ISSN : 2471-9803
Critical Care Obstetrics and Gynecology
Pregnancy, Diabetes Insipidus and Adrenal Insufficiency after Surgical Treatment in Clinically Non-functioning Pituitary Adenoma: A Case Report
1Endocrinology Service – Hospital, Clementino Fraga Filho University, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
2Coordinator of Studies Center Ricardo A. T., Castilho of the Medical Association of Teresopolis, Rio de Janeiro, Brazil
3Discipline of Endocrinology, University Hospital Clementino Fraga Filho, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- Corresponding Author:
- Alice Helena Dutra Violante
Discipline of Endocrinology
University Hospital Clementino Fraga Filho
Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
Tel: 021999592672 / 021998288150
E-mail: aliceviolante@infolink.com.br or aliceviolante@hucff.ufrj.br
Received Date: June 21, 2018; Accepted Date: June 28, 2018; Published Date: July 05, 2018
Citation: Ben JJ, Naliato ECO, Violante AHD (2018) Pregnancy, Diabetes Insipidus and Adrenal Insufficiency after Surgical Treatment in Clinically Non-functioning Pituitary Adenoma – A Case Report. Crit Care Obst and Gyne. Vol.4 No.2:6. doi:10.21767/2471-9803.1000159
Copyright: © 2018 Ben JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We report a case of successful pregnancy despite the development of central Diabetes Insipidus (DI) and adrenal insufficiency after surgical treatment of a clinically Non- Functioning Pituitary Adenoma (NFPA).
A 36-year-old female who underwent pituitary surgery to treat a clinically NFPA, presented features of central DI and adrenal insufficiency both treated and compensated, and successfully became pregnant.
The presence of both conditions in women who previously underwent pituitary surgery to treat NFPA and who became pregnant without intercurrences for either mother and the fetus is unusual. Additionally, in the present case, there was no need for adjustments in the pharmacological therapy.
The advent of central DI or adrenal insufficiency after transsphenoidal surgery are frequent complications reported as independent isolated phenomena during the immediate postoperative period or in the context of hypopituitarism. However, the presence of both conditions in women who previously underwent pituitary surgery to treat clinically NFPA and who became pregnant without intercurrences for either mother and the fetus is unusual, which prompted this case report.
Keywords
Pregnancy; Pituitary adenoma; Central diabetes insipidus; Hypothyroidism; Adrenal insufficiency
Abbreviations
DI: Diabetes Insipidus; NFPA: Non-Functioning Pituitary Adenoma; PRL: Prolactin; MRI: Magnetic Resonance Imaging; TSH: Thyroid Stimulating Hormone
Introduction
Pituitary tumors are classified according to the presence or absence of hormonal secretion. Among the secretors, the most common is the prolactinoma, which mainly affects women of childbearing age, produces Prolactin (PRL), leads to hypogonadism and/or hypopituitarism, and may, depending on size, have visual manifestations such as headache [1].
Serum PRL levels tend to relate to tumor size and frequency. A tumor is considered a microadenoma if it is smaller than 1 cm or a macroadenoma if it is 1 cm in size or larger. Macroadenomas have values exceeding 200 ng/mL. However, cystic tumors can exhibit lower PRL levels even when they present large dimensions. The treatment of prolactinomas with dopamine agonists has yielded excellent results [1,2].
Non-hormonally secreting pituitary adenomas are called “NFPAs” and also may be associated with hypopituitarism and visual symptoms such as headaches [1]. They can also be associated with secondary hyperprolactinemia-although of a lesser magnitude than that observed in prolactinomas-due to compression of the hypothalamus pituitary stalk, and treatment options include surgery. Complications may occur, including varying degrees of temporary or permanent panhypopituitarism and DI [3,4]. There is accordingly a need for differential diagnosis.
Case Report
A 36-year-old female visited the endocrinology ambulatory of the University Hospital Clementino Fraga Filho-Federal University of Rio de Janeiro, Brazil (HUCFF-UFRJ) in October 2009 complaining of menstrual irregularity and bilateral galactorrhea that had persisted for 3 years. Her laboratory tests revealed hyperprolactinemia (PRL ranging between 74 and 106 ng/mL; normal values=5-25 ng/mL) and hypogonadotropic hypogonadism with the remaining pituitary function normal somatotropic, thyroid stimulating, and corticotrophic axis (Table 1).
| Prolactin | Reference values (women) | July 2009 | November 2009 | May 2010 | May 2010 | September 2010 |
|---|---|---|---|---|---|---|
| 6.0-30.0 ng/mL | 74.0# | 0.32 & | 5.8* | 42 | 52 | |
| FSH | Follicular phase: 3.5-12.5 mUI/mL Ovulation peak: 4.7-21.5 mUI/mL Luteal phase: 1.7 -7.7 mUI/mL |
2.8 | 9.2 | 5.2 | 8.2 | 4.2 |
| LH | Follicular phase: 1.4-12.6 mUI/mL Ovulation peak: 14.0-95.6 mUI/mL Luteal phase: 1.0-11.4 mUI/mL |
4.8 | 18.2 | 9.4 | 7.5 | 4.6 |
| E2 | Follicular phase: 1.0-30.0 ng/dL Ovulation peak: 15.0-60.0 ng/dL Luteal phase: 5.0-30.0 ng/dL |
28 | 82 | - | - | 66 |
| TSH | 0.3-4.0 mUI/L | 2.03 | 3.56 | - | 1 | 1.07 |
| FreeT4 | 0.7-1.5 ng/dL | 0.99 | 1.2 | - | 0.9 | 0.8 |
| Cortisol | Between 7 and 9AM: 5.4-25.0 µg/dL | 15.6 | - | - | - | 12.3 |
| IGF-1 | 31-40 years: 130-354 ng/mL | 195 | - | - | - | - |
LH-Luteinizing Hormone
E2-Estradiol
TSH-Thyroid Stimulating Hormone
T4-Thyroxine
IGF-1-Insulin-Like Growth Factor-1
#Cabergoline treatment initiated (0.5 mg b.i.w.); Cabergoline dose decreased to 0.5 mg weekly
*Cabergoline interrupted
Table 1: Biochemistry (Pre-surgery).
Magnetic Resonance Imaging (MRI) conducted in September 2009 revealed the presence of suprasellar extension measuring 1.9 × 1.7 × 1.7 cm, contact with the optic chiasm, and visual campimetry without changes. The differential diagnosis was between NFPA with pituitary stalk compression or prolactinoma.
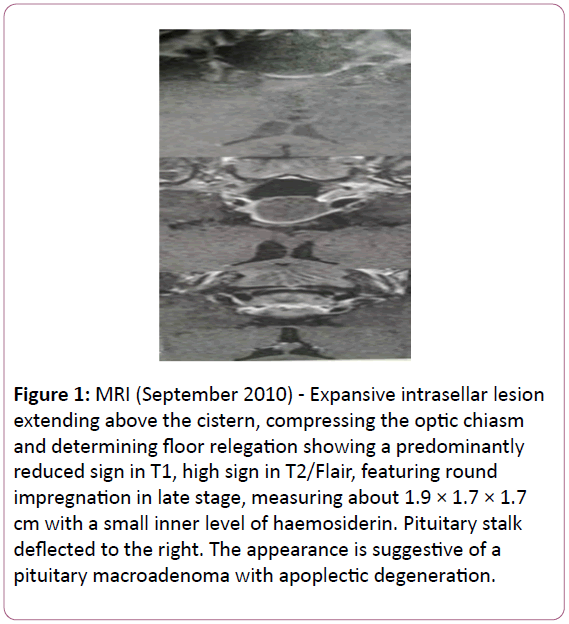
The patient received a dopamine agonist (Cabergoline) at a dose of 1.0 mg per week. Cabergoline treatment resulted in a significant decrease in the PRL levels (Table 1) with resolution of the galactorrhea, and the weekly dose was reduced to 0.5 mg. Nevertheless, the patient remained with menstrual irregularity and despite the biochemical response, the volume of the pituitary lesion increased. In September 2010, an MRI revealed a lesion measuring 2.2 × 1.5 × 2.0 cm (Figure 1).
Figure 1: MRI (September 2010) - Expansive intrasellar lesion extending above the cistern, compressing the optic chiasm and determining floor relegation showing a predominantly reduced sign in T1, high sign in T2/Flair, featuring round impregnation in late stage, measuring about 1.9 × 1.7 × 1.7 cm with a small inner level of haemosiderin. Pituitary stalk deflected to the right. The appearance is suggestive of a pituitary macroadenoma with apoplectic degeneration.
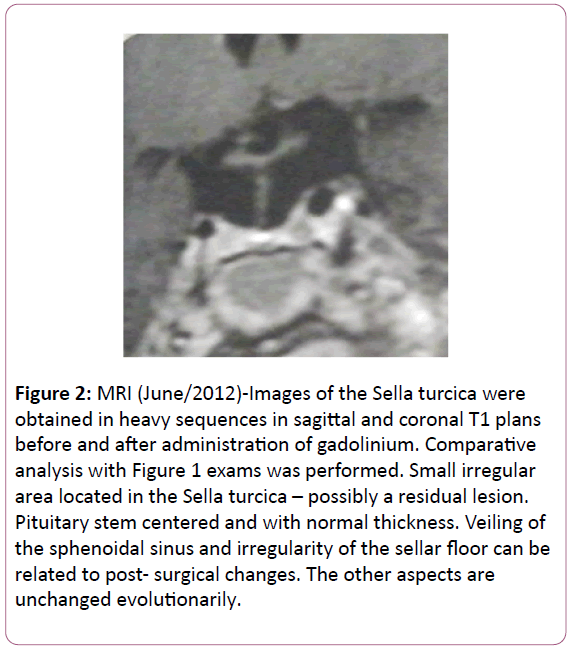
With the increase in tumor volume independent of PRL normalization, the diagnosis of prolactinoma was excluded and the patient was referred to surgical treatment of the adenoma. Surgery through transsphenoidal access was conducted in August 2011 by a neurosurgery team with extensive experience on pituitary surgery. The intervention was preceded by administration of 50 mg of hydrocortisone. The same dose was administered during the surgical procedure and postoperatively, with the evaluation of electrolytes and cortisol. Immunohistochemical analysis of the pituitary adenoma revealed positivity for FSH (Follicle-Stimulating Hormone) and LH (Luteinizing Hormone). Figure 2 exhibits the aspect of the pituitary gland obtained 10 months after the transsphenoidal intervention, suggesting the presence of a small irregular residual lesion.
Figure 2: MRI (June/2012)-Images of the Sella turcica were obtained in heavy sequences in sagittal and coronal T1 plans before and after administration of gadolinium. Comparative analysis with Figure 1 exams was performed. Small irregular area located in the Sella turcica – possibly a residual lesion. Pituitary stem centered and with normal thickness. Veiling of the sphenoidal sinus and irregularity of the sellar floor can be related to post- surgical changes. The other aspects are unchanged evolutionarily.
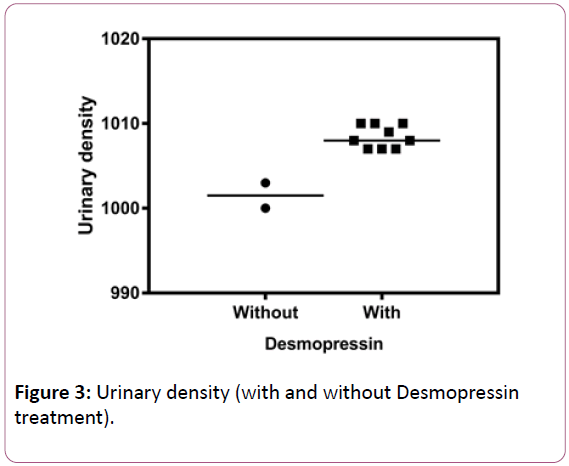
After the surgery, the patient developed permanent central diabetes insipidus (urinary density: 1000-1003 g/dL), polydipsia and polyuria (5-8 L/day) with a good clinical response to nasal Desmopressin (urinary density: 1007-1010 g/dL). The medication was interrupted soon after pituitary surgery to allow the investigation of DI (Table 2). After the definitive diagnosis, the medication was reintroduced with normalization of urinary density (Figure 3).
| August 2011 | Reference values | |
|---|---|---|
| Urinary density | 1000-1003 | >1005 |
| Plasma osmolarity (mOsm/Kg) | 270 | 275-295 |
| Urinary osmolarity (mOsm/Kg) | 452 | 300-900 |
| Sodium (plasma-mmol/L) | 135 | 135.0-145.0 |
| Potassium (plasma-mmol/L) | 4 | 3.5-5.5 |
Table 2: Post-surgical diagnosis of Diabetes Insipidus.
She also exhibited corticotrophic insufficiency (low basal cortisol in three distinct occasions: 4,6, 5.2 mcg/dL, and 6.2 mcg/dl; in one of them, the low basal levels were accompanied by symptoms of hypocortisolism such as asthenia, hyperemesis, and hypotension) (Table 3).
| Reference values (women) | June 2010 | February 2011 | June 2012 | February 2013 | June 2013 | December 2014 | March 2015 | June 2015 | December 2015 | |
|---|---|---|---|---|---|---|---|---|---|---|
| PRL | 6.0-30.0 ng/mL | 29.0 | 31.0 | 17.6 | - | 12.0 | 51.0 # | 1.0 | 3.7 | - |
| FSH | Follicular phase: 3.5-12.5 mUI/mL Ovulation peak: 4.7-21.5 mUI/mL Luteal phase: 1.7-7.7 mUI/mL |
- | 8.2 | 9.0 | - | - | - | - | - | - |
| LH | Follicular phase: 1.4-12.6 mUI/mL Ovulation peak: 14.0-95.6 mUI/mL Luteal phase: 1.0-11.4 mUI/mL |
- | 7.7 | 6.9 | - | - | - | - | - | - |
| E2 | Follicular phase: 1.0-30.0 ng/dL Ovulation peak: 15.0-60.0 ng/dL Luteal phase: 5.0-30.0 ng/dL |
- | 94.0 | 5.0 | - | - | - | - | 347.0 | - |
| TSH | 0.3-4.0 mUI/L | - | 2,29 | - | - | - | - | - | 3.04 | 3.7 |
| FT4 | 0.7-1.5 ng/dL | 1.1 | 0.9 | 1.1 | - | - | - | - | 0.9 | 0.7** |
| Cortisol | Between 7 and 9 AM: 5.4-25.0 µg/dL | 4.6! | 5.2* | - | - | 6.2* | - | - | - | - |
| ACTH | 7.2-63.3 pg/mL | - | - | - | 10.8 | - | - | - | - | - |
| IGF-1 | 31-40 years: 117-321 ng/mL | - | 177 | - | - | - | - | - | - | |
| Na | 135-145 mmol/L | - | 139 | 142 | 140 | - | 142 | - | - | |
| K | 3.5-5.5 mmol/L | - | 4.1 | 4.9 | 4.1 | - | 4.2 | - | - | |
| Progesterone | 1,700-2,000 pg/mL | - | - | - | 180 | - | - | - | - |
LH – Luteinizing Hormone
E2- Estradiol
TSH – Thyroid Stimulating Hormone
FT4 – Free Thyroxine
ACTH – Adrenocorticotropin Hormone
IGF-1 – Insulin-Like Growth Factor 1
#Cabergoline reinitiated (0.5mg, b.i.w.);
! with symptoms and treated with prednisone;
* without glucocorticoid treatment;
** treatment with levothyroxine initiated
Table 3: Biochemistry (Post-surgery).
Her gonadotropins and thyroid function returned to normal levels, and her hyperprolactinemia remained variable between 30 and 60 ng/mL. Her menstrual irregularity persisted. In 2015, the patient was submitted to an ITT (Insulin Tolerance Test) that confirmed the persistence of adrenal insufficiency (Table 3). Due to the patient’s desire to become pregnant, she was referred to the infertility clinic of our institution. By that time, her BMI was 39 kg/m2 and, in June 2015, her estradiol levels were 347.0 ng/dL. Her gonadotrophin levels were not evaluated concomitantly with estradiol. In the absence of another etiology for secondary infertility, and the patient began taking medroxyprogesterone, metformin and clomiphene. She successfully became pregnant after the fourth cycle, 4 years and 4 months after the pituitary surgery.
The pregnancy continued to term without complications, and the patient used prednisone 5 mg/day, Desmopressin 1 puff 12/12:00 and levothyroxine 75 mcg/day due to hypothyroidism diagnosed during the 10th week of pregnancy.
At the 38th week of gestation, the patient was submitted to an elective caesarean delivery under general anesthesia due to the great value of the pregnancy; the baby, a female, was born with an APGAR score of 9.9, a weight of 3,220 g and a length of 50 cm. She was breastfed without complications.
Discussion
A young woman visited our institution with a macroadenoma measuring 1.7 × 1.7 × 1.9 cm and complaints compatible with hypogonadism. Our first hypothesis was a diagnosis of prolactinoma, and we started treatment for this condition. Among the dopamine agonists, cabergoline exhibited excellent results not only in relation to the decrease and standardization of hormone levels but also a reduction in tumor size [1,2].
We were unsuccessful: the adenoma increased in size and attained a dimension of 2 cm (Figure 1). We considered the likelihood of it being an NFPA with the presence of a “stem effect" [1-3]. The patient was transferred to surgical treatment due to precise indications of tumoral compression/proximity to the optic chiasm, the patient’s desire to become pregnant, and the increased possibility of tumor growth due to the size of the lesion (macroadenoma) [3,4].
The surgical approach was transsphenoidal. Post-operatively, the patient presented clinical and laboratory manifestations of deficiency of ACTH (Adrenocorticotropic Hormone) and central DI.
Both morbidities are characterized by a low prevalence, (6.5% and 2.4%, respectively), and the association of these morbidities with the maintenance of the remaining pituitary function without changes is extremely unusual [3-6].
The occurrence of a post-operative pregnancy and successful gestational development reassured the medical team, and the patient was allowed to breastfeed. An MRI conducted 6 months after the patient had stopped breastfeeding revealed no growth of the lesion.
Hypothyroidism was only diagnosed during gestation. Central or secondary hypothyroidism is characterized by insufficient Thyroid Stimulating Hormone (TSH) production by the pituitary gland. This change affects 1 in 100,000 individuals and contributes minimally to cases of hypothyroidism. The treatment of secondary hypothyroidism, as well as in primary hypothyroidism, consists of replacement of Levothyroxine (LT4). However, the dose adjustment cannot be used to evaluate serum levels of TSH because if the etiology is pituitary deficiency, the levels of this hormone will be low or even undetectable [7-9].
Thyroid hormones are essential for growth and maturation of various tissues and organs, especially the brain and skeleton [8]. It is accordingly important to screen for thyroid function and, if necessary, replacement therapy before and after pregnancy [7-9]. In this case, the diagnosis of hypothyroidism was made during the first trimester of pregnancy. The patient started treatment without complications for herself or the fetus.
Corticotrophic insufficiency leads to increased morbidity and maternal-fetal mortality, with an increased prevalence of symptoms such as hyperemesis of pregnancy, postural hypotension and fatigue beyond the first trimester, as well as a risk of adrenal crisis due to the reduction of cortisol in the mother [10]. For the fetus, there is the increased incidence of oligohydramnios, low birth weight and intrauterine death [10]. During normal pregnancies, there is a physiological increase in total and free cortisol values. Based on that, it has been suggested that hydrocortisone doses are increased up to 20-40% in the last trimester [11]. Due to the lack of sufficient evidence, patients should be followed-up once every trimester with dosage adaptation based on the individual course of pregnancy [12].
The presence of DI also increases maternal-fetal morbidity and mortality [13,14]. Pregnancy is a risk factor for the development of subclinical DI [15]. In terms of treatment, there are controversies about the need for increased dosages of Desmopressin (DDAVP).
On the one hand, dose increases are justified by the increase in uterine vasopressinase; on the other hand, some practitioners advocate that an increase of this enzyme, which degrades vasopressin, would not have an effect as long as the replacement is done with DDAVP. Desmopressin, because it is synthetic, is more resistant to the action of vasopressinase [16], which removes the need for dose adjustment during pregnancy [17,18], in particular, as in the case in question, in patient who had been previously using this medication [19]. In terms of DDAVP, studies are emphatic and conclusive as to maternal-fetal safety [19-23].
Despite controversies regarding follow-up intervals and dose adjustments, levothyroxine, corticoids, and DDAVP are regarded as safe for the fetus during pregnancy [9,11,12,20,22,23]. The obstetrician team opted for a caesarean section in the present case. Du, et al. [24] indicated that the incidence of Caesarean deliveries is significantly higher in women with hypopituitarism. High-risk pregnancies in women with hypopituitarism have been attributed to a uterine defect secondary to endocrine deficiency [25]. In patients with childhood-onset combined pituitary hormone deficiencies, adequate hormonal replacement prior to ovarian stimulation resulted in successful pregnancies [26]. Though there have been reports of spontaneous pregnancies in patients with hypopituitarism, there is scarce data on the outcomes. Conception is reported to be achieved either spontaneously or as a result of ovulation induction, but pregnancies rarely are carried through to term [27-29]. Ovulation induction in these patients produces relatively low pregnancy rates, in comparison with other causes of anovulation, and high miscarriage rates [30]. Kubler et al. [29] analysed 31 pregnancies in women with hypopituitarism, observing increased risk for postpartum haemorrhage, transverse lie, and small for gestational age new-borns.
The subject of this case report developed central hypothyroidism during her pregnancy. The diagnosis was established in the late first trimester of the gestation, a period when there is a transition in the control of the production of T4 from the hCG to TSH [31]. A study from Fatemi et al. concluded that new unplanned hypopituitarism occurs in 5% of patients after transsphenoidal surgery and the likelihood of new hormonal loss is higher in patients with tumors larger than 20 mm, whereas hormonal recovery is more common in younger, non-hypertensive patients and those without intraoperative cerebrospinal fluid leak [32]. Besides having a large tumor (22 mm), our patient presented an MRI suggestive of a residual lesion, which has been indicated as a factor associated with lower probability of pituitary function recovery [33].
It has been reported that new-onset hypopituitarism and permanent DI affect, respectively, 11.6 and 4.3% of patients submitted to transsphenoidal surgeries [34].
The present case calls our attention for the occurrence of an association between permanent DI and adrenal insufficiency after pituitary surgery, followed, more than four years later, by a successful uncomplicated pregnancy, despite the advent of central hypothyroidism, which highlights the peculiarity of this case and the importance of long-term follow-up of patients with pituitary tumors by the endocrinologist.
Acknowledgments
The authors have no relevant conflict of interest to disclose.
References
- Melmed S, Casanueva FF, Hoffman AR (2011) Diagnosis & Treatment of Hyperprolactinemia: An Endocrine Society Clinical Practice Guideline. J Clil Endocrinol Metab 96: 273-288.
- Lamounier Fo LA, Violante AHD (2009) Prolactinomas. In: Rotinas diagnosticas e terapeuticas: serviço de endocrinologia do Hospital Universitario Clementino Fraga Filho. In: Vaisman M, Conceição FL, Vieira Neto L, (eds.) Editora Atheneu – Sao Paulo – Brazil, pp. 15-26.
- Fleserin M, Bordach MG, Tumialan LM (2016) Congress of Neurological Surgeon Systematic Review Evidence-Based and Guideline for Pretreatment Endocrine Evaluation of Patients with Non-functionig Pituitary Adenomas. Neurosurgery 79: 527-529.
- Karaca Z, Kelestimur F (2011) Pregnancy and other pituitary disorders. Best Pract Res Clin Endocrinol Metab 25: 897-910.
- Molitch ME (2006) Pituitary Disorders During Pregnancy. Endocrinol Metab Clin N Am 35: 99-116.
- Rasmussen UF, Mathiesen RE (2011) Endocrine disorders in pregnancy: Physiological and hormonal aspects of pregnancy. Best Pract Res Clin Endocrinol Metab 25: 875-884.
- Wiersinga WM, Duntas L, Fadeyev V, Nygaard B, Vanderpump MPJ (2012) ETA Guidelines: The Use of L-T4+L-T3 in the Treatment of Hypothyroidism. Eur Thyroid J 1: 55-71.
- Drahomira S, Lan J, Zdenka L, Tomas Z, Eliska P (2017) Thyroid in pregnancy: From physiology to screening. Crit Rev Clin Lab Sci 54: 102-116.
- De Groot L, Abalovich M, Alexander EK (2012) Management of Thyroid Dysfunction during Pregnancy and Postpartum: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 97: 2543-2565.
- Yuen KCJ, Chong LE, Koch CA (2013) Adrenal insufficiency in pregnancy: challenging issues in diagnosis and management. Endocrine 44: 283-292.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, et al. (2016) Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101: 364-389.
- Anand G, Beuschlein F (2018) Management of endocrine disease: Fertility, pregnancy and lactation in women with adrenal insufficiency. Eur J Endocrinol 178: 45-53.
- Mesmay M, Rigouzzo A, Bui T, Louvet N, Constant I (2013) Gestational diabetes insipidus during a twin pregnancy. Ann Fr Anesth Reanim 32: 118-121.
- Ananthakrishnan S (2009) Diabetes insipidus in pregnancy: etiology, evaluation, and management. Endocr Prac 15: 377-382.
- Yawasaki Y (1991) Aggravation of subclinical Diabetes Insipidus during Pregnancy N Eng J Med 324: 522-526.
- Robertson GL (2016) Diabetes insipidus: Differential diagnosis and management. Best Pract Res Clin Endocrinol Metabol 30: 205-218.
- Davison JM, Sheills EA, Barron MW, Robinson AG, Lindheimert MD (1989) Changes in the Metabolic Clearance of Vasopressin and in Plasma Vasopressinase throughout Human Pregnancy. J Clin Invest 83: 1313-1318.
- Durr JA, Lindheimer MD (1996) Diagnosis and management of diabetes insipidus during pregnancy. Endocr Pract 2: 353-361.
- Chanson P, Salenave S (2016) Diabetes insipidus and pregnancy – Ann Endocrinol 77: 135-138.
- Ray JG (1998) DDAVP use during pregnancy: an analysis of its safety for mother and child. Obstet Gynecol Surv 53: 450-455.
- Kalelioglu I, Uzum AK, Yildirim A, Ozkan T, Gungor F (2007) Transient gestational diabetes insipidus diagnosed in successive pregnancies: review of pathophysiology, diagnosis, treatment, and management of delivery. Pituitary 10: 87-93.
- Fleseriu M, Hashim IA, Karavitaki N (2016) Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101: 3888-3921.
- ANVISA (Brazilian National Sanitary Surveillance Agency).
- Du X, Yuan Q, Yao Y, Li Z, Zhang H (2014) Hypopituitarism and successful pregnancy. Int J Clin Exp Med 7: 4660-4665.
- Overton CE, Davis CJ, West C (2002) High risk pregnancies in hypopituitary women. Hum Reprod 17: 1464-1467.
- Correa FA, Bianchi PHM, Franca MM (2017) Successful Pregnancies After Adequate Hormonal Replacement in Patients With Combined Pituitary Hormone Deficiencies. J Endocrine Soc 1: 1322-1330.
- Lata I (2014) Spontaneous successful pregnancy in posthypophysectomy hypopituitarism: A rare case report. J Hum Reprod Sci 7: 218-220.
- Pilka L, Snajderova M, Rumpik D, Kaplanova T, Pilka R (2003) Pregnancy and delivery after traumatic panhypopituitarism. Ceska Gynekol 68: 277-279.
- Kubler K, Klingmuller D, Gembruch U, Merz WM (2009) High-risk pregnancy management in women with hypopituitarism. J Perinatol 29: 89-95.
- Hall R, Manski-Nankervis J, Goni N (2006) Fertility outcomes in women with hypopituitarism. Clin Endocrinol (Oxf) 65: 71-74.
- Ballabio M, Poshychinda M, Ekins RP (1991) Pregnancy-induced changes in thyroid function: role of human chorionic gonadotropin as putative regulator of maternal thyroid. J Clin Endocrinol Metab 73: 824-831.
- Fatemi N, Dusick JR, Mattozo C (2008) Pituitary hormonal loss and recovery after transsphenoidal adenoma removal. Neurosurgery 63: 709-718.
- Webb SM, Rigla M, Wägner A (1999) Recovery of hypopituitarism after neurosurgical treatment of pituitary adenomas. J Clin Endocrinol Metab 84: 3696-3700.
- Ammirati M, Wei L, Ciric I (2013) Short‑term outcome of endoscopic versus microscopic pituitary adenoma surgery: A systematic review and meta‑analysis. J Neurol Neurosurg Psychiatry 84: 843-849.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences