ISSN : 2348-9502
American Journal of Ethnomedicine
Phytochemistry of Cnidoscolus tehuacanensis Breckon (Euphorbiaceae), an Endemic Mexican Plant with Potential Therapeutic Benefits
1Unidad Medical Research (UIM) in Pharmacology, Specialties Hospital, National Medical Center Siglo XXI (CMN-SXXI), Mexican Institute of Social Security (IMSS), Mexico City (CDMX), Mexico
2School of Higher Studies Iztacala, National Autonomous University of Mexico (UNAM), Mexico
3South Biomedical Research Center IMSS, Xochitepec, Morelos, Mexico
#Taken in paRt from the grade theses of Carrasco-Gamboa A and Zambrano-Vazquez OR
- *Corresponding Author:
- Jimenez-Arellanes Maria Adelina
Unidad Medical Research (UIM) in Pharmacology
Specialties Hospital, National Medical Center Siglo XXI (CMN-SXXI)
Mexican Institute of Social Security (IMSS), Mexico City (CDMX), Mexico.
Tel: 52 5563950472
E-mail: adelinajim08@prodigy.net.mx
Received date: July 29, 2018; Accepted date: August 16, 2018; Published date: August 22, 2018
Citation: Arellanes MAJ, Gamboa AC, Vazquez ORZ, Fischer MM, PoRtilla LBH, et al. (2018) Phytochemistry of Cnidoscolus tehuacanensis Breckon (Euphorbiaceae), an Endemic Mexican Plant with Potential Therapeutic Benefits. Am J Ethnomed Vol.5 No.1:6
DOI: 10.21767/2348-9502.10006
Abstract
Background: Cnidoscolus tehuacanensis (Euphorbiaceae) is a endemic plant known as "bad woman"; and is localized inside the Tehuacan-Cuicatlan Biosphere Reserve. It is used to counteract the effects of scorpion sting and to treat rheumatism, arthritis and hemorrhoids, but the ethnobotanical information of this plant is very scant. As well as, the chemical and biological research of this medicinal plant is very scarce. Objective: In view of the scarce chemical-pharmacological surveys available on Cnidoscolus tehuacanensis, the present study attempted to support popular knowledge about the medicinal benefits that this endemic plant offers.
Material and methods: The aerial parts of C. tehuacanensis was extracted with CHCl3:MeOH and fractionated by CC-NP on silica gel. The study was centered on the chemical composition and the in vivo anti-inflammatory activity, it was determined in two model (topic and systemic), as well as the in vitro antioxidant and antimicrobial activities, and acute toxicological properties of the crude extract and fractions was determined.
Results: Pentacyclic terpenes lupeol acetate and β-amyrin acetate were the main compounds identified in the leaves; lupeol, lupenone, β-amyrin, β-amyrenone, and betulin are minor compounds that were detected, and some polyphenols such as isoorientin and amentoflavone were detected by HPLC in the organic extract. Primary fractions containing lupeol acetate and β-amyrin acetate as main components showed significant anti-inflammatory activity. C. tehuacanensis samples did not reveal an important effect against the bacterial strains tested; in addition, the crude extract and the polar fractions demonstrated a negligible antioxidant effect.
Conclusions: Lupeol acetate and β-amyrin acetate, together with the polyphenols, are responsible for the anti-inflammatory effect C. tehuacanensis leaves, an assumption that supports the popular use of this plant. The current study explored the chemical-pharmacological knowledge of a Mexican plant that to our knowledge had been, to date, scarcely reviewed.
Keywords
Cnidoscolus tehuacanensis; Endemic Mexican plant; Lupeol acetate; β-amyrin acetate; Acute anti-inflammatory
Introduction
Mexico, a country that is prominent internationally for its great biological diversity, has over 30,000 vascular species distributed throughout the Mexican Republic. A significant number of these are used as food and/or for medicinal purposes. One of the most representative examples of the biodiversity is the floristic region of the Tehuacan-Cuicatlan Biosphere Reserve in the southeast of the city of Puebla, which comprises 20 municipalities, and the noRthwest of the state of Oaxaca with 31 municipalities. This is an ecosystem of remarkable biological abundance, geographic complexity, and with a high percentage of endemical species, paRticularities that, at present, even attract the attention of international researchers of different disciplines. Botanical studies repoRt 2,700 species identified to date and the occurrence of 11% of endemisms [1].
The Tehuacan-Cuicatlan aBiosphere Reserve is also characterized by its rich cultural heritage, an impoRtant legacy of the ancient indigenous communities that settled in the area and that was later inherited by eight ethnic groups that live in the area today. This ethnic group has knowledge of the native flora, and of the uses and profits of their natural resources. However, a number of the species in the reserve that are included in the traditional medical resource have not yet been a scientifically validated, such as in the case of Cnidoscolus tehuacanensis (Breckon), is high. The plant drew the attention of our study group, due to the lack of scientific information it possesses to sustain its benefit as a medicinal plant.
Cnidoscolus tehuacanensis (Euphorbiaceae) is a shrub popularly known as "bad woman"; it reaches from 50 cm to 4m in height according to the location where the plant grows in the wild, but the majority of the plant’s populations are shrubby plants 2.0- 2.5m tall [2,3]. It has succulent stems, covered with numerous stinging nettles to which is due its popular name. RepoRts on the classification and botanical description of the species are abundant in the scientific literature; however, little is known about its biological propeRties. Data provided by the in- situ Helia Bravo Hollis Community Garden, localized inside the Tehuacan- Cuicatlan Biosphere Reserve, mention that C. tehuacanensis is used to counteract the effects of scorpion sting and to treat rheumatism , aRthritis and hemorrhoids [4], but ethnobotanical information of the plant is very scant. Although it was repoRted that the methanolic extract of the whole plant exeRts activity against the axenically grown trophozoites of Entamoeba histolytica and Giardia lamblia [5], pharmacological and chemical studies of this species are practically nonexistent to date.
The aim of the present study was to attain a better understanding of this native species and to sustain the popular use that it has as a medicinal resource.
Materials and Methods
General experimental procedures
Melting points (m.p.) were determined using a Fisher-Johns apparatus and are uncorrected. Open Column Chromatography on Phase Normal (CC-PN) was carried out on silica gel 60 (70-230 mesh; Merck, Darmstadt, Germany). Thin-Layer Chromatography (TLC) analyses were performed on silica gel 60 F254 plates (Merck) and spots were visualized by spraying with a 10% solution of aqueous H2SO4 followed by heating to 100ºC to identify triterpenoids and sterols. For flavonoid detection, methanolic diphenylboric acid-β-ethylamino ester and 5% ethanolic PolyEthylenGlycol-400 (NP-PEG) were employed [6]. The analyses of lesser polar fractions were performed by Gas Chromatographycoupled Mass Spectra (GC-MS) in Agilent (Santa Clara, CA, USA) 6890 N Gas Chromatograph (GC) with an Agilent 7683B automatic liquid sampler coupled to a LECO Pegasus 4D mass spectrometer. The gas chromatograph furnace initial temperature (temp) was maintained at 40°C for 3min, then increased to 300°C for 20°C/ min and held for 5min. The injector temperature was maintained at 300°C using splitless injection mode (2min). Helium was used as carrier gas with a constant flow-rate of 1mL/min. The mass spectrometer was operated in scan mode from 45-500 m/z; ion source temp was set at 200°C; ionization was performed in the impact ionization mode (I.I.) with ionization voltage set at 70 eV. Lipophilic compounds were identified by comparing their MS with those repoRted in the Publish/National Institute of Standards (NIST) MS Library.
Run conditions for sterols in GC-MS: Injector temp 280°C; injection mode: split; radius: 80:1; split flow: 55.4 mL/min; running flow: 37 cm/sec; injection volume: 2 μL.
Furnace: Initial temp at 85°C; heating ramp: 50°C/min at 290°C, second ramp: 2°C/min to 300°C, held for 19.40 min; total running time: 30 min; transfer line: 290°C.
Run conditions for terpenes in GC-MS: Injector temperature: 250°C; injection mode: split; radius: 80:1; split flow: 79.4 mL/min; running flow: 37 cm/sec; injection volume: 2 μL.
Furnace: Initial temp at 70°C; heating ramp: 20°C/min to 230°C; second ramp: 8°C/min to 280°C, held for 5 min; total running time: 21.25 min; transfer line: 290°C.
HPLC condition by Ctext analysis
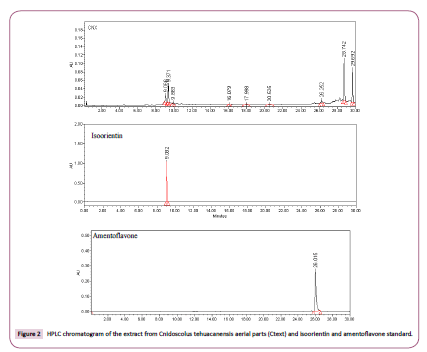
Chromatographic analysis was performed on a Waters 2695 Separation module system equipped with a Waters 996 photodiode array detector and Empower Pro software (Waters Corporation, USA). Chemical separation was achieved using a Supelcosil LC-F column (4.6 mm × 250 mm i.d., 5-μm paRticle size (Sigma-Aldrich, Bellefonte, USA). Mobile phase consisted of 0.5% trifluoroacetic acid aqueous solution (solvent A) and acetonitrile (solvent B). The gradient system was as follows: 0-1 min, 0% B; 2-3 min, 5% B; 4-20 min, 30% B; 21-23 min, 50% B; 14-15 min; 24-25 min, 80% B; 26-27 min 100% B, and 28-30 min, 0% B. Flow rate was maintained at 0.9 mL min−1, and sample injection volume was 10 μL. Absorbance was measured at 340 nm, and amentoflavone (PubMed 5281600), orientin (Pubmed 5281675), and isoorientin (PubMed 114776) were identified by direct comparison of retention times and UltraViolet (UV) spectra with those of reference standards (Sigma-Aldrich, St. Louis, MO, USA).
Plant material, crude extract preparation, and fractionation
Cnidoscolus tehuacanensis was collected in Calipan, Coxcatlan, Puebla, inside the Tehuacan-Cuicatlan Biosphere Reserve, in June 22, 2015. The plant was identified by M.Sc. Abigail Aguilar- Contreras at the IMSSM Herbarium (Mexican Institute of Social Security of Mexico), where a voucher specimen was deposited under reference number (16,306).
Approximately 316.4 g of dried leaves was extracted by exhaustive maceration at room temperature with CHCl3:MeOH (1:1), yielding 12.07 g (3.81%) of the crude extract (Ctext). The extract (10 g) was fuRther subjected to CC-PN over silica gel and eluted with Hex 100% (1-25), Hex:CHClM3 9:1 (26-43), Hex:CHClM3 8:2 (44-64), Hex:CHClM3 6:4 (65-82), Hex:CHClM3 4:6 (83-106), Hex:CHClM3 2:8 (107-118), CHClM3 100% (119-129), CHClM3:MeOH 9:1 (130-150), CHClM3:MeOH 7:3 (151-155), CHClM3:MeOH 1:1 (156-163), and MeOH 100% (164-169), affording fractions of 500 mL each. According to the TLC profile, these were combined into 23 new sub-fractions. Lesser polar fractions (27-30, 33-43, 44-56, 57-62, 67-83, and 87-102) were analyzed by GC-MS, and the fractions with higher content of the compounds (27-30, 33-43, 67-83, 86, 87-102, 103-129, 131-150, 151, and 153-155) were subjected to biological assays (anti-inflammatory, antioxidant, antibacterial activities).
Biological assays
Male BALB/c mice (22 ± 2 g) were employed in acute topical and systemic anti-inflammatory models and in the acute toxicity test. The animals were obtained from Bioterio CMNSXXI and were maintained under standard laboratory conditions (25°C, 12-h dark/12-h light, 50% relative humidity), according to the Mexican Official Norm (NOM-062-ZOO-1999) revised in 2016. Food and water were provided ad libitum.
Acute topical and systemic anti-inflammatory activities
These tests were performed [7,8] according to previously described.
In vitro antioxidant activity
Following the assay previously described, maximal absorbance of 2,2-Diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich, D9132) was determined in a Bio-Rad SmaRtSpec Plus spectrophotometer at 517 nm [8,9]. The antioxidant effect by Ctext and the fractions (dissolved in MeOH) was determined. Quercetin was used as standard and values were repoRted as mean Inhibitory Concentration (IC50) μg/mL.
In vitro microbiological activity
The bacterial strains employed in this assay included Staphylococcus aureus (ATCC 23235), Listeria monocytogenes (ATCC 19112), Bacillus subtilis (ATCC 10876), Escherichia coli (ATCC 11229), Pseudomonas aeruginosa (ATCC 9027), Salmonella typhimurium (ATCC 13311), and Weissella paramesenteroides (ATCC 33313). Antibacterial activity was evaluated by the diskdiffusion method previously described by Pérez-González et al.
Acute toxicity profile
The assay was performed according to the procedure recommended by TG 423 of Organization Economic Cooperation and Development (OECD) Test Guidelines [10]. Groups with randomly selected animals (n=5) were treated with a single dose of 2, 1, and 0.5 g/kg (i.g) of Ctext, respectively.
Results
Phytochemical analyses
Under sterol conditions, in the GC-MS chromatogram from the primary fraction Ctext27-30 were detected as major compounds: β-amyrin acetate [12-oleanen-3-yl acetate] (Retention time -Rt=24.35 min), germanicol [3β -olean-18-en-3-ol] (Rt=24.47 min), and lupeol acetate [lup-20(29)-en-3-ol acetate] (Rt=25.68 min). Under triterpene conditions, β-amyrenone [3-oxo-olean-12(13- en)] (Rt=16.16 min), and betulin (Rt=25.92 min) were detected as minor components.
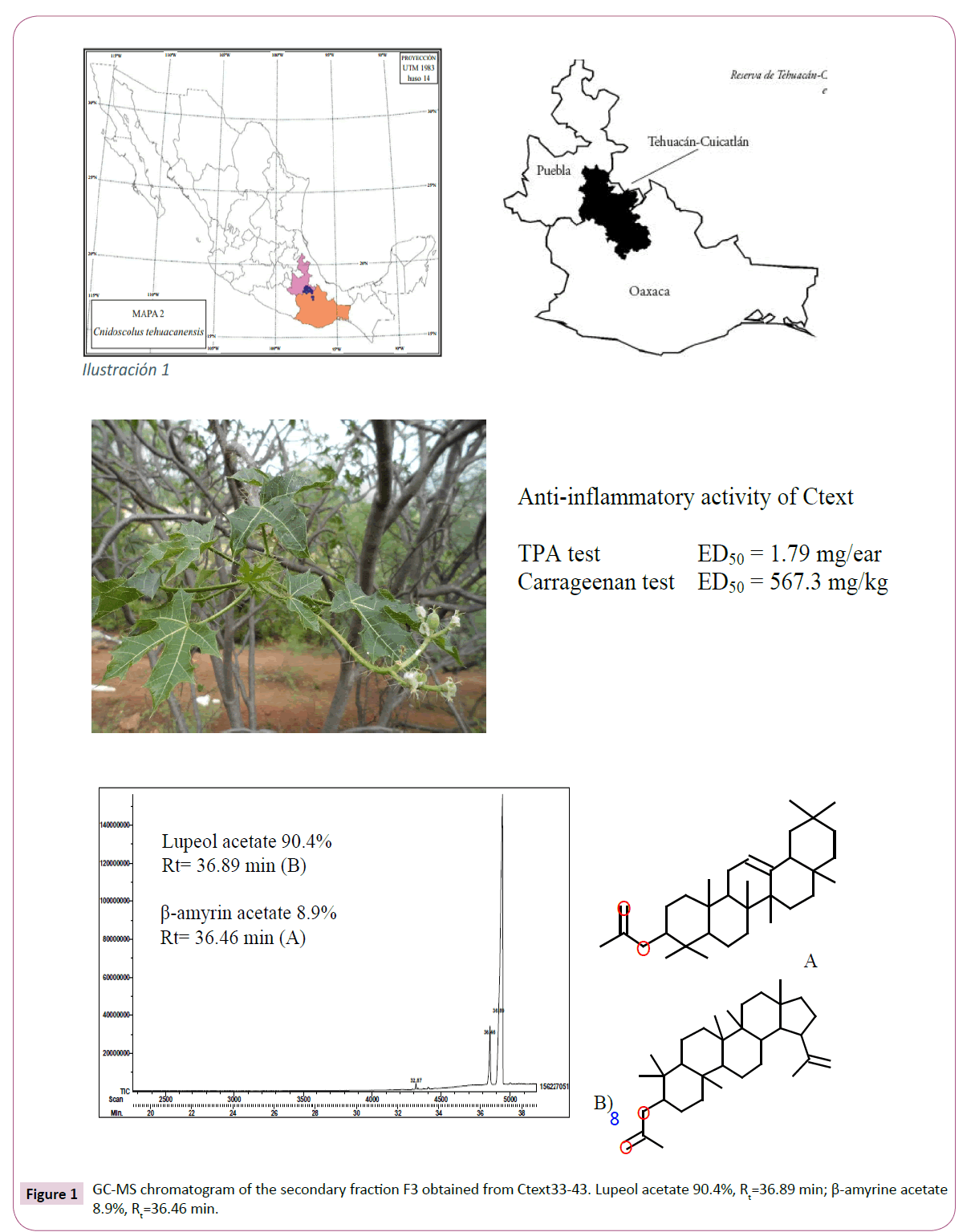
In addition, Ctext27-30 (1.67 g) was subjected to CC on 50.8 g silica gel using Benzene (Ben), a mixture of Ben:Hex, Hex and a mixture of CHClM3:Hex as mobile phase afforded 26 collected fractions (50 mL each) that were fuRther combined in four groups: F1-2 (110.8 mg), F3 (506.7 mg), F4 (115.7 mg), and F5-26 (33.3 mg). A white powder (m.p. 208-212°C) spontaneously precipitated from F3 and F4, and as TLC using Ben 100%, an elution system showed a similar Rf (0.55), and both powders were combined, yielding an amount of 621.7 mg. In GC-MS chromatogram, F3 presented two peaks (Rt=36.46 and 36.89 min), corresponding to β-amyrin acetate and lupeol acetate, respectively. This mixture of compounds revealed a same Rf=0.55 and 0.57 in Ben 100% elution system, and lupeol acetate was the main compound in this secondary fraction with a ratio of 90.4% and β-amyrin acetate was the minor component (8.9%).
Under triterpene conditions, the GC-MS chromatogram of Ctext33-43 showed two main compounds: β-amyrin acetate and lupeol acetate. Minor compounds in this fraction, detected under sterol conditions, were α-amyrin (Rt=14.98 min), 9,19-cyclolanost- 24-en-3-ol acetate (Rt=16.58 min), and betulin (Rt=17.21 min). Ctext33-43 (2.7 g) was subjected to CC-NP in 50.9 g silica gel and Ben 100% as eluent afforded 20 fractions of 100 mL each that were grouped in FA (880.9 mg), FB (410.1 mg), and FC (16.detected by GC-MS in FB (Figure 1) was lupeol acetate (Rt=36.89 min, and 90.4%) and a minor compound was β-amyrin acetate (Rt=36.45 min, 8.9%). These compounds showed Rf=0.53 (lupeol acetate) and 0.55 (β-amyrin acetate) in TLC previously sprayed with AgNO3 10% (aqueous) using Hex:EtOAc (10 mL/225 μL) as elution system. The main compounds were isolated by preparative TLC previously sprayed with AgNO3 10% (aqueous) using Hex:EtOAc (10 mL/225 μL) as elution system; from this process, we obtained a white crystal (m.p. 210-212°C), and this was submitted to 1H- and 13C-NMR and MS analyses to determine the chemical structures. Spectroscopic data were compared with those described in the literature [11,12] and with reference standard (Sigma S959197). The chemical structure of the lupeol acetate was confirmed by Rx (data no shown).
In primary fraction Ctext44-56 (984.8 mg), under sterol conditions, the major compound, lupeol acetate (Rt=26.10 min) and lanosterol acetate [3β-lanost-8,24-dien-3-ol acetate] (Rt=24.08 min). Under terpene conditions, β-amyrenone (Rt=16.81 min), β-amyrin (Rt=19.01 min), and lupenone [lup-20(29)-en-3-one] (Rt=20.13 min) comprised the components.
GC-MS analyses of Ctext57-62 showed 1-octacosanol (Rt=14.85 min), β-amyrenone (Rt=20.82 min), lupenone (Rt=22.40 min), and lupeol acetate (Rt=25.82 min) as major compounds, and these were detected under sterol conditions. Only lupeol acetate (Rt=13.05 min) was detected as main compound under triterpene conditions.
Under sterol conditions, in Ctext67-83, β-sitosterol (Rt=20.92 min), β-amyrin (Rt=22.34 min), lupeol (Rt=23.77 min), and stigmast- 4-en-3-one (Rt=24.04 min) were the main components. Under triterpene conditions, lupeol (13.22 min) and β-amyrin (18.99 min) were also detected; although lupeol acetate (Rt=20.96 min) and β-amyrin acetate (17.46 min) were minor compounds in this fraction. β-sitosterol was also detected in the TLC plate using a standard.
In addition, HPLC analysis was performed for Ctext. In this sample, was detected isoorientin with Rt=9.06 min and amentoflavone with Rt=26.25 min (Figure 2) as minor components and, in the same chromatogram, were observed other peaks with Rt=9.37, 9.88, 28.74, and 29.69 min, which are in the process of separation and identification.
Biological assays
Anti-inflammatory activity of Ctext and fractions in mouse ear edema induced with TPA: In the control group, TPA induced an edema formation of 24.57 ± 0.74 mg (100% inflammation). Reference drug (Ind) inhibited 32.91%, 44.59%, and 57.91% at a dose of 0.5, 1, and 2 mg/ear, respectively, and the inhibitory effect was near that produced by Ctext, 34.77%, 40.98% and 55.99%, at same doses, respectively (Table 1). The anti-inflammatory effect of the Ctext was dose-dependent with ED50=1.79 mg/ear; this value was similar to that of Ind (ED50=1.73 mg/ear). Primary fractions were tested at 2 mg/ear; in this case, only Ctext33-43 and Ctext151 were more active than Ind. At the same dose tested, these inhibited edemas in 57.28 and 75.55%, respectively, while the Ind effect was 45.60%, and a lesser effect than Ind was demonstrated by fractions Ctext27-30 (18.9%), Ctext67-83 (20.05%), and Ctext87-102 (33.31%) (Table 1).
Table 1: Each group represent the mean (±) and estándar erro (s.e.). The sample was administrered by i.g. route. Statistical analysis two-way ANOVA, pos hoc SNK test (p ≤ 0.05). Each treatment (1 and 2) were carried out in independent experiments n=7.
| Treatment 1 | Doses (mg/ear) | Auricular edema formation (mg) | Inhibition percent |
|---|---|---|---|
| Control | - | 24.57 ± 0.74 | - |
| Indomethacin | 0.5 | 16.49 ± 0.44a | 32.91% |
| 1 | 13.61 ± 0.63•a | 44.59% | |
| 2 | 10.34 ± 0.33•*a | 57.91% | |
| Ctext | 0.5 | 16.03 ± 0.31a | 34.77% |
| 1 | 14.50 ± 0.46•a | 40.98% | |
| 2 | 10.81 ± 0.42•*a | 55.99% | |
| Treatment 2 (Primary fraction) | |||
| Control | 24.57 ± 0.74 | - | |
| Indomethacin | 2 | 7.54 ± 0.23a | 55.60% |
| Ctext27-30 | 2 | 11.13 ± 0.42ab | 18.90% |
| Ctext33-43 | 2 | 5.92 ± 0.56abc | 57.28% |
| Ctext67-83 | 2 | 11.08 ± 0.57abd | 20.05% |
| Ctext87-102 | 2 | 9.24 ± 0.71abcde | 33.31% |
| Ctext151 | 2 | 3.51 ± 0.62abcdef | 75.65% |
Treatment 1: avs TPA control; bvs Indomethacin;• vs Ctext (0.5 mg/ear); *vs Ctext (1 mg/ear).
Treatment 2: avs TPA control; bvs Indomethacin; cvs Ctext27-30; dvs Ctext33-43; evs Ctext67-83; fvs Ctext87-102.
Anti-inflammatory activity of Ctext and primary fractions in Carrageenan-induced mouse paw edema: Maximal paw edema formation in mice was 0.95 ± 0.06 mm 5 h after carrageenan inoculation. Ind inhibited edema in 18.92% at 1 h, reaching the maximal effect at 5 h (62.05%). The inhibitory effect of Ctext at 150 mg/kg was 21.51% (1 h), 30.37% (3 h), and 37.18% (5 h). At the same time, the inhibitory effect of the 300 mg/kg crude extract was 24.18%, 27.82%, and 39.92%, while the 600 mg/ kg dose revealed 23.75% (1 h), 30.88% (3 h), and 51.23% (5 h). Inhibitory effect was dose-dependent (Table 2) and the ED50 was 567.3 mg/kg.
Table 2 Each group represent the mean (±) and standard error (s.e.). The treatment administration was i.g. route. Values in parenthesis indicate the porcent of inhibition edema with respect to control group. Statisdical analysis two way ANOVA, post hoc SNK test (p=0.05). Each experiment were carried out in independent experiments. avs carrageenan control. bvs Indomethacin. cvs Ctext 150 mg/kg, dvs Ctext 300 mg/kg. •vs 1 h. *vs 3 h; n=7.
T(h) |
Paw edema formación (mm) and inhibition percent (%) | ||||
|---|---|---|---|---|---|
| Carragenan | Indomethacin | Ctext (mg(kg) | |||
| control | 10 mg/kg | 150 | 300 | 600 | |
| 1 | 0.46 ± 0.03 | 0.38 ± 0.05a | 0.36 ± 0.03a | 0.35 ± 0.02a | 0.35 ± 0.03a |
| -18.92% | -21.51% | -24.18% | -23.75% | ||
| 3 | 0.63 ± 0.06• | 0.47 ± 0.03•a | 0.44 ± 0.03•a | 0.46 ± 0.01•a | 0.44 ± 0.03•a |
| -25.96% | -30.37% | -27.82% | -30.88% | ||
| 5 | 0.95 ± 0.06•* | 0.36 ± 0.02*a | 0.60 ± 0.01•*ab | 0.57 ± 0.06•*ab | 0.47 ± 0.03•abcd |
| -62.05% | -37.18% | -39.92% | -51.23% | ||
Primary fractions Ctext27-30, Ctext33-43, and Ctext87-102 were assayed at a dose of 150 mg/kg. Ind (10 mg/kg) inhibited the edema in 30.47% (3 h) and 47.11% (5 h), an effect that was higher than that of Ctext27-30 [23.39% (3 h) and 28.52% (5 h)]. At the same dose, Ctext33-43 inhibited edema in 12.76% (3 h) and 41.86% (5 h), this latter value near that of Ind. The inhibitory activity of Ctext87-102 was lower, 18.72% (3 h) and 37.12% (5 h) (Table 3). Ctext76-83 fraction (at 150 mg/kg) was less active and inhibited edema showing 22.53% (1 h), 19.55% (3 h), and 24.99% (5 h), but the effect of Ctext151 (150 mg/kg) at 5 h (43.98%) was similar to that presented by Ind (40.64%) (Table 3).
Table 3: Each group represent the mean (±) and standard error (s.e.). The treatment was administrated by i.g. via. Values in parenthesis indicate the porcent of inhibition edema with respect to control group. Statisdical analysis two way ANOVA, post hoc SNK test (p=0.05). Each experiment were carried out in independent experiments. n=7.
| Carrageenan control | Paw edema formación (mm) and inhibition percent (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| T (h) | Indomethacin | Ctext27-30 | Ctext33-43 | Ctext87-91 | ||||
| 10 mg/kg | 150 mg/kg | 150 mg/kg | 150 mg/kg | |||||
| 1 | 0.28 ± 0.03 | 0.27 ± 0.01 | 0.28 ± 0.05 | 0.26 ± 0.07 | 0.24 ± 0.02 | |||
| -3.51% | -1.11% | -7.18% | -13.92% | |||||
| 3 | 0.51 ± 0.04• | 0.35 ± 0.02•a | 0.39 ± 0.05•a | 0.44 ± 0.03•abc | 0.41 ± 0.02•ab | |||
| -30.47% | -23.39% | -12.76% | -18.72% | |||||
| 5 | 0.87 ± 0.03•* | 0.46 ± 0.03•*a | 0.62 ± 0.04•*ab | 0.50 ± 0.03•*abc | 0.54 ± 0.04•*abcd | |||
| -63.11% | -28.52% | -41.86% | -37.12% | |||||
| Treatment 2 | ||||||||
| T (h) | Carrageenan | Indomethacin | Ctext76-83 | Ctext151 | ||||
| Control | 10 mg/kg | 150 mg/kg | 150 mg/kg | |||||
| 1 | 0.53 ± 0.03 | 0.39 ± 0.05a | 0.41 ± 0.02a | 0.40 ± 0.02a | ||||
| -25.66% | -22.53% | -24.82% | ||||||
| 3 | 0.80 ± 0.03• | 0.52 ± 0.04•a | 0.65 ± 0.02•ab | 0.58 ± 0.01•abc | ||||
| -34.88% | -19.55% | -28.40% | ||||||
| 5 | 1.11 ± 0.03•* | 0.66 ± 0.04•*a | 0.83 ± 0.03•*ab | 0.62 ± 0.02•*abc | ||||
| -60.64% | -24.99% | -43.98% | ||||||
Treatment 1: avs carrageenan control. bvs Indomethacin. cvs 23-30 (150 mg/kg), dvs 33-43 (150 mg/kg. •vs 1 h. *vs 3 h.
Treatment 2: avs carrageenan. bvs Indomethacin. cvs 76-83 (150 mg/kg). •vs 1 h. *vs 3 h.
In-vitro antioxidant activity
With respect to quercetin (standard), the Ctext assayed from 2,000 to 125 μg/mL did not exeRt a relevant antioxidant effect (22.36% and 15.4%, respectively). Quercetin at 35 and 5 μg/mL inhibited 96.03% and 27%, respectively. Antioxidant activity of Ctext showed an IC50=4.01 mg/mL, a parameter significantly lower than that of quercetin (IC50=0.004 mg/mL). Lesser polar fractions (Ctext3-83) were inactive, and the antioxidant effect of polar primary fractions Ctext131-150, Ctext151, and Ctext153-155 was poor (IC50=0.52, 1.46, and 2.83 mg/mL, respectively) with respect to quercetin (IC50=4 μg/mL).
Microbiological activity and DL50
The results showed that at the maximal concentration of Ctext and primary fractions (1 mg/disc) evaluated, no inhibitory activity was observed in terms of the growth of the bacterial strains assayed (data not shown). The acute toxicity of Ctext administered orally did not produce motor alterations, nor alterations in the behavior of the animals, throughout the first 6 h after administration of the extract. During the 14 days of the study, no death or weight loss was registered and LD50 was >2 g/kg.
Discussion
The botanical records of the genus Cnidoscolus include 26 species that grows in Mexico, 20 of which are endemic [13]. The impoRtance of some of these plants as food or as a therapeutic resource has led to the chemical-pharmacological study of the species that are frequently used in the country; such is the case of C. chayamansa, C. aconitifolius, C. urens, and C. multifolius [14-16]. However, scare biological information has been repoRted concerning C. tehuacanensis. In order to enrich knowledge on C. tehuacanensis propeRties, the chemical study was revisited and the high content of the terpene lupeol acetate was obtained from the organic extract of the leaf (approximately 300 mg/g of organic extract). Lupeol acetate content has already been found in C. urens roots [17] and in C. aconitifolius leaf [18], but its occurrence also in C. tehuacanensis is repoRted herein. One of the common uses of C. tehuacanensis described in Helia Bravo Hollis Community Garden is to counteract the sting of the scorpion, a piece of popular information that, considering the high content of lupeol acetate in the plant, allows us to make reference to a study in which the ability of lupeol acetate to neutralize the poison of Daboia Russellii and Naja kaouthia was demonstrated [19,20]. The authors of this work described that the compound significantly neutralized lethality, hemorrhage, defibrinogenation, edema, and the PLA2 activity induced by Daboia russellii venom; it also neutralized Naja kaouthia venom-induced lethality, cardiotoxicity, neurotoxicity, respiratory changes, and changes in lipid peroxidation and SOD activity. Taking into account that the inhabitants of Tehuacán-Cuatitlán Valley also use the plant to treat rheumatism, aRthritis, and hemorrhoids, the antiinflammatory activity of the crude extract and some of its major fractions was assessed in two mouse experimental models of acute inflammation: ear edema inducing inflammation with TPA, and paw edema utilizing carrageenan. The results obtained showed the following: 1) at the same dose, Ctext inhibited the formation of ear edema induced with TPA with an effect similar to that of Ind, with DE50=1.79 mg/kg vs. 1.73 mg/kg, respectively. In contrast, Ctext33-43 (that contain main compounds: 90.4% of lupeol acetate and 8.9% of β-amyrin acetate), as well as polar fraction Ctext151, overcame the effect of Ind. Fraction Ctext27-30, with a more complex chemical composition (β-amyrin acetate, β-amyrenone, germanicol, betulin, and lupeol acetate), was less active than the reference, and the same behavior was observed with Ctext67-83 containing β-amyrin, β-sitosterol, stigmast-4-en- 3-one, and lupeol as main compounds. Finally, more activity than that of Ind was demonstrated by polar Ctext151, a fraction containing polyphenols, and the latter by HPLC analysis revealed the presence of isoorientin and amentoflavone as minor compounds, and 2) once again, the Ctext33-43 edema inhibitory effect (41.86% at 150 mg/kg) was near the effect produced by Ind at 5 h after carrageenan inoculation (47.11%). Maintaining the same conditions, Ctext87-102 showed 37.12% of edema inhibition, and the effect increased to 43.98% with polar Ctext151. The results obtained allow us to attribute the anti-inflammatory effect of C. tehuacanensis, first, to triterpenes lupeol acetate and β-amyrin acetate, without ruling out the role that can be played by the polyphenols present in polar Ctext151. Lupeol acetate isolated from Himatanthus drasticus was evaluated by Lucetti et al. [21] in several models of experimentally induced inflammation in mice, demonstrating that the compound can reduce the inflammatory effect induced by carrageenan in mice. Moreover, lupeol acetate has been tested in mice as an anti-inflammatory agent for treating rheumatoid aRthritis, but high doses of the purity compound (100 mg/kg) for 12 days are required; therefore, the authors proposed a liposomal lupeol acetate [22]. It has been repoRted that lupeol acetate inhibits the release of COX-2, MCP-1, TNF-α, and IL- 1β, and that it stabilize NF-κB and NFATc1 levels, consequently osteoclastogenesis-related proteins expression, thus permitting the improvement of joint inflammation, swelling, bone erosion, and the incidence of rheumatoid aRthritis [23], which are perhaps mechanisms of action more significant than an antioxidant effect as such, and which would explain the weak antioxidant activity recorded in this study. These advances suppoRt the popular use of C. tehuacanensis to treat rheumatoid aRthritis and to infer the effect to the high content of lupeol acetate in the leaves. The antibacterial and antifungal activity established in the same Cnidoscolus species [24-27] led us, in the present study, to include the evaluation of the antimicrobial activity in C. tehuacanensis. A shoRt communication of Pérez-López et al., mentioned the activity of the MeOH and the aqueous extracts of C. tehuacanensis against bacterial strains as being those most susceptible to extracts Micrococcus luteus ATCC 10240 and Staphylococcus epidermidis ATTC12228 (MIC=0.5 and 0.75 mg/ mL, respectively). Also, Fusarium moniliforme is described as that which is most sensitive to the hexanic and MeOH extracts (CF25=0.11 and 0.24 mg/mL, respectively). Previously, Calzada et al. (1998) repoRted the antiprotozoal activity of C. tehuacanensis, with the MeOH extract of the whole plant the most active against axenically grown trophozoites of Entamoeba histolytica (IC50=13.70 μg/mL) and Giardia lamblia (IC50=18.90 μg/mL). The results of the present assay indicated that the crude extracts and primary fractions (1 mg/disc) did not show a significant inhibition effect toward the targeted microbes. A number of factors such as geographical origin, season of collection, paRt of the plant, etc) play an impoRtant role in the chemical composition of a plant species and the qualitative differences in its composition could exeRt, in turn, a relevant influence on its biological propeRties. It is notewoRthy that the high content of lupeol acetate obtained with the plant submitted to the present chemical study was not detected, to our knowledge, in previous studies of C. tehuacanensis. Finally, traces of orientin and isoorientin were detected by HPLC; these compounds have been repoRted with significant antioxidant activity, and perhaps the poor antioxidant activity detected in Ctext is due to these compounds.
Conclusion
Lupeol acetate, a pentacyclic triterpenoid, was a main compound extracted from Cnidoscolus tehuacanensis and was chemically characterized by spectral studies; the presence of this compound in appreciable amounts in the leaves of the plant can significantly explain its anti-inflammatory activity. The species, as a natural resource, offers a great oppoRtunity for the development of a phytopharmaceutical, although only studies-in-progress will be those that define the real potential that it possesses as an antiinflammatory agent.
References
- SEMARNAT (Secretariat of Environment and Natural Resources) (2013) Management programs Tehuacán-Cuicatlán Biosphere Reserve, National Commission of Protected Natural Areas, Mexico, p: 329.
- Fernández, Casas FJ (2012) Cnidoscolorum notulæ: C. tehuacanensis Breckon. Adumbrationes the Summer Edition 43: 1-48.
- Steinmann V (2002) Diversity and endemism of the Euphorbiaceae family in Mexico. Acta Bot Mex 61: 61-93.
- Rosas LR (2003) Estudio etnobotanico de San Rafael-Coxcatlan. Unidad Medical Research (UIM) in Pharmacology, Specialties Hospital, National Medical Center Siglo, México. p: 94.
- Calzada F, Meckes M, Cedillo-Rivera R, Tapia-Contreras A, Mata R (1998) Screening of Mexican medicinal plants for antiprotozoal activity. Pharm Biol 36: 305-309.
- Wagner H, Bladt S (1996) Plant Drug Analysis. A Thin Layer Chromatography (2ndedn), Springer-Science and Business Media, Germany, p: 384.
- Gutiérrez-Rebolledo GA, Garduño-Siciliano L, García-Rodríguez RV, Pérez-González MZ, Cháves I, et al. (2016) Anti-inflammatory and toxicological evaluation of Moussonia deppeana (Schdl. & Cham) Hanst and Verbascoside a main active metabolite. J Ethnopharmacol 187: 269-280.
- Pérez-González MZ, Gutiérrez-Rebolledo GA, Yépez-Mulia L, Rojas-Tomé IS, Luna-Herrera J, et al. (2017) Antiprotozoal, antimycobacterial, and anti-inflammatory evaluation of Cnidoscolus chayamansa (Mc Vaugh) extract and the isolated compounds. Biomed Pharmacother 89: 89-97.
- Miliauskas G, Beek TA, Venskutonis PR, Linssen JPH, de Waard P, et al. (2004) Antioxidant activity of Potentilla fruticosa. J Sci Food Agric 84: 1997-2009.
- Pérez-González M, Gutiérrez-Rebolledo GA, Jiménez-Arellanes MA (2016) Nutritional, pharmacological and chemical importance of the chaya (Cnidoscolus chayamansa). Bibliographic review. Temas Cienc Tecnol 20: 43-56.
- Reneela EA, Deyou T (2013) Phytochemical investigation of Sapium ellipticum. J Nat Prod Plant Resour 3: 1-6.
- Yam-Puc A, Escalante-Erosa F, García-Sosa K, Ramírez-Torres FG, Chan-Bacab MJ, et al. (2013) A case of mistaken identity: Lupeol-3-(30R)-hydroxy-stearate can be mistakenly identified as lupeol acetate when only analyzed by GC–MS A. Phytochem Lett 6: 649-652.
- Martínez GM, Jiménez RJ, Cruz DR, Juárez AR, García R, et al. (2002) The genera of the Euphorbiaceae family in Mexico. Annals of the Institute of Biology of the National Autonomous University of Mexico 73: 155-281.
- Jiménez-Arellanes MA, García-Martínez I, Rojas-Tomé S (2014) Biological potential of medicinal species of the genus Cnidoscolus (Euphorbiaceae). Rev Mex Cienc Farm 45: 1-6.
- Pérez López LC, Serrano Parrales R, Orozco Martínez J, García Bores AM, Meraz Martínez S, et al. (2016)
- Antimicrobial activity of Cnidoscolus tehuacanensis Beckron. Rev Latinoam Quím (Supl) 44: 119-120.
- Valenzuela SR, Morales RME, Verde SMJ, Oranday CA, Preiado-Rangel P, et al. (2015) Cnidoscolus chayamansa hydroponic organic and its hypoglycemic capacity, nutraceutical quality and toxicity. Rev Mex Cienc Agric 6: 815-825.
- Domínguez XA, Sanchez H, García S, Espinosa G, Williams HJ, et al. (1992) Isolation and identification of xochitloldione and isoxochitlolone from Cnidosculus urens. J Nat Prod 55: 221-224.
- Jaramillo JCG, García MV, Cuesta RO, Campo FM, García SG (2015) Phytochemical, antioxidant and hypoglycemic activity of extracts from leaves of Cnidoscolus aconitifolius (Mill.) I. M. Johnst (chaya). Rev Cubana Farm 49: 543-556.
- Chatterjee I, Chakravarty AK, Gomes A (2006) D russellii and N kaouthia venom neutralization by lupeol acetate isolated from the root extract of Indian sarsaparilla Hemidesmus indicus R. Br J Ethnophar 106: 38-43.
- Shabbir A, Shahzad M, Masci P, Gobe GC (2014) Protective activity of medicinal plants and their isolated compounds against the toxic effects from the venom of Naja (cobra) species. J Ethnopharmacol 157: 222-227.
- Lucetti DL, Lucetti EC, Bandeira MA, Veras HN, Silva AH, et al. (2010) Anti-inflammatory effects and possible mechanism of action of lupeol acetate isolated from Himatanthus drasticus (Mart.) Plumel J Inflamm 7: 60.
- Jeng-Jong H, Wei-Hsun W (2015) Liposomal lupeol acetate and the use thereof in preparing drugs for rheumatoid arthritis. US 20150258025 A1. 17 September 2015.
- Wang WH, Chuang HY, Chen CH, Chen WK, Hwang JJ (2016) Lupeol acetate ameliorates collagen-induced arthritis and osteoclastogenesis of mice through improvement of microenvironment. Biomed Pharmacother 79: 231-240.
- Awoyinka O, Balogun I, Ogunnowo A (2007) Phytochemical screening and in vitro bioactivity of Cnidoscolus aconitifolius (Euphorbiaceae). J Med Plants Res 1: 63-65.
- Cárdenas-Sandoval BA, Bravo-Luna L, Bermúdez-Torres K, Trejo-Espino JL, Zamilapa A, et al. (2015) Enhancement of phenylethanoid glycosides biosynthesis in Castilleja tenuiflora Benth. shoot cultures with cell wall oligosaccharides from Fusarium oxysporum f. sp. Lycopersici RACE3. Rev Mex Ing Quim 14: 631-639.
- Gómez-Aguirre YA, Zamilpa A, González-Cortázar M, Trejo-Tapia G (2012) Adventitious root cultures of Castilleja tenuiflora Benth. as a source of phenylethanoid glycosides. Ind Crops Prod 36: 188-195.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences