ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Plant Extracts, BAU-Biofungicide and Fungicides in Controlling Some Important Diseases of Rice cv. BRRI Dhan40
Mahmud H1*, Hossain I2 and Ahmad MU2
1 Department of Agricultural Extension, Kushtia, Bangladesh
2 Department of Plant Pathology, Bangladesh Agricultural University, Mymensingh, Bangladesh
- *Corresponding Author:
- Mahmud H
Additional Deputy Director, Department of Agricultural Extension, Kushtia, Bangladesh
Tel: 8801727182615
E-mail: mhyat81@gmail.com
Received Date: August 08, 2016; Accepted Date: August 29, 2016; Published Date: August 31, 2016
Citation: Mahmud H, Hossain I, Ahmad MU (2018) Plant Extracts, BAU-Biofungicide and Fungicides in Controlling Some Important Diseases of Rice cv. BRRI Dhan40. Am J Phytomed Clin Ther Vol.6 No.2:7 DOI: 10.21767/2321-2748.100343
Abstract
Cercospora oryzae, Rhizoctonia solani, Sarocladium oryzae and Ustilaginoidea virens are main causal pathogen of four rice diseases viz; narrow brown leaf spot, sheath blight, sheath rot and false smut, respectively. An experiment was conducted to evaluate the extracts of garlic (Allium sativum L.) and neem (Azadirachta indica L.), BAU-Biofungicide (Trichoderma based preparation), Bavistin DF (Carbendazim) and Potent 250 EC (Propiconazole) under laboratory and field conditions for management of diseases of rice cv BRRI dhan40 during Aman season of 2011 and 2012. Significant reduction in mycelial growth of Cercospora oryzae, Rhizoctonia solani, Sarocladium oryzae and Ustilaginoidea virens were observed with BAU-Biofungicide (2 and 3%) in laboratory as well as reduced disease severity of narrow brown leaf spot, sheath blight, sheath rot and false smut in the field which is close to the effect of Propiconazole (0.1%). Carbendazim (0.1%) also showed significantly low severity of narrow brown leaf spot, sheath blight and sheath rot disease in the field and mycelial growth inhibition of Cercospora oryzae, Rhizoctonia solani and Sarocladium oryzae was recorded in-vitro test. Highest grain yield 6.07 t ha-1 was found in Propiconazole 250 EC (0.1%), whilst BAU-Biofungicide (3%) resulted 5.89 t ha-1yield. Most of the seed borne pathogens of harvested seeds were controlled by BAU-Biofungicide and Propiconazole when they were applied as foliar spray.
Keywords
BAU-Biofungicide; BRRI dhan40; Disease severity; Propiconazole; Management; Seed borne pathogens
Introduction
Rice (Oryza sativa L.) is one of the three major food crops of the world. It is the staple food for more than one and a half of the world’s population. It is a nutritious cereal crop providing 20% of the calories and 15% of protein of world’s population [1]. Rice is also the staple food crop in Bangladesh. The world average yield of rice is 4.50 t h-1 but the national average yield of rice is 4.35 t h-1 which is extremely lower in comparison to 7.01 t h-1 in South Korea and 6.91 t h-1 in China [2]. The production of rice is constrained due to different causes. Rice disease is one of the major threats for its production. Thirty two diseases of rice are reported to occur in Bangladesh. Ten diseases have the potentiality to cause major economic loss to the crop [3]. Losses have been estimated about 15.6% due to rice diseases [4]. Narrow brown leaf spot (NBLS) is one of the major foliar diseases of rice with yield losses up to 40% [5]. Narrow brown leaf spot (Cercospora oryzae Miyake) become very severe on susceptible varieties, and causes severe leaf necrosis. It also poses premature ripening, yield reduction and low grain milling quality [6]. Sheath blight (Rhizoctonia solani Kuhn) in rice is an important soil-borne and major fungal disease, reducing both grain yield and quality. Fifty percent yield losses were reported with sheath blight in rice fields [7]. Sheath rot pathogen (Sarocladium oryzae (Sawada) W. Gams and D. Hawksw) is seed borne and present in all rice growing countries all over the world. S. oryzae infection results in chaffy, discolored grains, and affects the viability and nutritional value of seeds [8,9]. The scientists [10] stated that sheath rot can lead to yield losses up to 85%. Rice False smut (RFS) has emerged in recent years as one of the most devastating grain disease [11]. Outbreaks of this disease often occur in cold weather and reduce the grain quality and yield [12], and late sowing and application of high doses of Nitrogen also favours the development of disease [13,14]. Upadhyay [15] reported that yield loss ranged from 1 to 75% due to RFS disease in many rice growing areas. The false smut (chlamydospores) contains mycotoxins (ustiloxins) that are toxic to animals and contaminate rice seeds and grains [13]. Moreover, false smut not only threatens rice production in yield and quality but also produces toxins that are dangerous to human health and livestock. Quality seed for planting is an important input for successful crop production. Good quality seed possesses major characteristics such as high yielding potentiality, viability, purity, free from varietal mixtures and free from infection by pathogens or having maximum acceptable tolerance limit of infection by a given pathogen in a given seed lot [16,17]. The scientist cited internally seed borne infection of rice seed by Pyricularia grisea (blast), Bipolaris oryzae (brown spot), Fusarium moniliforme (bakanae) and Alternaria padwiickii (stack burn) resulted in diseased seed and seedlings. These organisms cause grain discolouration of varying intensity and reduce commercial value. Chemical control of plant diseases is a common practice for reducing crop losses. Application of chemicals or fungicides is not a satisfactory method of control. Rice disease management strategies mainly aim at preventing outbreak through the use of host plant resistance and chemical pesticides. The continuous and indiscriminate use of chemicals has toxic effects on non-target organisms and can cause undesirable changes in the environment. However, the environmental pollution is caused by excessive use and misuse of agrochemicals, and the development of resistance over these chemicals among pathogens has led to remarkable changes to researchers and farmers using pesticides in agriculture. Various researchers tried to find out safe and economical control plant diseases by using extracts of different plant parts [18,19]. Control of plant disease by biological means instead of using chemicals has drawn special attention all over the world. Biocontrol assumes a special significance as an eco-friendly and cost-effective strategy which can be used as integrated with other strategies for a greater level of protection. Using of microorganisms (biocontrol) or chemical by-products made by microorganisms generate very effective and economically feasible biological control materials [20]. Trichoderma strains are among the most fungal biocontrol agents and are successfully used as biopesticides and biofertilizers in green house and field for plant production, and induced systemic resistance to pathogens in plant [21]. Trichodermacan function at the same time both as microbial antagonists and plant symbionts [22]. The present study has been designed to control rice diseases by using plant extracts and biocontrol agent as an alternative option in order to avoid haphazard using of chemicals. BAU-Biofungicide resulted in significant higher germination, plant stand, less disease incidence and higher yield of different crops [23-25]. Moreover the biochemical changes were found to occur in the rice plant as a response of T. harzianum [26] and Trichoderma have been used in the management of diseases of rice [27].
Materials and Methods
Preparation of plant extracts
Healthy leaves of neem (Azadirachta indica L.) was collected from medicinal garden (botanical garden), Bangladesh Agricultural University, Mymensingh and garlic cloves (Allium sativum L.) grown in spices research centre, Bangladesh Agricultural Research Institute, Gazipur, were collected and the samples were washed thoroughly under running tap water followed by sterile distilled water (SDW). The extracts were prepared by homogenizing 5 g of plant sample in 50 ml of SDW using a blender and the extracts were then prepared at 1% and 2% concentration by dilution with water, and kept in conical flasks separately before use [24].
Use of BAU-Biofungicide and fungicide
BAU-Biofungicide (Trichoderma based preparation) was used at 2 and 3%. BAU-Biofungicide is a Trichoderma based preparation [23]. Bavistin DF (Carbendazim) and Potent 250 EC (Propiconazole) were also used at 0.1 and 0.05% concentration, respectively.
Field experiments: Field experiments were carried out with rice cv BRRI dhan40 during two Aman seasons of 2011 and 2012 in the field laboratory of the Department of Plant Pathology, Bangladesh Agricultural University, Mymensingh. The experiment was conducted by using Randomized Complete Block Design having three replications. The field was fertilized as per recommendation of Bangladesh Rice Research Institute, Gazipur [28]. The individual plot size was 10 m2. Block to block, and plot to plot distances were 1.5 m and 1.5 m, respectively. Thirty four days old seedlings of susceptible variety BRRI dhan40 were uprooted from the seed bed and three seedlings per hill were transplanted in the field on August 4, 2011 and 2012. Hill to hill and row to row distances were 15 cm and 25 cm, respectively. The spray schedule was started just after commencement of disease symptom and three sprays were maintained at 15 days interval. Disease severity of each plot was assessed following the procedure of Standard Evaluation System for Rice [29]. Grain yield and Number of panicle/m2 of each treatment were recorded.
Isolation: Cercospora oryzae was isolated from infected leaves and seeds collected from the field. Rhizoctonia solani and Sarocladium oryzae infected sheath of rice plants were used. The diseased grains were collected for isolation of U. virens following the method of the researcher [30]. Isolation of fungi from seed [31] was followed. Pure culture of the pathogen was preserved in PDA with the help of hyphal tip culture method aseptically and stored in a refrigerator at 4°C for further study [32].
Bioassay of plant extracts, BAU-Biofungicide and fungicides on fungi: Potato dextrose agar medium was prepared and poured into 9 cm Petri plates. After solidification, three 5 mm discs of the medium were scooped from three places maintaining equal distance of 4 cm from the centre using a sterilized disc cutter. One milliliter of each of plant extracts, suspension of BAU-Biofungicide, Bavistin DF and Potent 250 EC were put into each hole and the plates were stored overnight. Next day, the plates were inoculated at the center with 6 mm blocks of 15 days old culture of fungi and incubated at 24 ± 1°C [24]. Each treatment was replicated thrice and only water was used for control treatment. Linear mycelial growth of fungi was measured up to 12 days of inoculation [33] and percent inhibition was calculated by the following formula [34]:
Inhibition (%)=X-Y/X
Where; X=Mean mycelia growth (radial) in control plate; Y=Mean mycelia growth (radial) in treatment.
Laboratory experiments: Blotter method of seed health test was carried out following ISTA rules [31]. Three layers of blotting paper (Whatman filter No.1) soaked in water and were kept at the bottom of a 9.0 cm dia. plastic petri dish and there after 25 seeds were kept on filter paper. Four hundred harvested seeds of each treatment were taken randomly from each sample of each year. The experiment was conducted in the net house of the Seed Pathology Centre, BAU, Mymensingh. The petri dishes containing seeds were incubated at 20 ± 2°C under alternating cycles of 12 hours near Ultra Violet light and darkness for 7 days. Incubated seeds were examined under stereo-binocular microscope to record the incidence of different seed borne fungi. Each seed borne infection was recorded and expressed in percentage [35].
Statistical analyses: All the recorded data on different parameters were analysed statistically using MSTAT-C computer program to find out the significance of variation resulting from experimental treatments. The difference between the treatment means were evaluated for significance using Duncan’s Multiple Range Test (DMRT) following the procedure as described [36].
Results
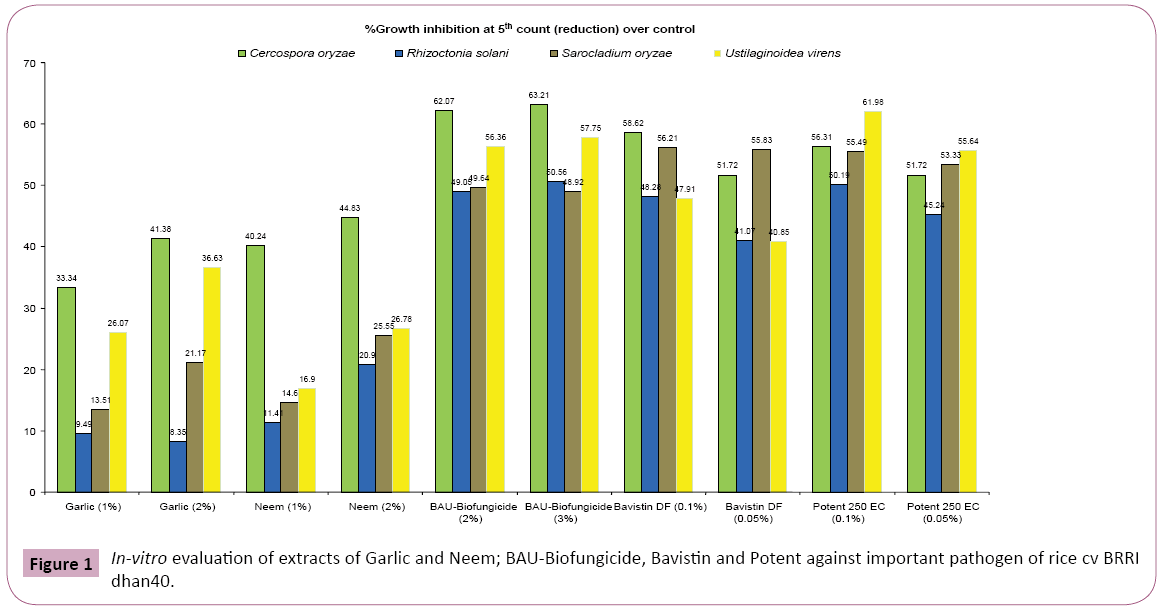
Lowest severity of narrow brown leaf spot disease was found in Potent (0.1%) and BAU-Biofungicide (2%) followed by Bavistin (0.1%). Severity of sheath blight disease was not observed with Potent (0.1 and 0.05%) at 105 DAT in 2011 and 2012, respectively. Significant reduction of severity of sheath blight disease was also found in Bavistin (0.1%) and BAU-Biofungicide (2%) (Table 1). Evidently, maximum (85.00%, 86.70%) reduction in severity of sheath rot disease was noted with Potent (0.1%) followed by Potent (0.05%), Bavistin (0.1%) and BAU-Biofungicide (2 and 3%) over control in 2011 and 2012, respectively. In case of false smut, lowest disease severity was recorded in Potent (0.1 and 0.05%) followed by Bavistin (0.1%) and BAU-Biofungicide (2%) over control during two successive years 2011 and 2012. Extract of garlic (2%) had good effect in reducing the disease severity between two plant extracts of garlic and neem (Table 2). Highest reduction of mycelial growth of C. oryzae and R. solani over control was found with BAU-Biofungicide (2 and 3%) preceded by Potent (0.1%) as shown in Figure 1. Maximum reduction (56.21%) of S. oryzae over control was observed in Bavistin (0.1%) followed by Potent (0.1%) and BAU-Biofungicide (2%). Highest reduction of mycelial growth of U. virens (61.98%) over control was recorded in Potent (0.1%) preceded by BAU-Biofungicide (2 and 3%). Maximum increase (27.67%) of grain yield over control was obtained with Potent (0.1%) followed by BAU-Biofungicide (2%) in 2012 and highest number of panicle/m2 was found in Potent (0.1%) followed by BAU-Biofungicide (3%) in 2011. Bavistin (0.1%) and Garlic (2%) also showed better result in increasing yield and number of panicle/m2 (Table 3). Effect of plant extracts, BAU-Biofungicide and fungicides on health status of harvested seeds of rice cv. BRRI dhan 40 was evaluated by standard blotter incubation test. It revealed that the seeds were found to be associated with 8 different seed borne fungi viz., Aspergillus flavus, Bipolaris oryzae, Curvularia lunata, Fusarium moniliforme, Fusarium oxysporum, Nigrospora oryzae, Sarocladium oryzae and Trichoderma harzianum (Table 4). Hundred percent reductions of seed borne infection of Aspergillus flavus was found with BAU-Biofungicide (3%) over control followed by Potent (0.1%) and neem (2%). Highest reduction of seed-borne infection (76.92%) of B. oryzae was obtained in harvested seeds by spraying plots both with BAU-Biofungicide (3%) and Potent (0.1%) over control followed by garlic (1 and 2%). Maximum reduction in seed-borne infection of Curvularia lunata (83.02%) was recorded with Potent (0.1%) followed by BAU-Biofungicide (2%) and garlic (2%). Highest reduction in seed borne infection (77.14%) of Fusarium moniliforme was observed with Potent (0.1%), while BAU-Biofungicide (3%) exhibited (66.67%) reduction over control. Maximum reduction (33.33%) of seed-borne infection of F. oxysporum was observed with BAU-Biofungicide (3%) over control. Hundred percent reduction of seed borne infection of Nigrospora oryzae was noted with Bavistin (0.1%) followed by BAU-Biofungicide (3%). Maximum reduction (79.31%) of seed-borne infection of Sarocladium oryzae was found in seeds of rice sprayed with BAU-Biofungicide (2%) followed by garlic (1 and 2%).
| Narrow brown leaf spot | Sheath blight | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (dose) | At 75 DAT | At 90 DAT | At 105 DAT | At 75 DAT | At 90 DAT | At 105 DAT | ||||||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | |
| Garlic (1%) | 4.67bc (33.29) |
5.00b (26.67) |
7.00cd (50.00) |
7.25cd (51.67) |
7.33cd (58.11) |
8.00c (55.56) |
15.67ab (7.82) |
8.25b (15.39) |
13.17b (31.87) |
9.50b (19.15) |
9.67b (54.66) |
7.50b (43.40) |
| Garlic (2%) | 4.33c (38.14) |
- | 6.00cd (57.14) |
- | 6.50d (62.86) |
- | 13.33abc (21.59) |
- | 10.33bc (46.56) |
- | 8.00bc (62.49) |
- |
| Neem (1%) | 5.00bc (28.57) |
6.00ab (20.00) |
10.00b (28.57) |
10.25b (31.67) |
11.00b (37.14) |
12.00b (33.33) |
13.00abc (23.53) |
7.75bc (20.51) |
10.00bcd (48.27) |
6.69c (43.06) |
8.00bc (62.49) |
4.89c (63.09) |
| Neem (2%) | 5.00bc (28.57) |
- | 8.33bc (40.50) |
- | 9.00c (48.57) |
- | 11.33bc (33.35) |
- | 8.33cde (56.91) |
- | 6.00cd (71.87) |
- |
| BAU-Biofungicide (2%) | 4.33bc (38.14) |
4.25b (43.33) |
5.00d (64.29) |
5.00d (66.67) |
3.50ef (80.00) |
3.25ef (81.94) |
12.17abc (28.41) |
7.00cd (28.21) |
8.00cde (58.61) |
6.00cd (48.94) |
4.33de (79.70) |
3.00d (77.36) |
| BAU-Biofungicide (3%) | 4.33bc (38.14) |
- | 5.00d (64.29) |
- | 3.00f (82.86) |
- | 11.33bc (33.35) |
- | 8.00cde (58.61) |
- | 4.33de (79.70) |
- |
| Bavistin DF (0.1%) | 5.00bc (28.57) |
5.25b (30.00) |
6.17cd (55.93) |
6.50cd (56.67) |
5.33de (69.54) |
5.50de (69.44) |
9.67c (43.12) |
5.00f (48.72) |
6.17de (68.08) |
3.50e (70.21) |
3.00e (85.94) |
0.00f (100.0) |
| Bavistin DF (0.05%) | 5.67b (19.00) |
5.50b (26.67) |
7.50bcd (46.43) |
8.00bc (46.67) |
7.00cd (60.00) |
7.25cd (59.72) |
11.00bc (35.29) |
6.50de (33.33) |
7.33cde (62.08) |
4.86de (58.64) |
4.00de (81.24) |
2.59d (80.45) |
| Potent 250 EC (0.1%) | 4.00c (42.86) |
4.25b (43.33) |
4.50d (67.86) |
5.00d (66.67) |
2.00f (88.57) |
2.50f (86.11) |
8.00c (52.94) |
4.50f (53.85) |
5.00e (74.13) |
3.50e (70.21) |
0.00f (100.0) |
0.00f (100.0) |
| Potent 250 EC (0.05%) | 4.67bc (33.29) |
4.50b (40.00) |
5.00d (64.29) |
5.00d (66.67) |
2.33f (86.69) |
2.75f (84.72) |
9.67c (43.11) |
5.50ef (43.59) |
6.00de (68.96) |
3.75e (68.09) |
0.00f (100.0) |
0.00f (100.0) |
| Control (water) | 7.00a | 7.50a | 14.00a | 15.00a | 17.50a | 18.00a | 17.00a | 9.75a | 19.33a | 11.75a | 21.33a | 13.25a |
Table 1: Effect of treatments on severity of brown spot and narrow brown leaf spot of rice cv. BRRI dhan40 in 2011 and 2012 Disease severity (%).
| Sheath rot | False smut | |||||
|---|---|---|---|---|---|---|
| Treatment (dose) | At 90 DAT | At 105 DAT | At 105 DAT | |||
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | |
| Garlic (1%) | 6.67bcd (42.84) |
6.00bcd (27.97) |
5.67bcd (57.46) |
5.00bc (50.00) |
5.67cd (31.93) |
4.50b (35.71) |
| Garlic (2%) | 5.33cde (54.33) |
- | 4.67cde (64.97) |
4.00bcd (60.00) |
5.00d (39.98) |
- |
| Neem (1%) | 9.00b (22.88) |
8.00ab (3.96) |
7.50b (43.74) |
6.00b (40.00) |
7.00b (15.97) |
5.00b (28.57) |
| Neem (2%) | 7.33bc (37.19) |
- | 6.17bc (53.71) |
5.00bc (50.00) |
6.00c (27.97) |
- |
| BAU-Biofungicide (2%) | 6.00cd (48.59) |
7.17abc (13.93) |
3.50ef (73.74) |
3.00cde (70.00) |
3.00e (63.99) |
3.00c (57.14) |
| BAU-Biofungicide (3%) | 5.00cde (57.16) |
- | 3.50ef (73.74) |
3.00cde (70.00) |
3.33e (60.02) |
- |
| Bavistin (0.1%) | 5.33cde (54.33) |
4.00def (51.98) |
3.33ef (75.02) |
3.00cde (70.00) |
2.67e (67.95) |
2.75c (60.71) |
| Bavistin (0.05%) | 6.00cd (48.59) |
5.00cde (4.80) |
4.00def (69.99) |
4.33bcd (56.70) |
3.33e (60.02) |
3.50c (50.00) |
| Potent 250 EC (0.1%) | 3.00e (74.29) |
2.00f (75.99) |
2.00ef (85.00) |
1.33e (86.70) |
0.00g (100.0) |
0.00e (100.0) |
| Potent 250 EC (0.05%) | 4.00de (65.72) |
3.00ef (63.99) |
2.67ef (79.97) |
2.33de (76.70) |
1.00f (88.00) |
1.00d (85.71) |
| Control (water) | 11.67a | 8.33a | 13.33a | 10.00a | 8.33a | 7.00a |
Table 2: Effect of treatments on severity of Sheath blight Sheath rot and False smut diseases of rice cv. BRRI dhan40 in 2011 and 2012 Disease severity (%).
| 2011 | 2012 | |||
|---|---|---|---|---|
| Treatment (dose) | No. of Panicle/m2 | Grain yield (t/ha) |
No. of Panicle/ m2 | Grain yield (t/ha) |
| Garlic (1%) | 250.00abc (5.93) |
5.51abc (14.32) |
242.00cd (3.64) |
5.02ab (16.67) |
| Garlic (2%) | 255.67abc (8.33) |
5.64abc (17.01) |
- | - |
| Neem (1%) | 240.00c (1.69) |
5.11bc (6.02) |
238.25d (2.03) |
4.72bc (9.77) |
| Neem (2%) | 248.00bc (5.08) |
5.16bc (7.05) |
- | - |
| BAU-Biofungicide (2%) | 280.67ab (18.93) |
5.75ab (19.29) |
277.50ab (18.84) |
5.23ab (21.63) |
| BAU-Biofungicide (3%) | 285.00ab (20.76) |
5.89ab (22.20) |
- | - |
| Bavistin (0.1%) | 252.67abc (7.06) |
5.63abc (16.80) |
245.00bcd (4.93) |
5.07ab (17.91) |
| Bavistin (0.05%) | 246.00bc (4.24) |
5.51abc (14.32) |
237.00d (1.50) |
4.82bc (12.09) |
| Potent 250 EC (0.1 %) | 289.33a (22.60) |
6.07a (25.93) |
283.00a (21.20) |
5.49a (27.67) |
| Potent 250 EC (0.05 %) | 284.00ab (20.34) |
5.74ab (19.09) |
273.00abc (16.92) |
5.20ab (20.93) |
| Control (water) | 236.00c | 4.82c | 233.50d | 4.30c |
Table 3: Effect of extracts of Garlic and Neem; BAU-Biofungicide, Bavistin and Potent on grain yield of rice cv. BRRI dhan40 in 2011 and 2012.
| Treatment (dose) | Aspergillus flavus | Bipolaris oryzae | Curvularia lunata | Fusarium moniliforme | Fusarium oxysporum | Nigrospora oryzae | Sarocladium oryzae | Trichoderma harzianum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | 2011 | 2012 | |
| Garlic (1%) | 2.00c (-42.86) |
2.00b (-33.33) |
4.50ef (-59.09) |
4.00e (-69.23) |
6.50ef (-66.67) |
5.25e (-60.38) |
8.25ab (-5.71) |
6.50c (-45.83) |
13.50d (-18.18) |
13.00de (-23.53) |
3.00bc (-40.0) |
2.50bc (-28.57) |
3.00f (-63.64) |
3.50cd (-51.72) |
0.00c | 0.00b |
| Garlic (2%) | 2.00c (-42.86) |
- | 4.00ef (-63.64) |
- | 6.00efg (-69.23) |
- | 6.00cd (-31.43) |
- | 13.50d (-18.18) |
- | 3.00bc (-40.0) |
- | 2.25f (-72.73) |
- | 0.00c | - |
| Neem (1%) | 2.00c (-42.86) |
2.00b (-33.33) |
12.00a (-9.09) |
11.50b (-11.54) |
7.50de (-61.54) |
6.50d (-50.94) |
7.00bc (-20.00) |
9.00b (-25.00) |
17.00ab (+3.03) |
20.00a (+17.65) |
5.00a (-0.00) |
3.00ab (-14.29) |
7.50bcd (-9.09) |
4.25c (-41.38) |
0.00c | 0.00b |
| Neem (2%) | 1.00d (-71.43) |
- | 10.50c (-4.55) |
- | 9.00cd (-53.85) |
- | 7.00bc (-20.00) |
- | 17.50a (+6.06) |
- | 3.50b (-30.0) |
- | 6.25cd (-24.24) |
- | 0.00c | - |
| BAU-Biofungicide (2%) |
1.00d (-71.43) |
1.00c (-66.67) |
3.00f (-72.73) |
3.00e (-76.92) |
5.00fg (-74.36) |
3.25f (-75.47) |
3.00e (-65.71) |
4.00ef (-66.67) |
13.50d (-18.18) |
12.25e (-27.94) |
2.00d (-60.0) |
2.00c (-42.86) |
2.00f (-75.76) |
1.50e (-79.31) |
7.50b | 6.50a |
| BAU-Biofungicide (3%) | 0.00e (-100.00) |
- | 3.00f (-72.73) |
- | 5.50efg (-71.79) |
- | 3.00e (-65.71) |
- | 11.00e (-33.33) |
- | 1.00e (-80.0) |
- | 2.25f (-72.73) |
- | 8.50a | - |
| Bavistin DF (0.1%) |
2.75b (-21.43) |
2.00b (-33.33) |
9.25d (-15.91) |
7.00d (-46.15) |
10.00c (-48.72) |
7.50c (-43.40) |
5.00d (-42.86) |
5.00de (-58.33) |
15.50bc (-6.06) |
14.00cd (-17.65) |
0.00f (-100.0) |
0.00e (-100.0) |
6.75bc (-18.18) |
5.25b (-27.59) |
0.00c | 0.00b |
| Bavistin DF (0.05%) |
3.25ab (-7.14) |
2.50ab (-16.67) |
10.00cd (-9.09) |
8.25c (-36.54) |
14.00b (-28.21) |
9.00b (-32.08) |
6.00cd (-31.43) |
5.50cd (-54.17) |
17.00ab (+3.03) |
15.00c (-11.76) |
2.25cd (-55.0) |
1.00d (-71.43) |
7.50ab (-9.09) |
6.50a (-10.34) |
0.00c | 0.00b |
| Potent 250 EC (0.1%) |
1.00d (-71.43) |
1.00c (-66.67) |
4.00ef (-63.64) |
3.00e (-76.92) |
4.00g (-79.49) |
2.25g (-83.02) |
2.00e (-77.14) |
3.50f (-70.83) |
14.00cd (-15.15) |
12.50de (-26.47) |
3.00bc (-40.0) |
2.00c (-42.86) |
4.75e (-42.42) |
3.25d (-55.17) |
0.00c | 0.00b |
| Potent 250 EC (0.05%) |
2.00c (-42.86) |
1.00c (-66.67) |
4.00ef (-63.64) |
4.00e (-69.23) |
4.50fg (-76.92) |
3.00fg (-77.36) |
3.00e (-65.71) |
4.25def (-64.58) |
15.50bc (-6.06) |
13.75cde (-19.12) |
3.25b (-35.0) |
2.25c (-35.71) |
5.50de (-33.33) |
4.00cd (-44.83) |
0.00c | 0.00b |
| Control (water) | 3.50a | 3.00a | 11.00a | 13.00a | 19.50a | 13.25a | 8.75a | 12.00a | 16.50ab | 17.00b | 5.00a | 3.50a | 8.25a | 7.25a | 0.00c | 0.00b |
Table 4: Effect of treatments on incidence of seed borne fungi of rice cv BRRI dhan40 in 2011 and 2012.
Figure 1: In-vitro evaluation of extracts of Garlic and Neem; BAU-Biofungicide, Bavistin and Potent against important pathogen of rice cv BRRI dhan40.
Discussion
Razu [25] reported that T. harzianum reduced the severity of narrow brown leaf spot disease compared to other bioagents. These findings were similar to the observation of the scientists [37]. Mahmud reported that BAU-Biofungicide and Tilt 250 EC were found to have excellent effect in controlling narrow brown leaf spot disease. Sheath blight of rice caused by R. solani was controlled by antagonist Trichoderma as observed [38]. These findings were correlated with the work of the researcher costa [39]. Spore suspension of T. harzianum was sprayed on the leaves and it significantly reduced disease severity and incidence as studied [40,41]. Mahmud and Hossain [37] reported that sheath blight of rice was controlled by Bavistin (0.1 and 0.05%). Application of garlic clove extract also marked reduction of disease severity of narrow brown leaf spot and sheath blight under field conditions as reported by the various researchers [25,37]. Gopalakrishnan and Valluvaparidasan [42] reported that significant reduction in severity of sheath rot disease was observed with biocontrol agents. Seven fungicides were tested against S. oryzae under field conditions, and Carbendazim 50% WP and Propiconazole 25 EC resulted in maximum reduction of sheath rot intensity and increased the grain yield [43]. The vibrant researchers Chen and Zhang [44] showed that two sprays of 50% propiconazole EC at 300 g a.i. ha-1 exhibited the best control of rice false smut [45]. The investigators also tested the efficacy of Trichoderma spp. compared with fungicides. They observed that bioagents showed fungicidal effect and reduced disease severity of false smut. Antagonistic effect of Trichoderma against C. oryzae was evaluatedin vitro test[32]. Mahmud observed the highest (59.03%) inhibition of mycelial growth. Similar works were also supported by the Manurung [46]. Significant reduction of mycelial growth in C. oryzae was found with Propiconazole (0.1%) as reported by the researchers Mahmud [32]. Mayo et al. [47]tested Trichoderma isolates for their potentiality to antagonize R. solani bydual culture and Trichoderma spp. over grew the pathogen, andobserved the inhibited growth of R. solani. Kalaiselvi and Panneerselvam [48] reported that T. harzianum was found to be most effective with 96% inhibition over control after 7 days of incubation by dual culture in S. oryzae. The authors Shamsi [49] reported that Bavistin at 300 ppm exhibited the profound effect in reducing radial growth of sheath rot pathogen. Kannahi [50] who evaluated the set of four isolates of Trichoderma spp. against U. virens by dual culture and Trichoderma species showed maximum antagonistic potential during incubation period. Various scientists Mahmud, Hossain and Ahmad [32] evaluated propiconazole against U. virens for its sensitivity in the stage of mycelial growth and observed significant inhibition. This finding was in accordance with the observation of scholars [44]. Razu [25] reported that foliar spray of T. harzianum showed the most effective in increasing grain yield of rice (20.25 to 23.13%). These findings were also in agreement with the observation of the authors mahmud and Hossain [37]. In this experiment, no significant result was noticed with the extract of neem leaf (A. indica L.) in reducing disease severity and increasing grain yield, while garlic 2% (A. sativum L.) showed good effect to increase grain yield which is supported by the razu [25]. Patel [51] reported that three species of Trichoderma showed antagonistic activity against A. flavus and observed the mycelial growth inhibition. Significant reduction of A. flavus was found with A. indica L of harvested seeds as reported by Mahmud [37]. The efficacy of T. harzianum against B. oryzae was tested and inhibitedthe highest growth (56.80%) of rice brown spot pathogen [32]. Farid [52] who evaluated four fungicides (Bavistin, Hinosan, Tilt 250 EC and Dithane M-45) against B. oryzae and highest mycelial growth inhibition was recorded by (95.58%) with Tilt 250 EC at 500 ppm. Garlic extracts were also effective in inhibiting the growth of B. oryzae at higher concentration [41]. Sarhan [53] also observed that T. harzianum inhibited the growth of C. lunata associated with rice seeds. The growth of C. lunata was evaluated with ethanol extract of A. sativum L. and significant reduction was obtained on test pathogen. Mahmud [37] reported that T. harzianum was effective in reducing seed borne infection of F. moniliforme. Various researchers Chowdhury Bashar, Shamsi [41] studied Bavistin against F. moniliforme in-vitro test and found significant inhibited growth. Trichoderma also inhibited the growth of seed borne pathogen (F. oxysporum) as reported by the investigators Mahmud [37]. Sempere [54] observed the fungal growth in dual cultures where T. harzianum inhibited pathogenic growth of N. oryzae. Trichoderma has the good potentialities to cause significant reduction of S. oryzae [32,37,49]. Similarly, extracts of garlic cloves (1 and 2%) inhibited the seed borne infection of S. oryzae [32,49]. Trichoderma harzianum was found to remain viable on the incubated seeds harvested from the plots sprayed with BAU-Biofungicide.
Conclusion
In this experiment, highest (25.93%) grain yield was increased in Propiconazole 250 EC (0.1%), while BAU-Biofungicide (3%) exhibited higher increase (22.20%) in yield over control. BAU-Biofungicide showed antagonistic activity and reduced disease severity in vitro test and field conditions, respectively. It also protects seed from seed borne pathogens for increasing yield and quality of rice by avoiding use of chemical that cause environmental pollution. The agriculture, facing various challenges is to increase productivity without ecological balance. Diseases of rice have to be given prioritized attention because of the importance of rice as staple food for majority of global population.
Acknowledgments
The author gratefully acknowledges the funding authority, Director of World Assembly of Muslim Youth, Dawah Program, Dhaka, Bangladesh.
References
- Jayaprakashvel M, Mathivanan N (2009) Biological control and its implications on rice diseases management. Research India Publications, pp: 440-455.
- USDA (2018) World agricultural production. United States Department of Agriculture, Foreign Agricultural Services, Circular Series. WAP 22: 4-18.
- Haq M, Mia MAT, Rabbi MF, Ali MA (2008) Incidence and severity of rice diseases and insect pests in relation to climate change. International Symposium on Climate Change and Food Security in South Asia, Dhaka, Bangladesh, pp: 1-37.
- Mondal D, Ghosh A, Roy D (2017) Yield loss assessment of rice (Oryza Sativa L.) due to different biotic stresses under system of rice intensification. J Entomology and Zoology Studies 5: 1974-1980.
- Zhou XZ, Uppala SS (2015) Field efficacy of new fungicides for management of narrow brown leaf spot of rice. Biology and Disease Management, Mycology. APS Annual Meeting, California, USA, p: 243.
- Groth D, Hollier C (2010) Narrow brown leaf spot of rice. Louisiana Plant Pathology: Identification and Management Series, LSU Ag Center, Baton Rouge.
- Kumar KVK, Reddy MS, Yellareddygari SKR (2011) Evaluation and selection of elite plant growth-promoting rhizobacteria for suppression of sheath blight of rice caused by rhizoctonia solani in a detached leaf bio-assay. Inter J Applied Biol and Pharmacy Technol 2: 488-495.
- Sakthivel N (2001) Sheath rot disease of rice: current status and control strategies. Major Fungal Diseases of Rice, pp: 271-283.
- Gopalakrishnan C, Kamalakannan A, Valluvaparidasan V (2010) Effect of seed-borne sarocladium oryzae, the incitant of rice sheath rot on rice seed quality. J Plant Prot Res 50: 98-102.
- Bigirimana Vincent de P, Gia Hua KH, Nyamangyoku OI, Hofte M (2015) Rice sheath rot: an emerging ubiquitous destructive disease complex. Front Plant Sci 6: 1066.
- Zhou YL, Pan YJ, Xie XW (2008) Genetic diversity of rice false smut fungus ustilaginoidea virens and its pronounced differentiation of populations in north china. Journal of Phytopathology 156: 559-554.
- Ashizawa T, Takahashi M, Moriwaki J, Hirayae K (2010) Quantification of the rice false smut pathogen from soil in Japan using real-time PCR. European Journal of Plant Pathology 128: 221-232.
- Ahonsi MO, Adeoti AA, Erinle ID, Alegbejo TA (2000) Effect of variety and sowing date on false smut incidence in upland rice in Edo State, Nigeria. IRRI Notes 25: 14.
- Li Y, Kang G, Zhang BJ, Zeng BD (1986) A preliminary study on false smut. Guangdong Agricultural Science 4: 45-47.
- Upadhyay A, Singh RV (2013) Yield loss assessment in rice due to false smut. Ann Plant Soil Res 15: 173-174.
- Fakir GA, Mia MAT (2004) The quality of farmer saved rice seeds in Bangladesh. National workshop on seed health improvement. BARC, Dhaka, Bangladesh.
- Kato HK, Ohata LP, Kauraw Lee YH (1998) Fungal disease of rice seeds, pp: 151-161.
- Akhter N, Begum MF, Alam S, Alam MS (2006) Inhibitory effect of different plant extracts, cow dung and cow urine on conidial germination of Bipolaris sorokiniana. Journal Bio Science 14: 87-92.
- Bdliya BS, Alkali G (2008) Efficacy of some plant extracts in the management of cercospora leaf spot of groundnut in the Sudan savanna of Nigeria. Archives of Phytopathology and Plant Protection, pp 1-12.
- Koike ST, Gaskell M, Fouche C (2011) Plant disease management for organic crops. Publication 7252. University of California, Division of Agriculture and Natural Resources, USA.
- Harman GE, Howell CR, Viterbo A (2004) Trichoderma species-opportunistic, avirulent plant symbionts. Nature Rev Microbiol 2: 43-56.
- Lorito M, Woo SL, Ruocco M (2006) In the molecular cross-talk between trichoderma, plants and pathogens provides new tools for disease control.
- Hossain I (2011) BAU-Biofungicide: unique eco-friendly means and new dimension of plant disease control in Bangladesh. Department of Plant Pathology, Bangladesh Agricultural University, Mymensingh.
- Hossain MM, Hossain I, Khalequzzaman KM (2015) Effect of seed treatment with biological control agent against Bipolaris leaf blight of wheat. International Journal of Scientific Research in Agricultural Sciences 2: 151-158.
- Razu MAU, Hossain I (2015) Eco-friendly management of rice diseases. Inter J Applied Sci Biotechnol 3: 80-88.
- Rasmy MR (1991) Studies on rice diseases in Egypt. PhD Thesis, Plant Pathology Department, Faculty Agriculture, Alexandria University, Egypt.
- Cumagun CJR (2012) Managing plant diseases and promoting sustainability and productivity with Trichoderma: The Philippine experience. J Agric Sci and Technol 14: 699-714.
- BRRI (2004) Modern rice cultivation. Bangladesh Rice Research Institute, Gazipur.
- IRRI (1996) Standard evaluation system of rice. International Rice Research Institute, Manila, Philippines.
- Liu MX, Oi S, Li Y, Wang S (2009) Study on isolation technique and culture condition of Ustilaginoidea virens. Lioning Agricultural Sciences 2: 20-22.
- ISTA (1996) International rules for seed testing. Seed Sci Technol 4: 3-49.
- Mahmud H, Hossain I, Ahmad MU (2017) In-vitro tests to determine the efficacy of plant extracts, BAU-biofungicide and fungicides on the inhibitory effects on some important rice pathogen. Current Research Journal of Biological Sciences 9: 36-43.
- Nene YL, Thaplial PN (1993) Fungicides in plant disease control. Oxford and IBH Publishing Co., New Delhi, India.
- Sundar AR, Das ND, Krishnaveni D (1995) In-vitro antagonism of Trichoderma spp. against two fungal pathogens of castor. Indian J Plant Protec 23: 152-155.
- Agarwal PC, Mortensen CM, Mathur SB (1989) Seed-borne diseases and seed health testing of rice, phytopathological Paper No. 30, CAB International Mycological Institute (CMI), Kew, Surrey, UK, pp: 58-59.
- Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley & Sons, New York, USA.
- Mahmud H, Hossain I (2017) Comparative efficacy of BAU-biofungicide and synthetic fungicides in management of diseases of rice (Oryza sativa L.) for quality seed production. Brazilian Journal of Botany 40: 389-397.
- Hasan MM, Ullah C, Nurunnabi M, Sagor SI, Ali MA (2010) Biological control of sheath blight of rice. J Envir Sci Natural Res 3: 161-164.
- Costa De DM, Samarasinghe SST, Dias HRD, Dissanayake DMN (2008) Control of Sheath blight by phyllosphere epiphytic microbial antagonists. Phytoparasitica 36: 52-65.
- Tewari L, Singh R (2005) Biological control of sheath blight of rice by trichoderma harzianum using different delivery systems. Indian Phytopathology 58: 35-40.
- Chowdhury P, Bashar MA, Shamsi S (2015) In vitro evaluation of fungicides and plant extracts against pathogenic fungi of two rice varieties. Bangladesh J Bot 24: 251-259.
- Gopalakrishnan C, Valluvaparidasan V (2006) Seed-borne biocontrol agents for the management of rice sheath rot caused by sarocladium oryzae (Sawada). J Biol Control 20: 197-204.
- Kindo A, Tiwari PK (2015) Efficacy of fungicides for the management of sheath rot disease in rice under field conditions. Plant Archives 15: 119-120.
- Chen Yu, Zhang Y, Yao J (2013) Frequency distribution of sensitivity of ustilaginoidea virens to four EBI fungicides, prochloraz, difenoconazole, propiconazole and tebuconazole, and their efficacy in controlling rice false smut in anhui province of china. Phytoparasitica 41: 277-284.
- El-Naggar MM, Elsharkawy MM, Almalla RA (2015) Control of ustilaginoidea virens, the causal agent of rice false smut disease in Egypt. Egyptian Journal of Biological Pest Control 25: 555-564.
- Manurung Ida R, Pinem MI, Lubis L (2014) Antagonism test of endophytic fungi against cercospora oryzae miyake and curvularia lunata (Wakk) boed. J Online Agroekoteknol 2: 1563-1571.
- Mayo S, Gutierrez S, Malmierca MG, Lorenzana A (2015) Influence of rhizoctonia solani and trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Frontier Plant Science 6: 1-11.
- Kalaiselvi S, Panneerselvam A (2015) In-vitro evaluation of fungicides and two species of trichoderma against sarocladium oryzae causing sheath rot of paddy (Oryza sativa L.). World J Pharmac Res 4: 1200-1206.
- Shamsi S, Chowdhury P (2016) In vitro evaluation of fungicides and some plant extracts against rice sheath rot pathogen sarocladium oryzae. Bangladesh J Sci Res 29: 47-54.
- Kannahi M, Dhivya S, Senthilkumar R (2016) Biological control on rice false smut disease using trichoderma species. Int J Pure App Biosci 4: 311-316.
- Patale SS, Mukadam DS (2011) Management of plant pathogenic fungi by using trichoderma species. Bioscience and Disease 2: 36-37.
- Farid A, Khalequzzaman KM, Islam N, Anam MK (2002) Effect of fungicides against bipolaris oryzae of rice under in vitro condition. Pakistan Journal of Plant Pathology 1: 4-7.
- Sarhan ART, Shibly MKA (2003) Biological control of pathogenic fungi associated with rice seeds. Arab J Plant Protect 21: 102-108.
- Sempere F, Santamarina MP (2008) Biological control of one species belonging to the dominant mycobiota of rice of Valencia. Annals Microbiology 58: 7-14.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences