ISSN : 0976-8505
Der Chemica Sinica
Preparation of Schiff base of 1, 2, 4-Triazole-4-amine with 3-Nitrobenzaldehyde, Its Complexation with Cu (II) and Zn (II) and Antimicrobial Activity of Complexes
Bharati KT1*, Gujarathi DB1, Tryambake PT1, Hase GJ1, Gaikwad RK1 and Khatal MB2
1S N Arts, D J M Commerce and B N S Science College, Sangamner, India
Abstract
This study includes the synthesis of new derivatives of 1, 2, 4-triazole-4-amine derived from 3-nitrobenzaldehyde as a Schiff base. Co-ordination compounds of this Schiff base were synthesized and characterized by UV-Visible Spectroscopy, Infra-Red Spectroscopy, NMR, elemental analysis and magnetic measurement. These co-ordination compounds show biological activity against bacterial strains.
Keywords
1, 2, 4- triazole-4-amine, Schiff base, Co-ordination compounds
Introduction
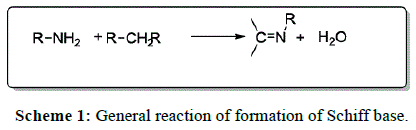
Schiff bases are the condensation products (following Scheme 1) [1] of primary amines and carbonyl compounds, named after Hugo Schiff, who discovered them in 1864 [2]. Schiff bases of aliphatic aldehydes are relatively unstable and are readily polymerized while those of aromatic aldehydes, having an effective conjugation system are more stable [3]. 1,2,4-triazole has been incorporated into a wide variety of therapeutically active drug [4,5] candidates including anti-inflammatory [6,7], antiviral, antimicrobial [8-11], analgesic [12], antitubercular [13-17] and anticancer properties [18-20]. People have synthesized some new 1, 2,4-triazole [21-24] and 1,3,4-triazole [25] derivatives with their antimicrobial activity [26,27].
Schiff base ligands are potentially capable of forming stable complexes with metal ion [28-30]. Schiff base metal complexes have been widely studied because they have industrial, antifungal, antibacterial, anticancer, antiviral and herbicidal applications [31-33]. The biological properties of 1,2,4-triazole derivatives and their complexes impelled us to synthesize new Schiff base of 1,2,4-triazole-4-amine and its metal complexes. In the present investigation, we report synthesis, characterization of Schiff base derived from 1,2,4-triazole-4-amine and 3-nitrobenzaldehyde and its complexes with Cu (II) and Zn (II) and their antimicrobial activity.
Materials and Methods
Preparation of ligand
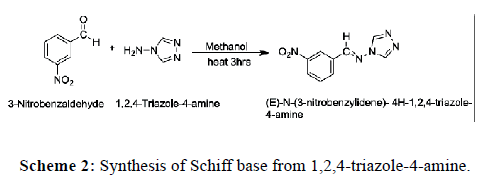
3-Nitrobenzaldehyde (2.172 mmol) and 1,2,4-triazole-4-amine (2.38 mmol) were mixed in 5 ml methanol and the mixture was refluxed for 3 hrs on water bath. The white color product was obtained after the addition of 10 ml of ice water. The Schiff base was filtered and washed with ice water and dried at room temperature. It shows practical yield of 80% and M.P. is 238˚C. Following reaction takes place (Scheme 2).
Characterization of ligand
The synthesized ligand was characterized by Spectrophotometer (Chemito UV-2100), Infra-Red Spectrophotometer (Bruker Optics, Model Alpha T), Bruker Advance II 400 NMR Spectrometer and elemental analysis by Perkin-Elmer 2400 CHN Analyzer. The physical parameters and result of elemental analysis were shown in Table 1.
| Sr. No. | Name and molecular formula of the ligand | Color | M.P. | % Yield |
Elemental analysis Calculated (Found) % |
|||
|---|---|---|---|---|---|---|---|---|
| C | H | N | O | |||||
| 1 | (E)-N-(3-nitrobenzylidene)-4H-1,2,4-triazole-4-amine (C9H7N5O2) |
White | 2380C | 80 | 49.76 (49.74 ) |
3.22 ( 3.20) |
32.25 (32.22) |
14.74 (14.70) |
Table 1: Characterization of ligand.
Synthesis of metal complexes
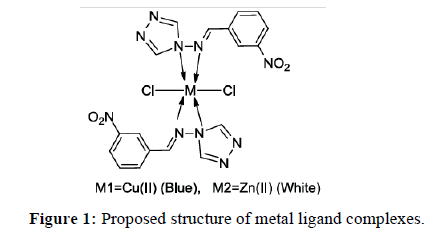
The solid complexes were prepared by mixing of aqueous solution of Copper chloride (0.264 mmol) and Zinc chloride (0.348 mmol) with 5 ml of 2% methanolic solution of ligand and refluxed for 2 hrs. Blue and white colored complexes were obtained for Copper (II) and Zinc (II) respectively (Figure 1).
Characterization of metal complexes
The metal complexes were characterized by UV Spectrophotometer (Chemito UV-2100), Infra-Red Spectrophotometer (Bruker Optics, Model Alpha T) and elemental analysis by Perkin-Elmer 2400 CHN Analyzer. The physical parameters and result of elemental analysis were shown in Table 2.
| Sr. No. | Name and molecular formula of the complex | Color | M.P. | % Yield | Elemental analysis Calculated (Found) % |
||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | M | |||||
| 1 | Bis-(E)-N-(3-nitrobenzylidene)-4H-1,2,4-triazole-4-amino Copper (II) chloride (C9H7N5O2)2.CuCl2 |
Blue | 3180C | 42.00 | 37.99 (37.96) | 2.46 (2.43) |
24.62 (24.60) | 11.2 (11.20) |
11.17 (11.15) |
| 2 | Bis-(E)-N-(3-nitrobenzylidene)-4H-1,2,4-triazole-4-amino Zinc (II) chloride (C9H7N5O2)2.ZnCl2 |
White | 2800C | 21.12 | 37.86 (37.84) |
2.45 (2.42) |
24.45 (24.40) |
11.22 (11.20) |
11.46 (11.40) |
Table 2: Characterization of metal complexes.
Antimicrobial activity
The antimicrobial activity of the metal complexes was evaluated with the help of ATCC cultures including gram positive (S. aureus) and gram negative (E. coli and P. aeruginosa) using Gentamicin as standard and antifungal activity was tested against Candida sp. using Nystatin as standard and adopting standard protocols [34]. Saturated solutions of complexes in DMSO were used for the antimicrobial studies.
Results and Discussion
The prepared complexes were found to be solids, insoluble in water but soluble in DMSO.
Electronic spectra
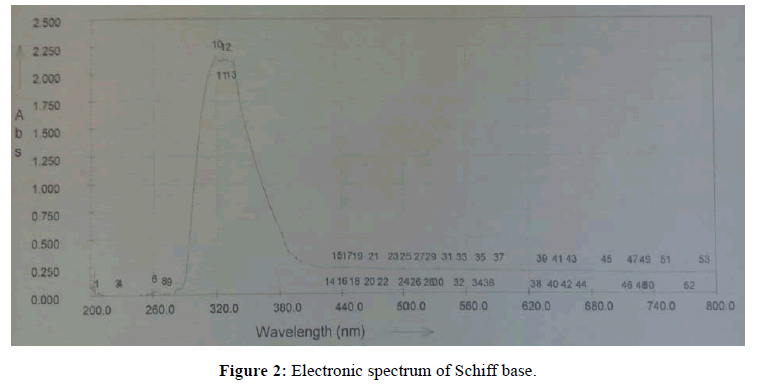
The characteristic peaks in electronic spectra of solutions of Schiff base in methanol and its complexes in DMSO are summarized in Table 3. The UV-Visible spectrum of Schiff base showed two bands at 319 and 327 nm (Figure 2). These two bands are observed due to π-π* transition. The UV-Visible spectrum of Cu (II) complex assigned to d-d transition. The spectrum of Cu (II) complex shows band at 332 nm which is attributed to the electronic transition of 2Eg →2T2g.
| Product | λmax (nm) | Abs | Wavenumber cm-1 | Transition | BM |
|---|---|---|---|---|---|
| L | 319, 327 | 2.158, 2.134 | 31347.96, 30581.03 | π- π*, | - |
| CuL2Cl2 | 332 | 1.666 | 3010.48 | π- π*, 2Eg →2T2g | 1.73 |
| ZnL2Cl2 | 316 | 1.355 | 31645.56 | π- π* | - |
Table 3: Electronic spectra of free ligand and its complexes.
The electronic spectrum of Zn (II) complex were diamagnetic as expected for d10 ions, so that no d-d transition can be expected in the Zn (II) complex. The magnetic susceptibility measurements provide data to characterize the structure of complex. The magnetic moment for Cu (II) complex was approximately 1.73 BM. Zn (II) complex were diamagnetic and were no magnetic moment [35].
FTIR spectra
The structure of prepared ligand and complexes were confirmed by Infrared Spectroscopy. The characteristic bands in the IR spectra of ligand and complexes were reported in Table 4.
| Product | Azomethine νC=N |
Triazole νN-N |
νM-N |
|---|---|---|---|
| L | 1653.88 | 1285.32 | - |
| CuL2Cl2 | 1645.45 | 1217.41 | 622.42 |
| ZnL2Cl2 | 1629.87 | 1211.07 | 620.86 |
Table 4: IR spectra of free ligand and its complexes.
Nuclear Magnetic Resonance
The 1H NMR spectra of ligand was recorded in DMSO. The 1H NMR spectrum show sharp signals at δ 9.31 for one proton which could be attributed to the CH=N groups, aromatic proton in the δ 7.80-8.38 and two triazole proton shows a sharp signal at δ 8.71-9.07.
Antimicrobial activity
The synthesized metal complexes exhibited a biological activity against one gram positive, two gram negative and one fungus Table 5.
| Compound | E. coli ATCC 25922 |
P. aeruginosa ATCC 27853 |
S. aureus ATCC 25923 |
Candida sp. |
|---|---|---|---|---|
| CuL2Cl2 | (-) | 08 mm | 08 mm | (-) |
| ZnL2Cl2 | (-) | (-) | 14 mm | (-) |
| Gentamicin(Standard) | 22 mm | 27 mm | 31 mm | (-) |
| Nystatin (Standard) | (-) | (-) | (-) | 22 mm |
Note: (-) indicates no activity.
Table 5: Effect of complexes on antimicrobial bacteria.
Conclusion
The complexes of Cu (II) and Zn (II) were synthesized by reaction of the synthesized ligand with the respective metal salts in 1:2 (M:L) ratio. The synthesized ligand and its complexes were characterized by UV-Visible, IR spectroscopic technique, NMR, magnetic susceptibility measurement, elemental analysis and their antimicrobial activity. The elemental and other spectral studies confirm the binding of Schiff base and metal ions and show the octahedral geometry of Cu (II) and Zn (II) complexes.
Acknowledgement
We are thankful to Principal Dr. KK Deshmukh for providing the facilities and motivation.
References
- Saxena A (2015) Metal complexes of Schiff bases: Preparation, characterization and synthesis of Schiff base derived from salicyladehyde and triazole and its transition metal complexes. Der Chem Sin 6: 29-33.
- Mahmud T (2010) Synthesis characterization and study of antibacterial activity of Enaminones complexes with Zinc and Iron. Arab J Chem 3: 219-224.
- Hasan A, Ameer AA, Ahmed A, Yousif E (2015) Synthesis and characterization of some transition metal (II) complexes with 1, 2, 4-triazole Schiff base. J Chem Pharm Res 7: 531-535.
- Paudyal R, Jamaluddin A, Warren JP, Doyle SM, Robert S, et al. (2014) Trafficking modulator TEN in1 inhibits endocytosis, causes endomembrane protein accumulation at the pre-vacuolar compartment and impairs gravitropic response in Arabidopsis thaliana. Biochem J 460: 177-185.
- Reddy LV, Nakka M, Suman A, Pal S (2011) Synthesis of novel quinoline analogues of nimesulide: An unusual observation. J Heterocyclic Chem 48: 555-562.
- Unangst PC, Shurum GP, Connor DT, Dyer RD, Schrier DJ (1992) Novel 1, 2, 4-oxadiazoles, and 1, 2, 4-thiadiazoles as dual 5-lipoxygenase and cyclooxygenase inhibitors. J Med Chem 35: 3691-3698.
- Mullikan MD, Wilson MW, Connor DT, Dyer RD, Schrier DJ (1993) Design of 5-(3,5-di-tert-butyl-4-hydroxyphenyl)-1, 3,4-thiadiazoles,-1, 3, 4-oxidiazoles and -1, 2, 4-triazoles as orally active, nonulcerogenic anti-inflammatory agents. J Med Chem 36: 1090-1099.
- Bhagyalakshmi N, Udupi RH, Niranjan MS (2012) Design, synthesis and evaluation of biological activity of certain novel triazole Schiff bases. Int J Pharm Chem Sci 1: 287-294.
- Wahi AK, Singh AK, Singh A (2011) Design and synthesis of novel Schiff bases having N-(4H-1, 2, 4-triazole-4-yl) benza-amide moiety as antimicrobial and anti-inflammatory agents. Pharm Chem 3: 146-154.
- Chandramouli, Shivanand MR, Navinbhai TB, Acharaya B, Udupi RH (2012) Synthesis and biological screening of certain new triazole Schiff bases and their derivatives bearing substituted benzothiazole moiety. J Chem Pharm Res 4: 1151-1159.
- Tahi M, Webster ER, Wilson R, Nithinchandra, Kalluraya B, et al. (2012) Regioselective reaction: synthesis, characterization and pharmacological activity of some new Mannich and Schiff bases containing sydnone. Eur J Med Chem 54: 597-604.
- Sughen J. K., Yoloye T (1978) Pharm Acta Helv 58: 64-68.
- Zahajska L, Klimesova V, Koci J, Waisser K, Kaustova (2004) Synthesis and antimycobacterial activity of Pyridylmethylsulfanyl and naphthylmethylsulfanyl derivatives of benzazoles, 1, 2, 4-triazole and pyridine-2-carbothioamide/-2-carbonitrile. J Arch Pharm (Weinheim) 337: 549-555.
- Foroumadi A, Kiani Z, Soltani F (2003) Antituberculosis agents VIII: Synthesis and in vitro antimycobacterial activity of alkyl α-[5-(5-nitro-2-thienyl)-1, 3, 4-thiadiazole-2-ylthio] acetates. Il Farmaco 58: 1073.
- Joshi RD, Vagdevi HM, Vaidya VP, Gadaginamath GS (2008) Synthesis of new 4-Pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxidiazole, triazole and pyrrole ring system: a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 43: 1989-1996.
- Malikarjuna BP, Sastry BS, Suresh Kumar GV, Rajendraprasad Y, Chandrasekhar SM, et al. (2009) Synthesis of new 4-isopropylthiazole hydrazide analogs and some derived clubbed triazole, oxidiazole ring system-a novel Class of potential antibacterial, antifungal and antitubercular agents. Eur J Med Chem 44: 4739-4746.
- Suresh Kumar GV, Rajendraprasad Y, Malikarjuna BP, Chandrasekhar SM, Kistayya C (2010) Synthesis of some novel 2-substituted-5-[isopropylthiazole] clubbed 1, 2, 4-triazole and 1, 3, 4-oxadiazoles as potential antimicrobial and antitubercular agents. Eur J Med Chem 45: 2063-2074.
- Chohan ZH, Supran CT (2005) In-vitro antibacterial and cytotoxic of cobalt (II), nickel (II) and zinc (II) complexes of the antibiotic drug cephalothin (Keflin). J Enz Inhib Med Chem 20: 463-468.
- Singh K, Kumar Y, Pundir RK (2010) Synthesis and characterization of biologically active organosilicon (IV) complexes with Schiff bases derived from o-aminothiophenol. Synth React Inorg Met Org Chem 40: 836-842.
- Altundas A, Nursen S, Colak N, Ogutchi H (2010) Synthesis and biological activity of new cycloalkylthiophene Schiff bases and their Cr (III) and Zn (II) complexes. Med Chem Res 19: 576-588.
- Nurhan G, Mevlut S, Elif C, Ali S, Neslishan D (2007) Synthesis and antimicrobial activity of some new 1, 2, 4-triazole derivatives. Turk J Chem 31: 335-348.
- Mobinkhaledi A, Foroughifar N, Khanpour M, Ebrahimi S (2010) Synthesis of some novel Schiff bases containing 1, 2, 4-triazole ring. Eur J of Chem 1: 33-36.
- Wajda-Hermanowicz K, Pieniążczak D, Zatajska A, Wróbel R, Drabent K, et al. (2015) A Study on the Condensation Reaction of 4-Amino-3,5-dimethyl-1,2,4-triazole with Benzaldehydes: Structure and Spectroscopic Properties of Some New Stable Hemiaminals. Molecules 20: 17109-17131.
- Ma L, Lu L, Zhu M, Wang Q, Li Y, et al. (2011) Mononuclear copper(II) complexes with 3,5-substituted-4-salicylidene-amino-3,5-dimethyl-1,2,4-triazole: synthesis, structure and potent inhibition of protein tyrosine phosphatases. Dalton Trans 40: 6532-6540.
- Neslishan D (2005) Synthesis and characterization of new trihetrocyclic compounds consisting of 1, 2, 4-triazole and 1, 3, 4-triazole rings. Turk J Chem 29: 125-133.
- Bagihalli GB, Avaji PG, Patil SA, Badami PS (2008) Synthesis, spectral characterization, in vitro antibacterial, antifungal and cytotoxic activities of Co (II), Ni (II) and Cu (II) complexes with 1, 2, 4-triazole Schiff bases. Eur J Med Chem 43: 2639-2649.
- da Silva Cleiton M, da Silva Daniel L, Modolo LV, Alves RB, Maria A, et al (2011) Schiff bases: A short review of their antimicrobial activities. J Adv Res 2: 1-8.
- Singh BP (2011) Synthesis, spectral characterization and biological activity of the Schiff base complexes. IJPRD 4: 51-57.
- Liu WS, Yang TL (2003) Synthesis and infrared and fluorescence spectra of europium and terbium complex with a novel Bis-Schiff base ligand derived from 2, 6-diaminopyridine and indole-2-carbaldehyde. Chem Pap 59: 60-65.
- Song L, Xie Y, Wang H (2000) Synthesis and antibacterial activity of benzimidazole Schiff base. Chem Abstr 10: 92-94.
- Cozzi P (2004) Metal-Salen Schiff base complexes in catalysis: Practical aspects. Chem Soc Rev 33: 410-421.
- Chandra S, Sangeetika J (2004) EPR and electronic spectral studies on Copper (II) complexes of some N-O donor ligands. Indian Chem Soc 81: 203-206.
- Majeed A (2008) Synthesis and characterization and study of the biological activity of some new metal complexes of 2-Hydroxy benzyledene-5-(2-Acetyloxyphenyl)-1, 3, 4-thiadiazole. Journal of Al-Nahrain University 11: 44-49.
- Lalitha MK, Manual of antimicrobial susceptibility testing. Indian J Med Microbiol, Christian Medical College and Hospital, Vellore, India.
- Chohan Z (2009) Antibacterial dimeric copper (II) complexes with chromone derived compounds. Trans Met Chem 34: 153-161.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences