ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
Piperonyl Butoxide: An Enhancing Arsenal for an Adomant Foe
Kweka EJ1,2*, Mazigo HD2, Mapunda G1 and Yewhalaw D3,4
1Division of Livestock and Human Diseases Vector Control, Tropical Pesticides Research Institute, Arusha, Tanzania
2Department of Medical Parasitology and Entomology, School of Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
3Tropical and Infectious Diseases Research Center, College of Public Health and Medical Sciences, Jimma University, Jimma, Ethiopia
4Department of Medical Laboratory Sciences and Pathology, College of Health Sciences, Jimma University, Jimma, Ethiopia
- *Corresponding Author:
- Kweka EJ
Division of Livestock and Human Diseases Vector Control
Tropical Pesticides Research Institute
Arusha, Tanzania
Tel: +255 754 368748
E-mail: kwekae@tpri.or.tz
Received Date: July 29, 2017; Accepted Date: August 28, 2017; Published Date: September 18, 2017
Citation: Kweka EJ, Mazigo HD, Mapunda G, Yewhalaw D (2017) Piperonyl Butoxide: An Enhancing Arsenal for an Adomant Foe. J Transm Dis Immun Vol.1 No.2:15
Abstract
Vector control using treated nets and recently industrially long lasting insecticidal nets (LLINs) have played a vital role in malaria control globally. The emergence of insecticides resistance among malaria vector against pyrethroids has raised a concern over achieved a milestone. Different insecticides resistance mechanisms have evolved against pyrethroids. This has given mosquitoes an opportunity to increase survivor fitness. The incorporation of synegistic effect of Piperonyl Butoxide (PBO) in LLINs has shown a significant impact in areas with pyrethroid resistant malaria vectors than LLIN without PBO. LLINs with PBO for management of resistance can revive pyrethroid strength against resistant malaria vectors. There is a necessity for National malaria control and other NGOs in Africa distributing LLINs to distribute nets with pyrethroids and PBO.
Keywords
Resistance; Anopheles gambiae; Malaria; Piperonyl butoxide; LLINs
Abbreviations
Indoor Residual Spray; LLINs: Long Lasting Insecticidal Nets; NGOs: Non-Governmental Organisations; PBO: Piperonyl Butoxide
Background
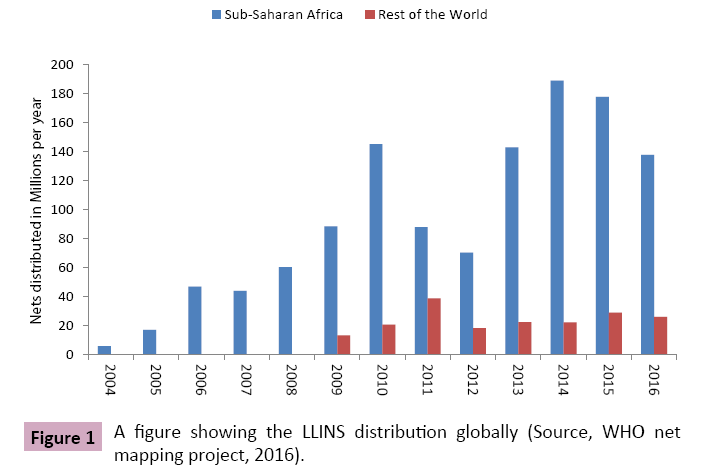
In most malaria endemic countries, long-lasting insecticidal nets (LLINs) are known to have an impact in malaria control by reducing mosquitoes survival fitness [1]. Malaria morbidity and mortality have declined from 1,500,000 to 480,000 per year from 2000 to date [2]. Malaria decline has been attributed to the use of LLINs distribution across Africa and rest of the world (Figure 1). Exposure of mosquitoes to LLINs and indoor residual spraying (IRS) insecticides significantly increased mortality for mosquitoes, women and children survorship [3]. Due to increased mortality, mosquitoes evolved mechanisms to inhence survivor for the fittest of forthcoming generations. Mosquitoes developed cuticular resistance to insecticides, Knockdown resistance through Sodium ions channel and metabolic resistance [4,5]. These mechanisms have led to increased tolerance of mosquitoes to pyrethroids insecticides [4,5]. The insecticides resistance selection pressure among vectors would increase as exposure to insecticides treated material is extended [6]. At present, LLINs are no longer showing the impact it has shown few decades ago in Africa where there has been reports of rapid decline of child mortality and mobidirty [7] due to mosquitoes insecticides resistance problem [8-11]. Changing of IRS compound have caused some resistant mosquitoes to be susceptible but with time they have built resistance [9,12-15]. Inseticides detoxification enzymes have been expressed in higher level and caused insecticides tolerance which increases the survivorship of mosquitoes and increase the disease risk [16]. There are also reports showing that, the efficacy of pyrethroid alone treated nets have declined drastically in areas with high pyrethroid resistant mosquito populations [17]. This may be a roadblock to sustain malaria control and initiate elimination by most malaria endemic African countries and transmission may not be interrupted despite large scale distribution of LLINs impregnated with pyrethroids alone [18].
The incorporation of a Synergistic effect of Piperonyl Butoxide (PBO) on roof and side panels of LLINs have improved the efficacy of LLINs against resistant mosquitoes [19-22]. It has a potency of increasing cuticular penetration of insecticides and increase mosquito susceptibility [23]. PBO has played a role in inhibiting esterases and P450s enzymes and their role in insecticide oxidative detoxification in insects [19]. LLINs with PBO have shown more efficacy against pyrethroid resistant mosquitoes than nets with pyrethroids only [21]. Metabolic resistant is widely spread in malaria vectors across sub-Saharan Africa and PBO could enhance the performance of LLINs by increasing the susceptibility status of the vectors to pyrethroids [21,22]. PBO works by inhibiting the activity of enzymes in insect body [24]. However, incorporation of PBO synegist in LLINs, has been reported to restore the efficacy of pyrethroids in the areas with high pyrethroid resistant population [21,22]. The LLINs with PBO have proved to be safe and no any side effect for human beings [25-27].
Currently, LLINs have an improved synergistic effect of PBO on top or on top and all side panels for managemenet of insecticide resistance [20,22].
What is the way forward
Malaria endemic areas government collectively should order their national malaria control programmes, donors and NGOs during campaigns for scaling up LLINs, its mandatory to use nets with PBO as synergist to pyrethroids either in one side or all sides of the net. Communities should be provided with names of available net brands with PBO as synergist for more awareness during purchasing. The information on benefits of PBO in nets should be clearly addressed.
Conclusion
Based on information gathered from different sources, the use of LLINs with PBO seems promising in increasing the susceptibility of malaria vectors and hence to revive malaria control elimination program in many malaria endemic sub-Saharan countries with pyrethroids based LLINs interventions.
Contributors
EJK conceived and drafted this commentary, HDM GM and DY revised critically and improved this commentary. All authors we have agreed upon its submission.
Acknowledgements
Authors thank all who in one way or another assisted the completion of this commentary
Ethics Committee Approval
This commentary was approved by Research and publication committee of Tropical Pesticides research Institute (TPRI)
References
- Lim SS, Fullman N, Stokes A, Ravishankar N, Masiye F, et al. (2011) Net Benefits: A Multicountry Analysis of Observational Data Examining Associations between Insecticide-Treated Mosquito Nets and Health Outcomes. PLOS Medicine 8:e1001091.
- Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, et al. (2015) The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526:207-211.
- Deribew A, Birhanu Z, Sena L, Dejene T, Reda AA, et al. (2012) The effect of household heads training about the use of treated bed nets on the burden of malaria and anaemia in under-five children: a cluster randomized trial in Ethiopia. Malaria Journal 11: 1-8.
- Balabanidou V, Kampouraki A, MacLean M, Blomquist GJ, Tittiger C, et al. (2016) Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proceedings of the National Academy of Sciences 113:9268-9273.
- Ibrahim SS, Ndula M, Riveron JM, Irving H, Wondji CS (2016) The P450 CYP6Z1 confers carbamate/pyrethroid cross-resistance in a major African malaria vector beside a novel carbamate-insensitive N485I acetylcholinesterase-1 mutation. Molecular Ecology 25:3436-3452.
- Ranson H, Lissenden N (2016) Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends in Parasitology 32: 187-196.
- D'Alessandro U, Olaleye B, Langerock P, Aikins M, Thomson M, et al. (1995) Mortality and morbidity from malaria in Gambian children after introduction of an impregnated bednet programme. The Lancet 345:479-483.
- Chang X, Zhong D, Lo E, Fang Q, Bonizzoni M, et al. (2016) Landscape genetic structure and evolutionary genetics of insecticide resistance gene mutations in Anopheles sinensis. Parasites & Vectors 9:228.
- Olé Sangba ML, Deketramete T, Wango SP, Kazanji M, Akogbeto M, et al. (2016) Insecticide resistance status of the Anopheles funestus population in Central African Republic: a challenge in the war. Parasites & Vectors 9:230.
- Lian L-Y, Widdowson P, McLaughlin LA, Paine MJI (2011) Biochemical Comparison of Anopheles gambiae and Human NADPH P450 Reductases Reveals Different 2′-5′-ADP and FMN Binding Traits. PLOS ONE 6:e20574.
- Oliver SV, Kaiser ML, Wood OR, Coetzee M, Rowland M, et al. (2010) Evaluation of the pyrrole insecticide chlorfenapyr against pyrethroid resistant and susceptible Anopheles funestus (Diptera: Culicidae). Tropical Medicine & International Health 15: 127-131.
- Matowo J, Jones CM, Kabula B, Ranson H, Steen K, et al. (2014) Genetic basis of pyrethroid resistance in a population of Anopheles arabiensis, the primary malaria vector in Lower Moshi, north-eastern Tanzania. Parasites & Vectors 7:274.
- Nkya TE, Poupardin R, Laporte F, Akhouayri I, Mosha F, et al. (2014) Impact of agriculture on the selection of insecticide resistance in the malaria vector Anopheles gambiae: a multigenerational study in controlled conditions. Parasites & Vectors 7:480.
- Olé Sangba ML, Sidick A, Govoetchan R, Dide-Agossou C, Ossè RA, et al. (2017) Evidence of multiple insecticide resistance mechanisms in Anopheles gambiae populations in Bangui, Central African Republic. Parasites & Vectors 10:23.
- Ossè RA, Aïkpon R, Gbédjissi GL, Gnanguenon V, Sèzonlin M, (2013) A shift from Indoor Residual Spraying (IRS) with bendiocarb to Long-Lasting Insecticidal (mosquito) Nets (LLINs) associated with changes in malaria transmission indicators in pyrethroid resistance areas in Benin. Parasites & vectors 6:73.
- Ishak IH, Riveron JM, Ibrahim SS, Stott R, Longbottom J, et al. (2016) The Cytochrome P450 gene CYP6P12 confers pyrethroid resistance in kdr-free Malaysian populations of the dengue vector Aedes albopictus. Scientific Reports 6:24707.
- Briët OJ, Penny MA, Hardy D, Awolola TS, Van Bortel W, et al. (2013) Effects of pyrethroid resistance on the cost effectiveness of a mass distribution of long-lasting insecticidal nets: a modelling study. Malaria Journal 12:77.
- Churcher TS, Lissenden N, Griffin JT, Worrall E, Ranson H (2016) The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 5:e16090.
- Demkovich M, Dana CE, Siegel JP, Berenbaum MR (2015) Effect of Piperonyl Butoxide on the Toxicity of Four Classes of Insecticides to Navel Orangeworm Amyelois transitella (Lepidoptera: Pyralidae). Journal of Economic Entomology 108:2753-2760.
- Bingham G, Strode C, Tran L, Khoa PT, Jamet HP (2011) Can piperonyl butoxide enhance the efficacy of pyrethroids against pyrethroid-resistant Aedes aegypti? Tropical Medicine & International Health 16:492-500.
- N’Guessan R, Asidi A, Boko P, Odjo A, Akogbeto M, et al. (2010) An experimental hut evaluation of PermaNet® 3.0, a deltamethrin–piperonyl butoxide combination net, against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in southern Benin. Transactions of the Royal Society of Tropical Medicine and Hygiene 104:758-765.
- Kweka EJ, Lyaruu LJ, Mahande AM (2017) Efficacy of PermaNet® 3.0 and PermaNet® 2.0 nets against laboratory-reared and wild Anopheles gambiae sensu lato populations in northern Tanzania. Infectious Diseases of Poverty 6:11.
- Motaâ€ÂÂSanchez D, Hollingworth RM, Grafius EJ, Moyer DD (2006) Resistance and crossâ€ÂÂresistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, Leptinotarsa decemlineata (Say)(Coleoptera: Chrysomelidae). Pest management science 62:30-37.
- Bingham G, Gunning RV, Gorman K, Field LM, Moores GD (2007) Temporal synergism by microencapsulation of piperonyl butoxide and αâ€ÂÂcypermethrin overcomes insecticide resistance in crop pests. Pest management science 63:276-281.
- Horton MK, Rundle A, Camann DE, Barr DB, Rauh VA, et al. (2011) Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics 127: e699-e706.
- Macedo PA, Schleier III JJ, Reed M, Kelley K, Goodman GW, et al. (2010) Evaluation of efficacy and human health risk of aerial ultra-low volume applications of pyrethrins and piperonyl butoxide for adult mosquito management in response to West Nile virus activity in Sacramento County, California. Journal of the American Mosquito Control Association 26:57-66.
- Beamand JA, Price RJ, Phillips JC, Butler WH, Jones GDG, et al. (1996) Lack of effect of piperonyl butoxide on unscheduled DNA synthesis in precision-cut human liver slices. Mutation Research/Genetic Toxicology 371:273-282.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences