Preventive Treatment with Diclofenac Suppositories May Improve BCG Induced Cystitis Symptoms without Affecting Its Oncologic Efficacy: A Prospective Double Blind Randomized Trial
Gal Rinott Mizrahi*, Avi Stein, Boris Friedman, Yuval Freifeld, Yael Hod and Yoram Dekel
Carmel Hospital, Haifa, Israel
- *Corresponding Author:
- Gal Rinott Mizrahi
Carmel Hospital
Haifa, Israel
Tel: +972-77-4450-480
E-mail: galrinott@gmail.com
Received Date: September 18, 2017; Accepted Date: September 22, 2017; Published Date: September 29, 2017
Citation: Mizrahi GR, Stein A, Friedman B, Freifeld Y, Hod Y, et al. (2017) Preventive Treatment with Diclofenac Suppositories May Improve BCG Induced Cystitis Symptoms without Affecting Its Oncologic Efficacy: A Prospective Double Blind Randomized Trial. J Clin Med Ther. 2:25.
Abstract
Purpose: To evaluate whether prophylactic treatment with Diclofenac suppositories may relief bladder irritative symptoms following BCG irrigation, and to assess its effect on the oncologic outcome.
Methods: A prospective, randomized, double-blind, placebo-control trial on 36 patients diagnosed with Non- Muscle Invasive Bladder Cancer (NMIBC) treated with induction installations of BCG. Patients received either a transrectal Diclofenac suppository (50 mg) or a placebo suppository immediately prior to and 24 hours after bladder irrigation.
Following each irrigation patients completed a Quality of Life and a specific side effect questionnaire. Patients were thereafter followed prospectively for tumor recurrence.
Results: For each irrigation, patients in the treatment group had statistically significant lower complains of dysuria (presented as the percent change from baseline; 473% vs. 161% in the control and treatment groups respectively, p=0.026). Other BCG side effects affected by the suppositories are reported. During a mean follow up of 22.8 month, 56% and 58% of patients in the treatment and control groups respectively had tumor recurrence (p=0.96). No significant side effects have been observed related to the study drug.
Conclusion: Diclofenac suppositories are easy and safe to use and according to our results on this small study cohort, their use may suggest a beneficial effect in preventing complains caused by BCG induced cystitis without increasing tumor recurrence rate.
Keywords
Urinary bladder neoplasms; Immunotherapy; Diclofenac; Cystitis; Recurrence
Introduction
Urothelial Carcinoma of the bladder (UCB) is the most common malignancy involving the urinary system, and the 9th most common malignancy worldwide [1]. Approximately 75% of patients have Non Muscle Invasive Bladder Cancer (NMIBC) at the time of diagnosis with a natural history of high recurrence and progression rates (50-80% and up to 30% respectively) [2]. Patients with NMIBC are stratified into three risk groups according to their risk of both recurrence and progression in order to determine the follow up and treatment approach [3-5]. Current evidence supports the use of adjuvant intra-vesical chemotherapy and/or immunotherapy for NMIBC patients in the intermediate and high risk groups [6]. Intravesical Bacillus Calmette-Guérin (BCG) is one of the most effective and commonly used adjuvant immune-therapy. BCG is a live, attenuated mycobacterium in the same family as tuberculosis that is capable of inducing a local inflammatory response upon instillation which is responsible for its antitumor effect [7]. Available evidence suggests that urothelial cells including tumor cells and cells of the immune system have crucial roles in the therapeutic anti-tumor effect of BCG. The possible mechanism includes attachment and internalization of BCG by urothelial cells, secretion of cytokines and chemokines, and presentation of BCG and cancer cell antigens to cells of the immune system. Immune system cell subsets that have potential roles in BCG therapy include CD4+ and CD8+ lymphocytes, natural killer cells, granulocytes, macrophages, and dendritic cells. It is shown that tumor cells are killed directly by cytotoxicity of the immune cells, indirectly by secretion of soluble factors such as TRAIL (tumor necrosis factor-related apoptosis-inducing ligand), and to some degree, by the direct action of BCG [7]. The treatment protocol with intra-vesical BCG is composed of an induction instillation which is classically given according to the empirical 6-weekly schedule, followed by a maintenance schedule for a minimum of 1 year. The superiority of Trans Urethral Resection of Bladder Tumor (TURBT) combined with intra-vesical BCG treatment over TURBT alone or TURBT combined with intravesical chemotherapy, in preventing tumor recurrence, has been confirmed by several meta-analyses and RCTs [5]. However, intra-vesical BCG treatment is associated with more side effects and is therefore less tolerated by patients [8]. Complications can be classified as local, as a result of direct effects on the bladder, or systemic, as a result of systemic absorption of the agent. Local side effects include BCGinduced cystitis, bacterial infection, frequency and macroscopic hematuria. Systemic side effects include malaise, fever and arthritis. According to different large scale studies side effects appear in 50-95% of cases [9]. According to the EORTC trial 30911, 19% of the patients stopped treatment due to toxicity [10]. More recently, in the largest published EORTC study which included more than 1,300 patients, the number of patients stopping treatment was 7.8% [11] which is obviously lower but still significant and bothersome. Various medications have been suggested to prevent BCG toxicity, including lowering the dose, Nonsteroidal Anti-Inflammatory Drugs (NSAIDS), antibiotics, spasmolytics and anti-cholinergic drugs. However, to date none of these methods has been validated [9]. Diclofenac is a proven, commonly prescribed NSAID that has analgesic, anti-inflammatory, and antipyretic properties. As with all NSAIDs, diclofenac exerts its action via inhibition of prostaglandin synthesis by inhibiting cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2), but has been proven to have also other multimodal and novel mechanisms of action including inhibition of substrate P, alteration of interleukin-6 production and inhibition of thromboxane-prostanoid receptor [12]. The rout of analgesia administration might also influence its effect. Specifically, rectal analgesia has the advantages of bypassing the possible systemic discomfort caused by oral administration (i.e. gastric discomfort, nausea and vomiting) and has lower proportion of hepatic first pass metabolism since approximately half of the drug absorbed rectally bypasses the liver. This results in faster pain relief and longer action of treatment. Diclofenac suppositories are easily administered and safe in the majority of patients. To our knowledge no placebo controlled studies have assessed the role of rectal administration of Diclofenac as prophylaxis for BCG side effects. Therefore we conducted a prospective, randomized double-blind, placebo controlled trial to evaluate the advantage of such treatment in the prevention of side effects following treatment with intra-vesical BCG.
Materials and Methods
Patients and randomization
The present study was performed at the Department of Urology, Lady Davis Carmel Medical Center, Haifa, Israel during the period between 1/2012 and 12/2013, and has been approved by the Institutional Ethic Committee. Patients with NMIBC, candidates for a set of 6 BCG bladder irrigations, who met the inclusion criteria, were invited and informed about the present study. Inclusion criteria were as follows: males and females, aged 20-90, capable of signing informed consent. Exclusion criteria were: diclofenac hypersensitivity, pregnancy or lactation, elevated creatinine at baseline (male >1.2 mg/dL and female >1.0 mg/dL), peptic ulcer, rectal/anal inflammation or gross hematuria.
Endpoints
The primary endpoint was to compare the percent of patients suffering from each symptom.
Secondary end point was to compare the recurrence rate of tumors.
Randomization
Patients enrolled to the study were randomly allocated to receive either diclofenac or placebo rectal suppositories. Randomization was done in the hospital pharmacy by code labeling and random code number generation by computer software. Suppositories were identically packaged and numbered while both patient and operator were blinded to the exact nature of the suppository.
Drug administration and bladder irrigation protocol
Patients randomized to the treatment group received one Diclofenac suppository (50 mg) immediately prior to bladder irrigation. Patients randomized to the placebo group received an identical looking placebo suppository. As for BCG bladder irrigations they were handled as largely elsewhere described. In brief, patients were placed in supine position and BCG suspension at a concentration of 50 × 108 CFU in 50 mL sterile saline was administrated using urethral catheterization as accepted. Patients were instructed to withhold urination for an hour following administration of intra-vesical BCG suspension. 24 h following irrigation patients were instructed to administer another single identical rectal suppository according to their study group.
Outcomes measures
Patients data was recorded including- gender, age, time from surgery to BCG irrigation, comorbidities, administered medications, drug allergies and baseline serum creatinine levels. Patients were asked to fill out for each irrigation a Quality of Life (QOL) questionnaire (COOP1,2,3) and a side effect form both related to the last passing week. Both questioners have been validated for patients during Intravesical BCG therapy [13] and validated for the Hebrew language [14]. Following termination of the study patients were followed prospectively for tumor recurrence.
Statistics
We were planning a study of independent cases and controls. Prior data indicate that the probability of a side effect like dysuria among controls after BCG bladder irrigation is 0.35. To demonstrate a decrease of 30% in patients suffering from a side effect, we need to study 30 treatment patients and 30 control patients to be able to reject the null hypothesis that the side effect rates for treatment and controls are equal with power=0.8. The Type I error probability associated with this test of this null hypothesis is .05. For 19 cases in the treatment group and 17 in the control group the power calculated was 0.71. In case of Continuous variable, this sample size allows us to detect different of 1 SD between the two study groups. Demographic and clinical variables where compared using the following statistical tests; ANOVA for age and history of bladder irrigations, Chi-square for sex, presence of comorbidities and risk group, and T test for baseline creatinine levels. The Mann–Whitney U test for small samples was used to compare the presence of side effects and the QOL between the two study groups. A Kaplan-Mayer analysis was used to compare tumor recurrence during the period of follow up. All p-values were two-sided, and statistical significance is defined as p<0.05.
Results
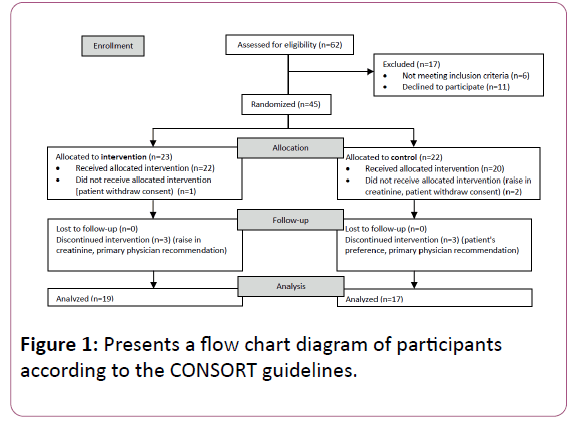
Of the 62 patients assessed for eligibility, 17 patients were excluded by exclusion criteria (n=6) or personal preference to decline participation (n=11). Therefore 45 patients have been randomized: 23 patients were enrolled to the intervention arm and 22 to the control arm. In the intervention arm, 1 patient did not start treatment due to consent withdrawal for personal reasons. In 3 patients, treatment was discontinued due to a slight rise in serum creatinine (n=2) or due to primary physician recommendation (n=1). In the control arm, 2 patients did not start treatment due to consent withdrawal (n=1) or rise in serum creatinine (n=1) and 3 patients who started treatment discontinued intervention due to consent withdrawal (n=2) or primary physician recommendation (n=1). Finally, analysis was done for 19 patients in the treatment group and 17 patients in the control group (Figure 1).
There was no statistically significant difference between the two groups in baseline variables including mean age, sex, baseline creatinine, comorbidities, history of bladder irrigations, mean time from surgery (TURBT) to the first bladder irrigation and risk group (Table 1).
| Demographic parameter | Treatment group (n=19) | Control group (n=17) | Significance (P value) |
|---|---|---|---|
| Mean age (years) | 62.36 | 62.5 | 0.972† |
| Male sex (%) | 73.7 | 58.25 | 0.34†† |
| Baseline creatinine (mg/dL) | 0.89 | 0.79 | 0.163††† |
| Diagnosis of diabetes (%) | 31.6 | 41.2 | 0.358†† |
| History of bladder irrigations (%) | 15.8 | 11.8 | 1† |
| Mean time from surgery to first irrigation (days) | 64.44 | 66.53 | 0.913††† |
| High risk (%) | 43.8 | 64.3 | 0.261†† |
| Intermediate risk (%) | 56.3 | 35.7 | |
| †ANOVA ††Chi square ††† T test |
|||
Table 1: Presents the demographic and clinical variables of patients in the treatment vs. control group.
Table 1: Presents the demographic and clinical variables of patients in the treatment vs. control group.
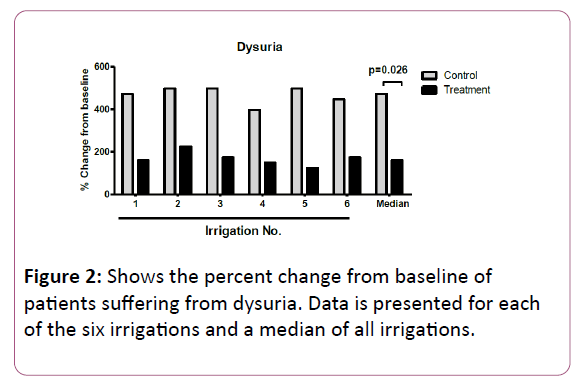
Figure 2 displays the percent change from baseline of patients suffering from dysuria. For each of the six irrigations, patients in the control group had a higher increase in the percent change from baseline compared with the treatment group, with a statistically significant difference in the median percent change between the two groups (473% vs. 161% in the control and treatment groups respectively, p=0.026).
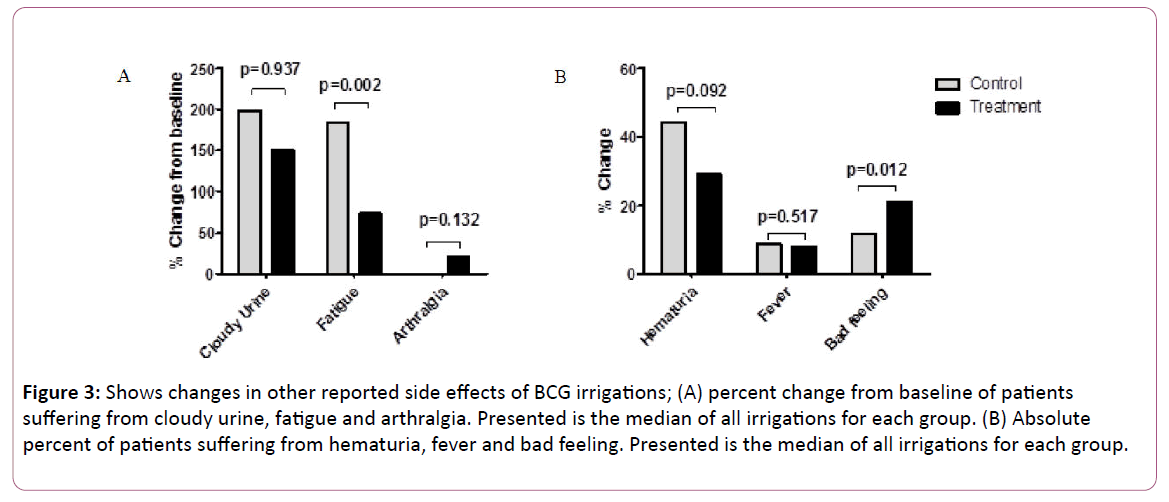
Figure 3A displays the median percent change from baseline of patients suffering from cloudy urine, fatigue and arthralgia. For cloudy urine and arthralgia there was no statistically significance difference between the two groups. For fatigue, patients in the control group had a statistically significant higher increase in the median percent change from baseline compared with the treatment group (184% vs. 74.5% in the control and treatment groups respectively, p=0.002).
Figure 3: Shows changes in other reported side effects of BCG irrigations; (A) percent change from baseline of patients suffering from cloudy urine, fatigue and arthralgia. Presented is the median of all irrigations for each group. (B) Absolute percent of patients suffering from hematuria, fever and bad feeling. Presented is the median of all irrigations for each group.
Figure 3B displays the median percent of patients in each group suffering from hematuria, fever and bad feeling. Regarding hematuria and fever there was no statistically significant difference between the two groups.
A higher percent of patients suffering from bad feeling, was observed in the treatment group vs. the control group with statistical significance (21.1% vs. 11.8% in the treatment and control groups respectively, p=0.012).
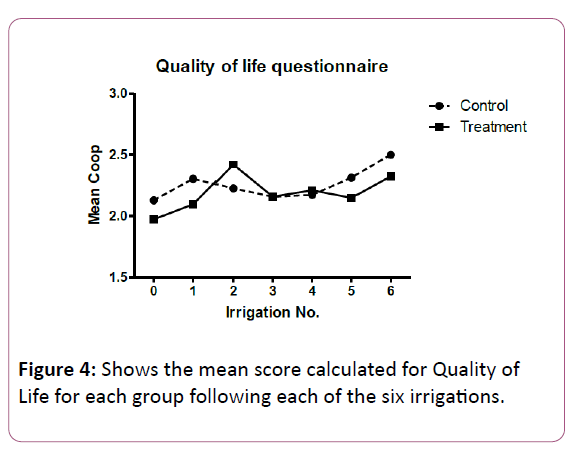
Figure 4 displays the mean score calculated for Quality of life for each group following each of the irrigations. For all irrigations, there was no statistically significant difference between groups.
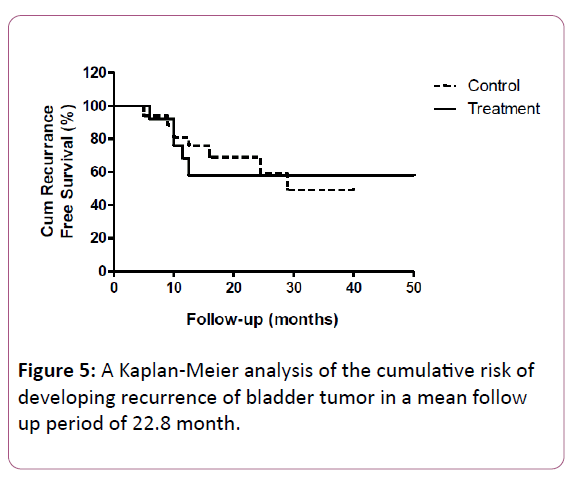
Figure 5 displays a Kaplan-Meier analysis of the cumulative risk of developing recurrence of bladder tumor in a mean follow up period of 22.8 month. During this follow up period, 56% of patients in the treatment group and 58% of patients in the control group had tumor recurrence. Overall there was no statistically significance difference in the recurrence rate between groups (p=0.96).
Discussion
Bladder irrigations with BCG are considered the most effective prophylactic treatment for patients with high-risk NMIBC. In intermediate risk disease, it was also found to be more effective than intra-vesical chemotherapy but it's use should be outweighed against potential severe toxicity [15]. The frequent side effects are a major reason to avoid the use of BCG or for poor compliance of patients to this treatment. In the EORTC study which included more than 1300 patients treated with BCG, 62.8% reported local side effects and 30.6% had some form of systemic side effects [11]. This is in line with previous reports on this subject which also illustrate high frequency of side effects [16]. The most frequent side effect which has been reported by patients in the EORTC study was BCG-induced cystitis (35%) [11]. Depending on the definition used, the incidence of cystitis reported by other previous studies is highly variable (5% to 90%) [17]. This common bothersome side effect is a consequence of immune stimulation, lymphokine release and associated inflammatory response. Symptoms include dysuria and frequency which may be accompanied by flu-like symptoms, such as low grade fever and malaise that commonly last for 24 to 48 hours. In accordance with the literature, in our current study dysuria was reported by almost 40% of patients. Recommendations for prevention and treatment of intra-vesical BCG adverse events were published by the International Bladder Cancer Group, in October 2008 [18].
This was based on a literature review and to a large extent on expert opinion because literature did not reach a level of evidence greater than level 3. A more recent review with treatment recommendations was published by Decaestecker et al. [9]. Taken together recommendations regarding the prevention and treatment of BCG induced cystitis state that treatment is advised when symptoms persist for longer than 1-2 days following BCG installation or when symptoms become intolerable for the patient.
Reported treatments include spasmolytics, anticholinergics, and analgesics. None of these drugs however, have been tested in this condition versus placebo, except for oxybutynin, which failed to be effective. In the current study we aimed on evaluating the advantage of prophylactic treatment with Diclofenac suppository in relieving cystitis symptoms following BCG irrigation. We elected the transrectal route for administration partially based on the experience acquired by the use of Diclofenac suppositories in preventing pain following Transrectal Ultrasound (TRUS) guided biopsies [19] and episiotomies [17] in which they have been found to be beneficial. Our hypothesis was that given the antiinflammatory effects, the treatment will lower the incidence of symptomatic BCG induced cystitis and thus will lower patient's experience of adverse effects following installation and hopefully improve compliance. Our modest study showed that preventive treatment with Diclofenac suppositories has an advantage over the control group in lowering the incidence of dysuria and fatigue. Although a small number of participants were included in this study, these differences reached a statistical significance.
The presumed mechanism by which treatment with Diclofenac reduces dysuria is by down regulating the inflammatory response that underlies BCG induced cystitis. Not surprisingly other symptoms that were not expected to improve with treatment since they are not physiologically related to Diclofenac's anti-inflammatory activity, such as hematuria and cloudy urine, were not different between the treatment and placebo groups. We could not find a logical explanation for the difference found in fatigue between the groups.
Our concern whether the anti-inflammatory effect of Diclofenac might reduce BCG treatment efficacy and result in higher recurrence rate, was denounced by showing non-significant difference in recurrence rate between groups in a mean follow up period of 22.8 month. Limitations of the current study include; a relatively small number of participants (n=36) which limits the strength of the results. Furthermore, no reliable information about patient's compliance was collected and the only measures for outcome assessment have been subjective.
Conclusion
Diclofenac suppositories are easy and safe to use and according to our results on this small study cohort, have a beneficial effect in preventing complains caused by BCG induced cystitis without increasing tumor recurrence rate. Larger studies are needed to strengthen this observation and decide on clinical recommendations.
References
- Ploeg M, Aben KK, Kiemeney LA (2009) The present and future burden of urinary bladder cancer in the world. World J Urol 27: 289-293.
- Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A (2014) International variations in bladder cancer incidence and mortality. Eur Urol 66: 59-73.
- Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, et al. (2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49: 466-465; discussion 475-477.
- Brausi M, Witjes JA, Lamm D, Persad R, Palou J, et al. (2011) A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol 186: 2158-2167.
- Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, et al. (2013) EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol 64: 639-653.
- Resnick MJ, Bassett JC, Clark PE (2013) Management of superficial and muscle-invasive urothelial cancers of the bladder. Curr Opin Oncol 25: 281-288.
- Redelman-Sidi G, Glickman MS, Bochner BH (2014) The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nat Rev Urol 11: 153-162.
- Shang PF, Kwong J, Wang ZP, Tian J, Jiang L, et al. (2011) Intravesical Bacillus Calmette-Guerin versus epirubicin for Ta and T1 bladder cancer. Cochrane Database Syst Rev.
- Decaestecker K, Oosterlinck W (2015) Managing the adverse events of intravesical bacillus Calmette-Guerin therapy. Res Rep Urol 7: 157-163.
- van der Meijden AP, Sylvester RJ, Oosterlinck W, Hoeltl W, Bono AV, et al. (2003) Maintenance Bacillus Calmette-Guerin for Ta T1 bladder tumors is not associated with increased toxicity: results from a European Organisation for Research and Treatment of Cancer Genito-Urinary Group Phase III Trial. Eur Urol 44: 429-434.
- Brausi M, Oddens J, Sylvester R, Bono A, van de Beek C, et al. (2014) Side effects of Bacillus Calmette-Guerin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 65: 69-76.
- Gan TJ (2010) Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin 26: 1715-1731.
- Bohle A, Balck F, von Weitersheim J, Jocham D (1996) The quality of life during intravesical bacillus Calmette-Guerin therapy. J Urol 155: 1221-1226.
- Ben-Eli D, Hermoni D (1994) Evaluation of functional status in primary care--use of WONCA/COOP charts. Harefuah 127: 327-333.
- Sylvester RJ, van der MA, Lamm DL (2002) Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol 168(5):1964-1970.
- Lamm DL, van der Meijden PM, Morales A, Brosman SA, Catalona WJ, et al. (1992) Incidence and treatment of complications of bacillus Calmette-Guerin intravesical therapy in superficial bladder cancer. J Urol 147: 596-600.
- Koya MP, Simon MA, Soloway MS (2006) Complications of intravesical therapy for urothelial cancer of the bladder. J Urol 175: 2004-2010.
- Witjes JA (2008) Clinical Practice Recommendations for the Prevention and Management of Intravesical Therapy–Associated Adverse Events. Eur Urol.
- Haroon N, Ather MH, Khan S, Kumar P, Salam B (2015) Diclofenac Suppository as a Preemptive Analgesia in Ultrasound-guided Biopsy of Prostate: Randomized Controlled Trial. Urology 86: 682-685.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences