KC, Wilson RS, Smith EE, Kusek JW, Van Bokhoven A, et al. (2014) Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: Findings from the MTOPS study. J Urol 194:454-461.
- Taoka R, Kakehi Y (2017) The influence of asymptomatic inflammatory prostatitis on the onset and progression of lower urinary tract symptoms in men with histologic benign prostatic hyperplasia. Asian J Urol 4:158-163.
- Madersbacher S, Sampson N, Culig Z (2019) Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: A mini-review. Gerontol 65:458-464.
- Neill MG, Appu S, Zlotta AR (2009) Strategies to preserve prostate health. Drugs of Today (Barcelona, Spain: 1998). 45:63-80.
- Comhaire F, Mahmoud A (2004) Preventing diseases of the prostate in the elderly using hormones and nutriceuticals. The Aging Male 7:155-169.
- Keehn A, Lowe FC (2015) Complementary and alternative medications for benign prostatic hyperplasia. Can J Urol 22:18-23.
- Keehn A, Taylor J, Lowe FC. (2016) Phytotherapy for benign prostatic hyperplasia. Cur Urol Rep 17:53.
- Suzuki M, Ito Y, Fujino T, Abe M, Umegaki K, et al . (2009) Pharmacological effects of saw palmetto extract in the lower urinary tract. Acta Pharmacol Sin 30:271-281.
- Ooi SL, Pak SC (2017) Serenoa repens for lower urinary tract symptoms/benign prostatic hyperplasia: Current evidence and its clinical implications in naturopathic medicine. J Altern Complement Med 23:599-606.
- Latil A, Pétrissans MT, Rouquet J, Robert G, De La Taille A (2015) Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. The Prostate 75:1857-1867.
- Russo A, Capogrosso P, La Croce G, Ventimiglia E, Boeri L, et l .(2016) Serenoa repens, selenium and lycopene to manage lower urinary tract symptoms suggestive for benign prostatic hyperplasia. Expert opinion on drug safety. 15:1661-1670.
- Minutoli L, Bitto A, Squadrito F, Marini H, Irrera N, et al. (2013) Serenoa repens, lycopene and selenium: A triple therapeutic approach to manage benign prostatic hyperplasia. Curr Med Chem 20:1306-1312.
- Imenshahidi M, Hosseinzadeh H (2016) Berberis vulgaris and berberine: An update review. Phytother Res 30:1745-1764.
- Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, et al. (2017) Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br J Pharmacol 174:1325-1348.
- Iram F, Khan SA, Husain A (2017) Phytochemistry and potential therapeutic actions of Boswellic acids: A mini-review. Asian Pac J Trop Biomed 7:513-523.
- Takooree H, Aumeeruddy MZ, Rengasamy KR, Venugopala KN, Jeewon R, et al. (2019) A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit Rev Food Sci Nutr 59:S210-243.
- Semalty M, Adhikari L, Semwal D, Chauhan A, Mishra A.et al. (2017) a comprehensive review on phytochemistry and pharmacological effects of stinging nettle (Urtica dioica). Curr Tradit Med.3:156-167.
- Jurikova T, Skrovankova S, Mlcek J, Balla S, Snopek L (2019) Bioactive compounds, antioxidant activity, and biological effects of European cranberry (Vaccinium oxycoccos). Molecules 24:24.
- Wong CP, Bray TM, Ho E (2009) Induction of pro inflammatory response in prostate cancer epithelial cells by activated macrophages. Cancer Lett 276:38-46.
- Assinder SJ (2008) Oxytocin increases 5α‐reductase activity of human prostate epithelial cells, but not stromal cells. The Prostate 68:115-1121.
- Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, et al. (2002) Which increase PSA secretion and modify actin cytoskeleton? The FASEB J 16:1429-1431.
- Catanzaro D, Rancan S, Orso G, Dall’Acqua S, Brun P, et al. (2015) Boswellia serrata preserves intestinal epithelial barrier from oxidative and inflammatory damage. PloS One. 10:e0125375.
- Cosentino V, Fratter A, Cosentino M (2016) Anti-inflammatory effects exerted by Killox®, an innovative formulation of food supplement with curcumin, in urology. Eur Rev Med Pharmacol Sci 20: 1390–1398.
- Kumar R, Gupta YK, Singh S (2016) Anti-inflammatory and anti-granuloma activity of Berberis aristata DC. in experimental models of inflammation. Indian J Pharmacol 48:155-161.
- Holy J (2004) Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell Motil Cytoskelet 58:253-268.
- Vacherot F, Azzouz M, Gil‐Diez‐de‐Medina S, Colombel M, De La Taille A,et al. (2000) Induction of apoptosis and inhibition of cell proliferation by the lipido‐sterolic extract of Serenoa repens (LSESr, Permixon®) in benign prostatic hyperplasia. The Prostate. 45:259-266.
- Kim EH, Brockman JA, Andriole GL (2018) The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia. Asian J Urol 5:28-32.
- Chua T, Eise NT, Simpson JS, Ventura S (2004) Pharmacological characterization and chemical fractionation of a liposterolic extract of saw palmetto (Serenoa repens): Effects on rat prostate contractility. J Ethnopharmacol 152:283-291.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences