ISSN : ISSN: 2576-1412
Journal of Applied Microbiology and Biochemistry
Phytochemistry and Hepatoprotective Potential of Wood Apple against Paracetamol Induced Hepatotoxicity in Albino Rats
Arshed Iqbal Dar*
Department of Zoology, University of Islamia College of Science and Commerce, Srinagar, India
- *Corresponding Author:
- Arshed Iqbal Dar, Department of Zoology, University of Islamia College of Science and Commerce, Srinagar, India Tel: 6005222540; E-mail: drarshediqbal@gmail.com
Received date: April 23, 2022, Manuscript No. IPJAMB-22-12790; Editor assigned date: April 25, 2022, PreQC No. IPJAMB-22-12790 (PQ); Reviewed date: May 09, 2022, QC No. IPJAMB-22-12790; Revised date: June 23, 2022, Manuscript No. IPJAMB-22-12790 (R); Published date: July 01, 2022, DOI: 10.36648/2576-1412.6.7.034
Citation: Dar AI (2022) Phytochemistry and Hepatoprotective Potential of Wood Apple against Paracetamol Induced Hepatotoxicity in Albino Rats. J Appl Microbiol Biochem Vol:6 No:7
Abstract
Liver is a major organ system involved in the metabolism of various drugs, xenobiotics and toxins. During the metabolism, excessive free radicals are generated and may cause liver damage. Therefore, damage to the liver inflicted by hepatotoxic substances is of grave consequences. Toxicity from paracetamol is not from the drug itself but from one of its metabolites, N-Acetyl-P-Benzoquinoneimine (NAPQI). In overdoses, large amounts of paracetamol are metabolized by oxidation because of saturation of the sulphate conjugation pathway, but once the protective intracellular glutathione stores are depleted, hepatic damage may ensue. Since the modern system of medicine is known for inducing liver damage as a part of side effects, a hepatoprotectant of herbal origin can be considered as a useful, safe and effective co-supplement to minimize the mentioned manifestations. In this background Feronia limonia L. commonly known as wood apple was assessed for hepatoprotective activity against paracetamol as hepatotoxin. The results obtained from the experimental bioassay portrays that the damage of liver due to paracetamol over dosage was confirmed by elevated levels of various biochemical parameters viz. AST, ALT, ALP, Serum bilirubin, total cholesterol, triglycerides etc. Oral administration of various doses of the purified fraction of wood apple resulted in gradual normalization of the activities of AST, ALT, ALP, Serum bilirubin, total cholesterol and triglycerides. This evidently suggests the protective effect of the extracts in improving the functional integrity of liver cells. From our results we may infer that the mode of action of purified fraction of wood apple in affording the hepatoprotective activity against paracetamol induced hepatotoxicity may be due to the cell membrane stabilization, hepatic cell regeneration and normalizing the serum parameters. Hence the present study justified the traditional uses of Feronia limonia L. in the treatment of liver disorders. Thus in future this herbal extract may be used as strong constituent for the formulation of hepatoprotective herbal drug.
Keywords
N-Acetyl-P-Benzoquinoneimine (NAPQI), Hepatoprotective, Paracetamol, Feronia limonia L
Introduction
Herbal medicines are being increasingly utilized to treat a wide variety of diseases, though the knowledge about their mode of action is relatively scanty. So there is a growing interest regarding the pharmacological evaluation of various plants used in traditional system of medicine. Many diseases (atherosclerosis, diabetes mellitus, asthma, nephritis, liver diseases) are due to the specific organ damage. The organ damage may be due to the excessive generation of free radicals [1]. Several drugs from natural sources are known to scavenge off such free radicals and prevent the organ damage [2]. Liver is a major organ system involved in the metabolism of various drugs, xenobiotics and toxins. During the metabolism, excessive free radicals are generated and may cause liver damage. In spite of tremendous strides in modern medicine, there are hardly any drugs that stimulate liver function, offer protection to the liver from damage or help regeneration of hepatic cell [3]. There are however, number of drugs employed in traditional system of medicine for liver afflictions [4]. Therefore drugs from natural source are being adopted to treat hepatitis/liver diseases. The use of plants, plant extracts or pure compounds isolated from natural products to treat diseases is a therapeutic modality, which has stood the test of time even if much of the science behind such therapy is still in its infancy. There has been a resurgence of scientific interest in medicinal plants during the past 20 years, being rekindled by the worldwide importance of medicinal plants and crude drugs in traditional medicine.
Liver is the largest glandular organ of the body. It weighs about 3 lb (1.36 kg.). It is reddish brown in color and is divided into four lobes of unequal size and shape. The liver lies on the right side of the abdominal cavity beneath the diaphragm. Blood is carried to the liver via two large vessels called the hepatic and the portal vein. The hepatic artery carries oxygen-rich blood from the aorta (a major vessel in the heart). The portal vein carries blood containing digested food from the small intestine. These blood vessels subdivide in the liver repeatedly, terminating in very small capillaries. Each capillary leads to a lobule. Liver tissue is composed of thousands of lobules, and each lobule is made up of hepatic cells, the basic metabolic cells of the liver. Liver is the most important organ, which plays a pivotal role in regulating various physiological processes in the body. It is involved in several vital functions, such as metabolism, secretion and storage viz. to produce substances that break down fats, convert glucose to glycogen, produce urea (the main substance of urine), make certain amino acids (the building blocks of proteins) filter harmful substances from the blood (such as alcohol), storage of vitamins and minerals (vitamins A, D, K and B12) and maintain a proper level of glucose in the body. The liver is also responsible for producing cholesterol. It produces about 80% of the cholesterol in our body. It has great capacity to detoxicate toxic substances and synthesize useful principles. Therefore, damage to the liver inflicted by hepatotxic agents is of grave consequences [5]. The liver is the key organ regulating homeostasis in the body. It is involved with almost all the biochemical pathway related to growth, fight against disease, nutrient supply, energy provision and reproduction [6]. Liver is expected not only to perform physiological functions but also to protect against the hazards of harmful drugs and chemicals. The liver is sometimes referred to as the “great chemical factory” of the body, because the body depends on the liver to regulate, synthesize, store and secrete many important proteins, nutrients, chemicals and to purify and clear toxin or unneeded substances from the body. Most importantly, the liver is considered to be the center of metabolic transformation of drugs and other toxins entering from the gastrointestinal tract as such the normal or healthy functioning of the liver determines the health status of an individual. The liver is an organ of prime importance and plays a significant role not only in metabolism and detoxification of exogenous toxins and therapeutic agents, but also in the bioregulation of fats, carbohydrates, amino acids, proteins, blood coagulation and immunomodulation. Impairment of the liver generally occurs from excessive exposure to xenobiotics, alcohol, chemotherapeutic agents, viruses and protozoan infections. Depending upon the severity of the hepatic cell injury, viral acute hepatitis can lead to chronic hepatitis, which if left untreated can result in cirrhosis or malignant lesions. Most of hepatotoxic chemicals damage liver cells mainly by inducing lipid per oxidation and other oxidative damages.
Throughout the ages, humans have relied on nature for their basic needs for the production of food stuffs, shelters, clothing, fertilizers, flavours as well as medicines. Plants have formed the basis of sophisticated traditional medicine systems that have been in existence for thousands of years and continue to provide mankind with new remedies. Plants have potent biochemicals and have components of phytomedicine. Plant based natural constituents so called secondary metabolites make them resistant to various environmental stress e.g. scarcity of water, salinity, pathogens etc. The curative properties of medicinal plants are mainly due to the presence of various complex chemical substances of different composition which occur as secondary metabolites [7,8]. They are grouped as alkaloids, glycosides, flavonoids, saponins, tannins; carbohydrates and essential oils. Any part of the plant may contain active components [9]. The medicinal action of plants are unique to particular plant species or groups of plants and are consistent with this concept as the combination of secondary products in a particular plant is taxonomically distinct [10]. Arid and semi-arid plants are good sources for the production of various types of secondary metabolites which include alkaloids, flavonoids, steroids, phenolics, terpens, volatile oils, saponins, tannins, lignins and so many other metabolites. Now a day there is a renewed interest in medicinal plants because of their less side effects and safety to the communities.
Paracetamol as hepatotoxin
Overdoses of the analgesic and antipyretic acetaminophen represent one of the most common pharmaceutical product poisonings in the United States [11]. Although considered safe at therapeutic doses, in overdose, acetaminophen produces a centrilobular hepatic necrosis that can be fatal [12]. Whereas the initial biochemical and metabolic events that occur in the early stages of toxicity have been well described, the precise mechanisms of hepatocyte death are poorly understood. Necrosis is recognized as the mode of cell death and apoptosis has been ruled out [13,14]. Several recent excellent reviews on acetaminophen toxicity have been recently published [15,16]. Acetaminophen is metabolically activated by cytochrome p450 to form a reactive metabolite that covalently binds to protein [17]. The reactive metabolite was found to be N-acetyl-p-benzoquinone imine (NAPQI), which is formed by a direct two-electron oxidation [18]. More recently, the cytochromes 2E1, 1A2, 3A4, and 2A6 have been reported to oxidize acetaminophen to the reactive metabolite [19-21]. NAPQI is detoxified by Glutathione (GSH) to form an acetaminophen- GSH conjugate. After a toxic dose of acetaminophen, total hepatic GSH is depleted by as much as 90%, and as a result, the metabolite covalently binds to cysteine groups on protein, forming acetaminophen-protein adducts [17]. To better understand the role of specific adducts in acetaminophen toxicity, developed immunochemical assays that were very specific for acetaminophen covalently bound to cysteine groups on protein [22-24]. Using this antiserum, the relationship between acetaminophen covalent binding and toxicity in mice in a competitive ELISA to quantify the adducts. Western blot assays were used to determine the molecular nature of the acetaminophen-protein adducts and immunohistochemical analyses were used to determine the specific cells that contained the adducts [25,26]. These studies have been reviewed. Western blot assays for acetaminophen protein adducts have also been used to study toxicity in human overdose patients. In these studies, adducts have been detected in the blood of human overdose patients with severe toxicity (hepatic transaminases above 5000 IU/l) [27].
Feronia limonia L. (Wood Apple)
Taxonomical classification of Feronia limonia L.
Kingdom - Plantae
Division - Magnoliophyta
Class - Magnoliopsida
Order - Sapindales
Family - Rutaceae
Genus - Feronia
Species - limonia
Premodern medicinal uses of Feronia limonia L. (Wood Apple)
Feronia limonia L. (Family Rutaceae) commonly known as Wood Apple or Kaitha and is widely distributed in most tropical and subtropical countries. The Feronia limonia is native and common in India, Sri Lanka, China and Indonesia, where it is cultivated along roads and edges of fields and occasionally in orchards [28]. Feronia limonia was known as a medicinal plant already in ancient Greek and Roman times and one of the most important plants of ‘Ayurved’, the traditional Indian medicine. In India, the fruit is used as a stomachic, diuretic, cardiotonic and tonic to the liver and lungs. Some recent reports identified its use in gastrointestinal disorders [29]. The tree grows up to an elevation of 450 m in the western Himalayas, fruits 5 to 6.3 cm; globose, hard; pericarp woody, rough, grey colored, seeds embedded in an edible pulp. Decoction of Feronia limonia leaves is consumed by some Indian tribes for treating acidity and related gastrointestinal problems [30].
The unripe fruits are sour, aromatic, astringent, constipating, alexipharmic and are useful in diarrhea, pruritus and pharyngodynia. The unripe fruit is alexipharmic, astringent to the bowels; removes itching of the body; increases “vata”, “pita”, and “kapha”. Useful in whooping cough. The Hindus consider the unripe fruit to be a useful astringent in diarrhoea and dysentery. It is used as substitute for bael (Eagle marmelos) in the treatment of diarrhoea and dysentery.
Ripe fruits are considered as sour, sweet, acrid with flavor and taste; difficult to digest; refrigerant, aphrodisiac, alexipharmic; cures cough, dysentery, heart diseases, vomiting; removes biliousness, “vata”, “tridosah”, and blood impurities, fatigue, thirst, hiccough; good for throat, asthma, constipation, tumours, opthalmia, leucorrhoea, the juice put in the ear cures earache. According to yunani the fruits are cardiotonic, tonic to the liver and the lungs, astringent and binding, diuretic, strengthening the gums; the juice is good for stomtitis and sore throat; useful in biliousness; topically it relieves pain due to stings of wasps and other insects. They are beneficial in scurvy and sore throat. Fruit pulp is sour, sweet, edible stomachic, stimulant and astringent. The pulp is applied externally as a remedy for the bites of venous insects. Pulp with honey and pipli is given for hiccup and difficulty for breathing. Pulp is used for infections of gums and throat and to tone the breast. Fruit pulp is also used by tribal of Vidisha District of Madhya Pradesh against boils and amoebiosis. 50 g pulp of the ripe fruit is mixed in butter milk and taken once daily, for 3 days as vermifuge. 10 g of ripe fruit along with 5 g jiggery is given once a day for one month against diabetes.
This plant recently gained a great therapeutically relevance owing to their high coumarins and monoterpenoids content, which is explored for treatment of snake bite [31]. Fruits, leaves and stem bark of Feronia limonia L. have been studied for antitumor, larvicidal and antimicrobial activity [32,33].
From the traditional knowledge it is very clear that the fruit pulp of Feronia limonia L. have the hepatoprotective activity. But still no scientific and methodical investigations have so far been reported in the literature regarding their hepatoprotective activity against paracetamol induced hepatotoxicity in albino rats.
Plant collection:The fruits of Feronia limonia L. used for the present study were collected from the Vidisha district of M.P in India and the details of the same is mentioned in Table 1.
Table 1: Showing plant material and its part used.
| Sr. No. | Plant species | Common name | Family | Part used | Month of collection | Season of collection |
|---|---|---|---|---|---|---|
| 1 | Feronia limonia L. | Kaitha, Kavat. | Rutaceae | Fruit pulp | Aug-Oct. | Summer and Autumn |
The fruit of the plant was identified, confirmed and authenticated by Prof. P.N.Srivastav, Department of Botany S.S.L Jain College, Vidisha. The herbarium specimen of the plant was kept in Herbarium of Pest Control and Ayurvedic Drug Research Laboratory, S.S.L Jain P.G College, Vidisha (M.P) under voucher specimen no. PCAADRL/FL/2010. The fruits were shade dried and pulverised.
Materials and Methods
Preparation of extract
Extraction of the plant material is needed for any phytochemical or phytopharmacological experimental work. The extraction of shade dried powdered material (40-60 mesh size) of the fruit pulp of Feronia limonia L. (Wood Apple) was carried out by soxhlet apparatus in the laboratory using different solvents of increasing order of polarity. The different solvents used for the extraction purpose depends on the chemical nature of the active principles to be separated out from the particular plant material. The extraction procedure adopted was of [34]. The mode of action of plant material depends on the texture and water content of the plant material and substance to be extracted either in any solvent. The method used for the extraction of plant material is as under.
Soxhlet extraction method: Soxhlet apparatus of 5000 ml mesh size fitted with heating mental of the same size was utilized for extraction of crude material. In order to get an idea about the percentage yield and nature of biologically active portion, a sample of known amount of the shade dried and crushed material was then extracted successively with different solvents according to increasing order of polarity viz. n-hexane, Pet-ether, Chloroform, Ethyl acetate, Ethanol and distilled water. N-hexane and petroleum ether was used for deffating; while as, ethanol and distilled water are polar solvents, which can dissolve the polar compounds present in the cytoplasmic part of the plant material. Extraction was carried out for duration of 48 hours or till 8 cycles is completed. The crude extracts thus obtained were filtered with Whatsman filter paper number 1. The filtrate was vacuum evaporated in rotavapour (RE-100) to obtain a semisolid crude material. The vacuum evaporation was done under reduced pressure and at temperature below 40oC. The crude extract gave different percentage of yields in different solvents. The same has been described in Table 4. The concentrated extract was tested for bioassay and chromatographic separation of the flavonoid compound. A trace of toluene was added with the crude extracts to prevent fungal growth. Extracts were kept in glass vials with stoppers. They were placed in refrigerator for further isolation, purification, spectral analysis and bioassay experiments.
Determination of percentage yield: The percentage yield of extracts will be calculated by using following formula:
Percentage Yield = Weight of extract /Weight of powder drug ×100
Determination of moisture content or loss on drying: An excess of water in medicinal plant materials will encourage microbial growth, the presence of fungi or insects, and deterioration following hydrolysis. Limits for water content should therefore be set for every given material. This is especially important for materials that absorb moisture easily or deteriorate quickly in presence of water. Loss on drying is the loss of mass expressed as per cent w/w. The test for loss on drying determines both water and volatile matter in the crude drug. Moisture is an inevitable component of crude drug, which must be eliminated as far as possible. An accurately weighed quantity of about 2 g of powdered drug was taken in a tared glass petri dish. The powder was distributed evenly. The petri dish kept open in vacuum oven and the sample was dried at a temperature between 100 to 150°C for 2 h until a constant weight was recorded. Then it was cooled in desiccators to room temperature, weighed and recorded.
% loss on drying will be calculated by using the following formula:
% loss on drying = Loss in weight of the sample/Weight of the sample ×100
Phytochemical screening:In order to determine the presence of alkaloids, glycosides, flavones, tannins, terpenes, sterols, saponins, phenolics, fats and sugars, a preliminary phytochemical study (color reactions) with various plant extracts was performed.
Test of Carbohydrates
Molish test: To 2-3 ml extract, add few drops of α-naphthol solution in alcohol shake and add conc. H2SO4 from sides of the test tube. Purple to Violet ring appeared at the junction. It indicates presence of carbohydrates.
Fehling soultion test: Mix 1 ml. Fehling’s A and 1 ml. Fehling’s B solutions, boil for one minute. Add equal volume of test solution. Heat in boiling water bath for 5-10 minute. First a yellow, then brick red ppt. was observed.
Test of Glycosides
Borntrager’s test for anthraquinone glycosides: To 3 ml. extract, add dil. H2SO4. Boil and filter. To cold filtrate, add equal volume of benzene or chloroform. Shake well. Separate the organic solvent. Add ammonia. Ammoniacal layer turns pink or red.
Test of Saponin
Froth formation test (Saponification): 2 ml. solution of crude was mixed in water in a test tube, and then shaken well, stable froth was formed, with the presence of saponin.
Test of volatile oils: Hydro distillate material. Separate volatile oil from distillate and perform the following tests:
- Volatile oils have characteristic odour.
- Filter paper is not permanently stained with volatile oil.
- Volatile oils are soluble in 90% alcohol.
Test of flavonoids
Alkaline reagent test: In the test sample few drops of NaOH solution were added immense yellow colour was formed which turns to colorless on addition of few drops of acid which indicated presence of flavonoids.
Zinc hydrochloride test: In the test sample a mixture of zinc dust and conc. H2SO4 was added which gave red color after few minutes.
Ammonia test: Filter paper strips were dipped in the alcoholic and aqueous solution of the extract and ammoniated; the filter paper changes its color to yellow which indicates the presence of flavonoids.
Pew test: To 1 ml of the each extract, a piece of metallic magnesium/ zinc was added followed by addition of 2 drops of concentrated hydrochloric acid; a brownish color confirmed the presence of flavonoids in all the extracts.
Test of Alkaloids
Mayer’s reagent: Few drops of reagent were added in each extract and observed formation of the white or cream colored precipitate.
Dragendorff’s reagent: Few drops of Dragendorff’s reagent were added in each extract and observed formation of the orange yellow or brown colored ppt.
Wagner’s reagent: Few drops of Wagner’s reagent were added in each extract and observed formation of the reddish brown precipitate.
Test for Phenolic compounds
Ferric chloride test: On addition of ferric chloride solution (5%), color was observed in all three portions due to the presence of Phenolic compounds.
Lead acetate test: Few drops of lead acetate solution (5%) were added to the alcoholic extract. White precipitate was appeared which confirm the presence of Phenolic compounds.
Purification and chromatography
Purification of crude extract: There are various secondary metabolites viz. Alkaloids, flavonoids, glycosides, saponins, phenolics, tannins etc. Present in the plant extracts, and further isolation and purification is required to find the pure active compound that is most effective in the treatment of hepatotoxicity. In the present study, the crude extract obtained from vacuum evaporation of Feronia limonia L. was subjected to isolation, purification, chemical examination, spectral analysis and characterization of compound present in it. The methods used for purification is as under.
Column chromatographic purification of crude extract: Column chromatography is a method used to purify individual chemical compounds from mixture of compounds [35]. The main advantage of column chromatography is the relative low cost and disposable of stationary phase used in the process. It prevents cross contamination and stationary phase degradation due to recycling. Two methods are generally used to prepare a column, the dry method and the wet method. In column chromatography the stationary phase, a solid adsorbent is placed in a vertical glass column and the mobile phase, a liquid is added to the top which flows down through the column (by either gravity or external pressure). Column chromatography is advantageous over most other chromatographic techniques, because it can be used in both analytical and preparative applications. Not only can column chromatography be used to determine the number of components of a mixture, but it can also be used to separate and purify substantial quantities of those components for subsequent analysis.
The glass column was thoroughly washed with detergent and water and kept for drying. The column was prepared with silica gel grade A (40-60 mesh size). Silica gel was first digested with water like other gels and then was poured in column. Application of crude extract as concentrated solution was done with a pipette placed against a column wall just above the cotton plug soaked in the solvent kept to protect the top of the column while the concentrated extract slowly drained from the pipette. Care was taken that it did not touch the adsorbent material or the walls. When all the crude extract had been adsorbed on the top of the column, the vacant space above it was filled with solvents and the column was allowed to run and on no account, the column was not allowed to run dry, during packing or during separation. The supply of the solvent and combinations of solvents was poured again into the column until we got all the diverse fractions. The various fractions thus obtained were collected in small glass vials. The fractions Dd4, Ee5, Ff6 for Feronia limonia L. obtained in the course of column chromatography were subjected for hepatoprotective commotion to perceive the compelling hepatoprotective activity among above different fractions.
Thin layer chromatography: TLC was produced with the aim of identifying the individual substances in a mixture and also testifying for purity or for separation of mixtures. TLC is performed on a sheet of glass, plastic or aluminum foil which is coated with a thin layer of adsorbent material, usually with silica gel or cellulose. This layer of adsorbent is known as stationary phase. The plate or sheet is placed in a chamber containing a small amount of solvent which acts as mobile phase. The height of the solvent front and center of spots were measured in the form of Rf value. The Rf value indicates the position at which a substance was located in the chromatogram.
The biologically active compound was separated from the crude extract by the Thin Layer Chromatography. The commercially prepared glass plates were used, touching the edges of the plates with fingers avoided on surface; the sample was applied with the help of a capillary tube as a minute spot at the spot line marked at 1 cm from the edge of the plate. The plate was then placed into a saturated tank/chamber with suitable solvents to just wet the lower edge of the plate sorbent but not enough to wet the part of the plate where the spots were applied (origin). The solvent front migrates up the plate through the sorbent by capillary action, a process known as development. The information provided by the finished chromatogram includes the migrating behavior of the separated substances. It is given in the form of Rf value defined by Brimly and Bgrrett (Relative to front).
Rf=Compound distance from origin (midpoint)/Solvent front distance from origin
The Rf value is always <1.
Acid hydrolysis: 5 to 10 ml each fraction from column chromatograph was mixed with 2 ml of methanolic HCL (10%) was refluxed for four hours in evaporator at the temperature 35-40°C. After 4 hours reaction mixture was diluted with 3 ml of distilled water and then again evaporated to remove methanol, it was performed two times then the aqueous solution was extracted with CHCl3, after evaporation the aqueous layer was neutralized with 10% NaOH and concentrated under reduced pressure. Then it was compared on TLC to confirm the presence of hepatoprotective compound.
Methylation: The acid hydrolyzed fractions were methylated. After the removal of methanol, the solution was extracted three times with ethyl acetate. The extract was washed with methanol. It was crystallized with methanol repeatedly till the crystals were obtained.
Animal experimental design
Breeding and maintenance of experimental animals: Swiss albino rats weighing between 150-200 g, bred in Animal House of Pest Control and Ayurvedic Drug Research Laboratory, were used for the study. The animals were procured and housed in the animal house maintained under standard hygienic conditions, at a temperature of 25 ± 1°C with 50 ± 10% relative humidity and with a 12: 12 hr light/dark cycle. Food pellets (Hindustan lever Ltd. Mumbai, India) and tap water were provided ad libitum. Studies were performed in accordance with the CPCSEA guidelines.
Determination of LD50 value and selection of doses: Healthy and hygienic swiss albino rats of either sex weighing 150-200 g maintained under standard laboratory conditions were used for acute oral toxicity test according to Organization for Economic Co-operation and Development guidelines 423. Animals were fasted prior to dosing (only water was withheld overnight). On next day, the fasted body weight of each animal was determined and the dose was calculated according to the body weight. 20 animals were divided into four groups for giving dose 100, 500, 1000 and 2000 mg/kg. The rodents did not die at 100 mg/kg (mean dose was tolerated) so subsequent doses were increased by a factor of 0.7. Maximum dose was 2000 mg/kg. Animals were observed individually at least once during the first 30 minutes after dosing, periodically during the first 24 hours (with special attention given during the first 4 hours), and daily thereafter, for a total of 14 days. Behavioral changes viz. awareness, neurological profile and autonomic profiles were examined. 1/10th and 1/5th of the maximum tolerated safe dose (LD50) were selected as treatment dose for further hepatoprotective activity.
Animal model: Albino rats between 150-200 g, bred in Animal House of Pest Control and Ayurvedic Drug Research Laboratory, were used for the study. The animals were procured and housed in the animal house maintained under standard hygienic conditions, at a temperature of 25 ± 1°C with 50 ± 10% relative humidity and with a 12:12 hr light / dark cycle. Food pellets (Hindustan lever Ltd. Mumbai, India) and tap water were provided ad libitum. Studies were performed in accordance with the CPCSEA guidelines. The animals were divided into six groups of six animals in each group. Group 1 served as normal control which received distilled water only. Group 2 served as paracetamol control and received paracetamol at a dose of 500 mg/kg bw (p.o) at every 72 h for 10 Days. Groups 3 to 5 received ethanolic extract of the fruit pulp of Feronia limonia at 100, 200 and 300 mg/kg bw/day for 7 days (p.o). Group 6 served as standard control and received Silymarin (100 mg/kg p.o). Groups 3 to 6 were intoxicated with paracetamol (500 mg/kg bw) 1 h before the administration of extract or Silymarin for 10 days.
Biochemical investigation: When the plasma membrane of a hepatocyte is damaged, a variety of enzymes that are normally located in the cytosol are released into the bloodstream. Numerical analysis of these enzymes in the serum serves as a useful quantitative marker for the proper evaluation of liver damage. A number of serum enzymes involved are evaluated to distinguish and assess the hepatocellular injury. The rats were kept overnight fasting after 10 days and blood samples were collected by retro orbital puncture under ether anesthesia and the serum was used for the estimation of biochemical markers like AST, ALT, ALP, Total Bilirubin, Total Cholesterol, and Triglycerides using standard kits [36]. The animals were sacrificed by overdose of ether and autopsied. Livers from all animals were removed, washed with ice cold saline, weighed. Small piece of liver tissue was collected and preserved in 10% formalin solution for histopathological studies. Livers of animals were homogenized with ice-chilled 10% KCl solution and centrifuged at 2000 rpm for 10 min.
Histopathological investigation
Fixation: Ten percent buffered neutral formalin was considered the best general fixative for pathological specimens because it preserves the widest range of structures, requires a relatively short fixation time, can be used for long-term storage, and penetrates rapidly and evenly without over hardening. Wet tissue specimens can remain in 10% buffered neutral formalin for many months without adverse effects or unwanted precipitates, and it preserves nuclear and cytoplasmic detail adequately as reported by Edna et al. [37].
Tissue processing: dehydration, clearing and infiltration: The three stages of tissue processing-dehydration, clearing, and infiltration-were sequential steps designed to remove the extractable water from tissue specimens and replace it with a medium that solidifies to allow sectioning. The first step in processing was dehydration. Water is present in tissues in free and bound (molecular forms). Tissues were processed to the embedding medium by removing some or all of the free water. Ethanol is the usual solvent of choice for dehydration.
Clearing is the transition step between dehydration and infiltration with the embedding medium. Xylene is used for routine paraffin embedding because of its compatibility with many types and sizes of tissue specimens.
Infiltration is the saturation of tissue cavities and cells by a supporting substance which is the medium in which they were finally embedded. Tissues were infiltrated by immersion in a paraffin wax, which is fluid when hot and solid when cold as reported by Edna et al.
Hand processing schedule: (Total processing time-14 to 16 hours)
All specimens were placed in cassettes and loaded into tissue baskets. Since paraffin wax is not miscible with water, the tissues were exposed to a series of graded dehydrant and clearing agent before immersion in paraffin wax. The following procedure was designed for hand processing viz. the tisssues were exposed to 70% alcohol for 1 hour, 80% alcohol for 1 hour, 95% alcohol three changes one hour each, absolute alcohol three changes one hour each, xylene three changes one hour each, paraffin four changes one each hour each and After finishing from the processing program, the basket including the cassettes was taken out for embedding.
Embedding: Embedding is the process of surrounding tissue with paraffin wax to facilitate the cutting of thin sections. Stainless steel molds were used for casting/shaping liquid paraffin into blocks.
Requirements for embedding are as follows:
- A supply of clean, filtered paraffin wax held at 2-4°C above its melting point.
- A cold plate to rapidly cool the wax.
- A supply of moulds in which to embed the tissues.
General embedding procedure
- Opening the tissue cassette, checking against worksheet entry to ensure the correct number of tissue pieces is present.
- Selecting the mould; there should be sufficient room for the tissue cassette with allowance for at least a 2 mm surrounding margin of wax.
- Placing the labeled cassette base onto the mould.
- Filling the mould with paraffin wax.
- Using warm forceps to orient the tissue, taking care that it does not cool in the air; at the same time.
- Chilling the mould on the cold plate. This ensures that the correct orientation is maintained and the tissue surface to be sectioned is kept flat.
- Releasing the wax block and cassette from the mould.
- Trimming excess wax from sides, front, and back of cassettes.
- Placing cassettes in numerical order for trimming and cutting.
Microtomy: Microtomy is the mean by which the tissues are sectioned and attached to a surface so that examination microscopy can take place. The rotary microtome was used for making thin sections (5 μm).
Equipmment required for paraffin section cutting
Water bath (Flotation): The flotation bath was heated to a few degrees below the melting point of the paraffin. The distilled water in the water bath was used to help eliminate air bubbles and wrinkles.
Hot plate: Hot plate especially designed for drying tissue sections on slides were used. With a temperature setting at the melting point of the wax no obvious damage was done to the sections, and drying was complete in 30 minutes.
Fine pointed forceps (13 cm in length) and small brush:These instruments are used for the handling of sections during cutting and for removing of folds and creases in the sections during the floating.
Scalpel
Slide rack
Clean slides
Ice tray
Pencil
Trimming of tissue blocks: A knife is used for this task. The trimming was done by either setting the thickness adjuster at 15 μm or by advancing the block using the coarse feed mechanism. The blocks were trimmed carefully to expose the surface of the tissues. The trimmed cassettes were placed on a cooling tray to produce wrinkle-free sections.
After completion of trimming, the block was placed on melting ice for few minutes for cooling both the tissue and the wax and giving them a similar consistency, so making sectioning easier.
Cutting sections: For routine tissue sectioning, the thickness selector was set at 5 µm. With the aid of a small brush and a pair of forceps, a ribbon of six sections has been cut and lifted from knife edge and floated out shiny side onto water bath containing clean water at a temperature of approximately 46-50°C. The ribbon was gradually lowered onto the floatation bath to eliminate wrinkles and entrapped air. Air bubbles were removed with a small brush or by submerging a slide under the ribbon. The slide was drawn gently under the section containing bubbles and the bubbles were removed. We allowed the sections to remain on the water bath until they flattened. The sections were separated and placed onto clean pre-marked slides. The slides were drained vertically for several minutes before placing them onto a warming table that is set at 37°C to 40°C. The slides were ready for staining.
Haemotoxilin and eosin (Staining): The haemotoxilin and eosin stain is probably the most widely used histological stain. Its popularity is based on its comparative simplicity and ability to demonstrate clearly an enormous number of different tissue structures. Haematoxylin can be prepared in numerous ways and has a widespread applicability to tissues from different sites. Essentially, the haemotoxilin component stains the cell nuclei blue/black, with good intranuclear detail, whilst the eosin stains cell cytoplasm and most connective tissue fibers in varying shades and intensities of pink, orange and red. Following the application of haemotoxilin, eosin solutions were used conventionally to counter stain. Eosin-phloxine gives the widest range of contrast — pink to bright red.
Mayer’s Haemotoxilin and Eosin procedure
- Deparaffinizing slides and hydrating to water.
- Staining in Mayer’s haemotoxilin solution for 15 minutes.
- Washing in lukewarm running tap water for 15 minutes.
- Placing in distilled water.
- Placing in 80% ethyl alcohol for 1 to 2 minutes.
- Counterstaining in eosin-phloxine solution for 2 minutes.
- Dehydrating and clear through 2 changes each of 95% ethyl alcohol, absolute ethyl alcohol, and xylene, 2 minutes each.
- Mounting with Canada balsam.
Photomicrography: Light microscopic examination of the sections was then carried out and micrographs produced using Olympus BX-60 photographic microscope at Jawaharlal Nehru Cancer Hospital and Research Centre. Bhopal.
Statistical analysis: Numerical data obtained from this study were expressed as the mean value ± standard error of mean. Differences among the control and treatment groups were determined using statistical package (Graph Pad Instant). A probability level of less than 5% (p<0.05) was considered significant.
Results and Discussion
Herbal medicines are being increasingly utilized to treat a wide variety of diseases, though the knowledge about their mode of action is relatively scanty. So there is a growing interest regarding the pharmacological evaluation of various plants used in traditional system of medicine. Many diseases (atherosclerosis, diabetes mellitus, asthma, nephritis, liver diseases) are due to the specific organ damage. The organ damage may be due to the excessive generation of free radicals. Several drugs from natural source are known to scavenge off such free radicals and prevent the organ damage.
Feronia limonia showed higher % of loss in weight i.e. (78%) on drying as mentioned in the Table 2. Total ash content, acid soluble and water soluble ash content of the plant material has been portrayed in the Table 3. Percentage yield of the ash content after burning of plant material was found to be (58.8 gm) in Feronia limonia L. Acid soluble ash content was found to be 17.6 gm while as, water soluble component of the ash was found to be (41.2 %) in Feronia limonia L. Ash content shows the moisture present in the powdered material as well as it indicates the nutrients and mineral composition. The mineral composition in the ash also gives an indication of the particular nutrient present in it. Different solvents were used according to increasing order of polarity i.e. n-hexane, petroleum ether, chloroform, ethyl acetate, ethanol and water (Tables 2, 3).
Table 2: Showing percentage of loss in weight of plant material on drying.
| Sr. No. | Plant species | Wet weight of plant material | Dry weight of plant material | Loss in weight on drying | % loss in weight |
|---|---|---|---|---|---|
| 1 | Feronia limonia L. | 2 Kg 500 g | 550 g | 1950 g | 78% |
Table 3: Showing ash content of Feronia limonia L. with acid soluble and water soluble ash content.
| Plant material | Weight of plant material before burning | Weight of ash content after burning of plant material | % yield of ash content after burning of plant material | % of acid soluble ash content | % of water soluble ash content |
|---|---|---|---|---|---|
| Feronia limonia L. | 5 gm. | 2.94 gm. | 58.8 gm. | 17.6 gm. | 41.2 gm. |
Table 4 shows the percentage yield of Feronia limonia in different solvents by soxhlet extraction method. Feronia limonia yielded (4.2 gm.) in n-hexane, (2.98 gm.) in petroleum ether, (0.4 gm.) in chloroform, (1.42 gm.) in ethyl acetate, (1.25 gm.) in ethanol and (2.34 gm.) in water respectively. Sukumar et al., have reported that percentage yield of the same species grown at different climatic conditions may be different [38,39]. The yield also depends on the month of collection of the plant material as well as soil texture of the region. Therefore, the present study is in agreement with the previous workers in the field that percentage yield gives an indication of the availability of particular bioactive material in the plant. In the present study TLC of the crude extracts of the the plant material have been worked out using different solvent systems. The TLC plates were visualized in iodine chamber, UV light and by naked eyes to visualize the color pattern of each spot as depicted in Table 5.
Table 4: Showing percentage yield of Feronia limonia L. in different solvents by soxhlet extraction method.
| Plant species | Solvent used (ml) | Weight of powdered plant material (g) | Volume of solvent (ml) | Weight of extract obtained (g) | % yield of extract (g) |
|---|---|---|---|---|---|
| Feronia limonia L. | n-hexane | 150 g | 200 ml | 6.37 g | 4.24 g |
| Petroleum ether | 150 g | 200 ml | 4.48 g | 2.98 g | |
| Chloroform | 150 g | 150 ml | 0.6 g | 0.4 g | |
| Ethyl acetate | 150 g | 200 ml | 2.13 g | 1.42 g | |
| Ethanol | 150 g | 200 ml | 1.88 g | 1.25 g | |
| Water | 150 g | 200 ml | 3.51 g | 2.34 g |
Table 5: Thin layer chromatography of 90% ethanolic extract of plant material with Rf value and color characteristics.
| Crude extract of plant materials | Solvent systems | Rf values | Visual light | Iodine chamber | UV light |
|---|---|---|---|---|---|
| Feronia limonia L. | n-hexane: ethyl acetate (9:1) | 0.46, 0.52 | Reddish brown, Reddish. | Dark brown, Yellow. | Dark blue,Blue. |
Two spots in Feronia limonia L. were obtained with Rf (0.46, 0.52) respectively. Column chromatography is advantageous over most other chromatographic techniques, because it can be used in both analytical and preparative applications. Not only can column chromatography be used to determine the number of components of a mixture, but it can also be used to separate and purify substantial quantities of those components for subsequent analysis. Feronia limonia L. yielded three fractions having light brown, greenish yellow and reddish yellow chromatographic color characteristic by using n-hexane: ethyl acetate in the ratio of 9:1 as portrayed in Table 6. Preliminary phytochemical screening of the successive extracts of Feronia limonia were assessed as reported in Table 7. Which depicts the strong positive results for alkaloids, glycosides and flavonoids in alcoholic extract? Proteins, amino acids and vitamins were found to be present in both aqueous as well as in alcoholic extract. Fluorescence characteristic of the Feronia limonia extract revealed the different color characteristics when observed under UV (254 and 366 nm) and visible light with different extracts as mentioned in Table 8.
Table 6: Showing column chromatography of the 90% ethanolic extract of the plant materials.
| Crude extract of plant materials | Solvent system (ml.) | Weight of fractions (gm) | Chromatographic color of fractions. |
|---|---|---|---|
| Feronia limonia L. | n-hexane: ethyl acetate(9:1) | 10 gm 6 gm14 gm | Light brown,Greenish yellow,Reddish yellow |
Table 7: Phytochemical tests of the successive extracts of fruit pulps of Feronia Limonia L.
| Sr. No. | Chemical constituents | Aqueous extract | Pet. ether extract | Alcoholic extract | Chloroform extract |
|---|---|---|---|---|---|
| 1 | Alkaloids | - | + | ++ | - |
| 2 | Carbohydrates | - | - | + | - |
| 3 | Glycosides | + | + | ++ | - |
| 4 | Saponins | + | - | + | - |
| 5 | Flavonoids | + | + | ++ | - |
| 6 | Tannins | - | + | + | + |
| 7 | Phenolic Compounds | - | + | + | + |
| 8 | Proteins and Amino acids | + | - | + | - |
| 9 | Vitamins | + | - | + | - |
| +=Present, -=Absent, ++=Strong positive. | |||||
Table 8: Fluorescence characters of the Feronia limonia L. extracts were observed under UV (254 and 366 nm.) and visible light with different solvents.
| Sr. No. | Name of the Extract | UV Light | Visible Light | |
|---|---|---|---|---|
| Short (254 nm) | Long (366 nm) | |||
| 1 | Petroleum ether (40-60°C) | Yellowish brown | Brown | Light brown |
| 2 | Chloroform | Yellowish brown | Yellow | Light brown |
| 3 | Methanol | Green | Blackish brown | Yellowish brown |
| 4 | Aqueous | Greenish brown | Dark brown | Light brown |
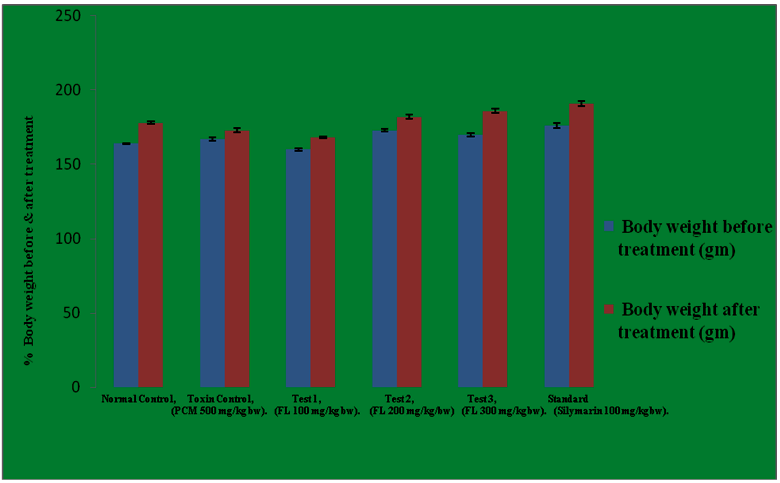
The normal values for the body weight (before and after treatment) were calculated to be 164.0 and 178.0 which considerably got decreased in diseased group i-e in paracetamol intoxicated group and the values were recorded to be 167.0 and 173.0 respectively. 100 mg/kg b.w of 90% ethanolic extract of Feronia limonia promotes the body weight from 160 gms to 168 gms. 200 mg/kg b.w elevates the body weight from 173 gms to 182 gms. Similarly, 300 mg/kg b.w of Feronia limonia extract and 100 mg/kg b.w of Silymarin increases the body from 170 to 186 and 176 to 191 gms respectively. The results were found to be quite significant as compared to the normal control group at 5% level (P<0.05). In this study, the results obtained at 300 mg/kg body weight of 90% ethanolic extract of Feronia limonia were almost similar to the 100 mg/kg body of Silymarin and the same is mentioned in the Table 9 and Figure 1.
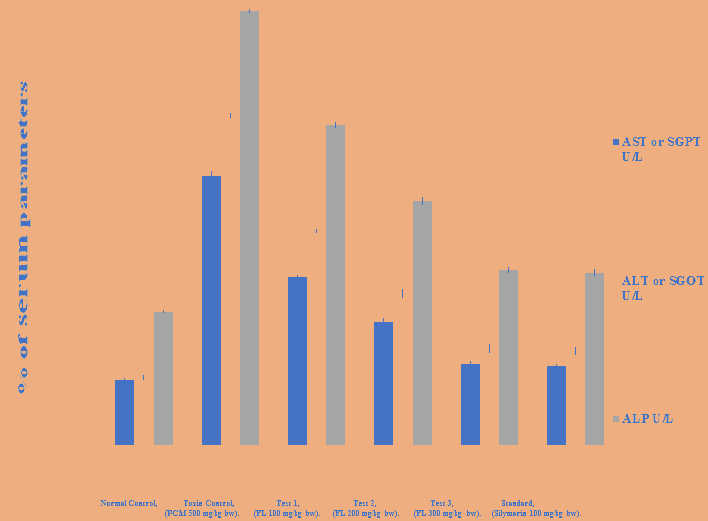
It was noticed that the levels of AST, ALT and ALP parameters increases after the paracetamol intoxication i-e in diseased group. The AST, ALT and ALP levels got increased from normal control i-e 63.54 to 261.2, 65.21 to 320.5 and 129.7 to 422.1 in diseased control respectively. Oral administration of 100, 200 and 300 mg/kg b.w of 90% ethanolic extract of Feronia limonia significantly depletes the levels of AST, ALT and ALP parameters in albino rats. 100 mg/kg b.w of plant extract depletes the levels of AST, ALT and ALP from 261.2, 320.5 and 422.1 in diseased group to 163.5, 207.7 and 311.7 in T1 group respectively. 200 mg/kg b.w of plant extract decreases the AST, ALT and ALP levels from 261.2, 320.5 and 422.1 in diseased group to 119.6, 147.3 and 237.3 in T2 group respectively. Similarly, 300 mg/kg b.w of plant extract effectively reduced the above parameters from 261.2, 320.5 and 422.1 in diseased control to 78.43, 93.97 and 170.4 in T3 group respectively. The microsomal enzyme (ALP) was found to be present in higher concentration among all the above parameters in diseased group, followed by ALT and then AST. Oral administration of 300 mg/kg b.w of 90% ethanolic extract of Feronia limonia and 100 mg/kg b.w of standard drug Silymarin effectively reduced all the biochemical parameters and showed the significant results when compared with the normal control at 5% level (P < 0.05) as reported in Figures 2,3 and Tables 9-11.
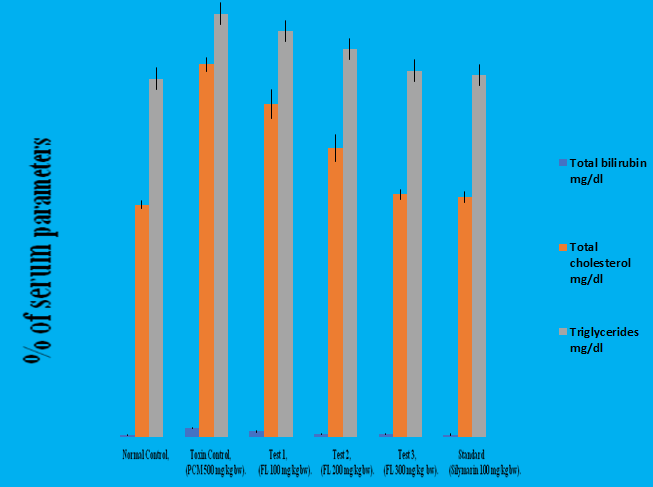
Different doses of this plant extract significantly normalize the total bilirubin, total cholesterol and triglyceride levels. 100 mg/kg b/w of Feronia limonia extract depletes the levels above parameters from 4.320 to 2.693, 182.4 to 163.1 and 207.3 to 198.8 respectively. 200 mg/kg b/w of Feronia limonia extract decreases the same parameters from 4.320 to 1.537, 182.4 to 141.1 and 207.3 to 198.8 respectively. Similarly, 300 mg/kg b.w of Feronia limonia depletes the levels of above parameters from 4.32 to 1.463, 182.4 to 118.7 and 207.3 to 179.4 respectively. The triglycerides were found to be present in higher concentration followed by total cholesterol and total bilirubin in diseased control group i.e in paracetamol intoxicated group. 300 mg/kg b/w of Feronia limonia and 100 mg/kg b/w of Silymarin showed the prominent results. The results were found to be quite significant when compared with the normal control group at 5% level (P<0.05) as mentioned in Table 11 and Figure 3.
Table 9: Effect of ethanolic extract of fruit pulp of Feronia limonia L. on the body weight (before and after treatment) in albino rats.
| Groups | Body weight before treatment (gm) | Body weight after treatment (gm) |
|---|---|---|
| Normal | 164.0 ± 0.3651 | 178.0 ± 0.9661 |
| Diseased | 167.0 ± 1.065 | 173.0 ± 1.238 |
| Test 1. | 160.0± 0.9309 | 168.0 ± 0.5774 |
| Test 2. | 173.0 ± 0.9309 | 182.0 ± 1.265 |
| Test 3. | 170 .0± 1.155 | 186.0 ± 1.571 |
| Standard | 176.0 ± 1.807 | 191.0 ± 1.693 |
Table 10: Effect of 90% ethanolic extract of fruit pulp of Feronia limonia L. on biochemical markers in paracetamol induced hepatotoxicity in albino rats.
| Treatment | AST or SGPT U/L | ALT or SGOT U/L | ALP U/L |
|---|---|---|---|
| Normal | 63.54 ± 1.817 | 65.21 ± 1.993 | 129.7 ± 1.263 |
| Diseased | 261.2 ± 4.600 | 320.5 ± 1.546 | 422.1 ± 1.159 |
| T1 | 163.5 ± 1.666 | 207.7 ± 1.465 | 311.7 ± 2.462 |
| T2 | 119.6 ± 3.070 | 147.3 ± 3.527 | 237.3 ± 3.357 |
| T3 | 78.43 ± 2.250 | 93.97 ± 3.891 | 170.4 ± 2.615 |
| Standard | 76.96 ± 1.376 | 91.42 ± 3.262 | 167.5 ± 3.161 |
Table 11: Effect of 90% ethanolic extract of fruit pulp of Feronia limonia L. on biochemical markers in paracetamol induced hepatotoxicity in albino rats.
| Treatment | Total bilirubin mg/dl | Total cholesterol mg/dl | Triglycerides mg/dl |
|---|---|---|---|
| Normal | 0.87 ± 0.1012 | 113.7 ± 1.815 | 175.5 ± 1.299 |
| Diseased | 4.320 ± 0.4359 | 182.4 ± 3.503 | 207.3 ± 2.080 |
| T1 | 2.693 ± 0.3328 | 163.1 ± 6.965 | 198.8 ± 5.471 |
| T2 | 1.537 ± 0.3342 | 141.4 ± 6.490 | 190.0 ± 5.311 |
| T3 | 1.463 ± 0.3319 | 118.7 ± 2.460 | 179.4 ± 7.753 |
| Standard | 1.100 ± 0.3077 | 117.3 ± 2.737 | 177.3 ± 2.062 |
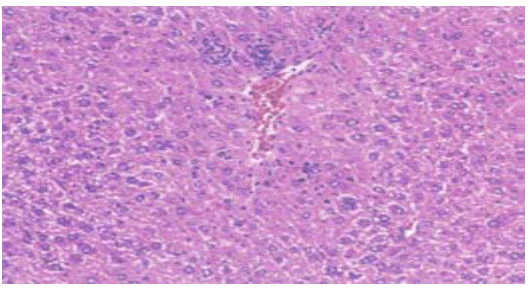
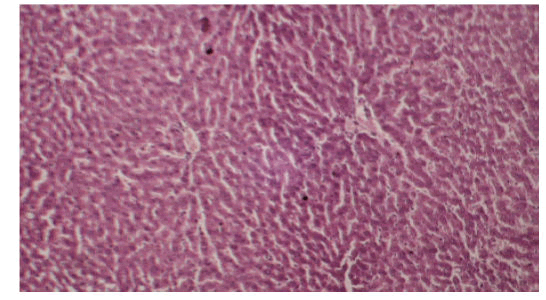
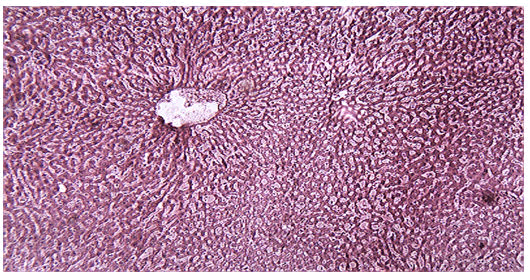
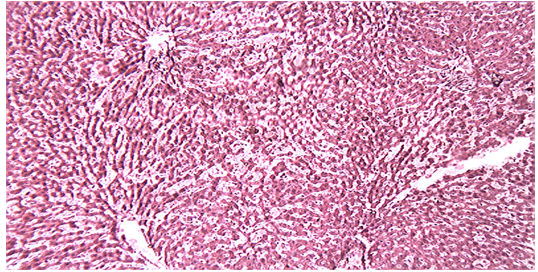
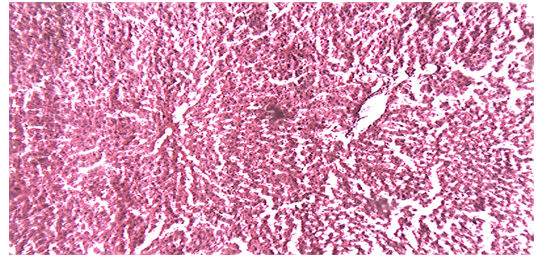
Histological examination of the liver sections reveals that the normal liver architecture of sinusoids, hepatocytes and the central vein from which chords of hepatocytes are radiating as shown in (Figures 4 and 5) was disturbed by hepatotoxins intoxication which shows centrizonal necrotic areas, hydropic changes, fatty degeneration, apoptotic hepatocytes and conjested central veins with piknotic nucleus as depicted in (Figures 6 and 7).
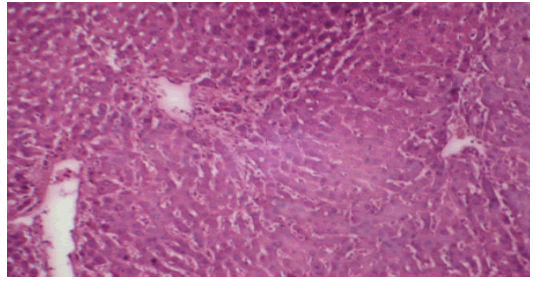
Treatment of rat with 90% ethanolic extract of Feronia limonia L. exhibit regenerative changes like normal architecture of liver parenchyma, absence of fatty changes, haemorrhages and necrosis, normal appearance of hepatic cells with nucleus, less vacuolization and fatty change supplements the protective effect of the extract as portrayed in (Figures 8 and 9).
Standard drug Silymarin treatment improved the functional integrity of the hepatocytes and normalizing the hepatic architecture as shown in (Figure 10).
Conclusion
Liver is a major organ of human body that plays a crucial role in elimination and biotransformation of toxic substances. Toxicity from paracetamol is not from the drug itself but from one of its metabolites, N-Acetyl-P-Benzoquinoneimine (NAPQI). Paracetamol biotransformation involves conjugation with glucuronide and sulphate. A small amount of paracetamol is metabolized by mixed function oxidase enzymes to form highly reactive compound NAPQI, which is immediately conjugated with glutathione and subsequently excreted as cysteine and mercapturic conjugates. In overdoses, large amounts of paracetamol are metabolized by oxidation because of saturation of the sulphate conjugation pathway, but once the protective intracellular glutathione stores are depleted hepatic damage may ensue. Since the modern system of medicine is known for inducing liver damage as a part of side effects, a hepatoprotectant of herbal origin can be considered as a useful, safe and effective co-supplement to minimize the mentioned manifestations. Indian subcontinent has historical tradition of using medicine of herbal origin that is often considered to be protective and curative with minimal side effects. Ethnobotany and ethnopharmacology are interdisciplinary fields of research that look specifically at the empirical knowledge of indigenous peoples concerning medicinal substances, their potential health benefits and their health risks associated with such remedies. As can be seen, many of the plant derived pharmaceuticals and phytomedicnes currently in use were used by native people around the world. Some of this knowledge has been documented and codified or studied scientifically. Also of the hundreds of thousands of species of living plants, only a fraction has been investigated in the laboratory. The importance of ethno-botanical inquiry as a cost-effective means of locating new and useful tropical plant compounds cannot be overemphasized. Most of the secondary plant compounds employed in modern medicine were first discovered through ethno-botanical investigation. Modern allopathic usually aims to develop a patentable single compound or a “magnetic bullet” to treat specific conditions. Traditional medicine often aims to restore balance by using chemically complex plants, or by mixing together several different plants in order to maximize a synergistic effect or to improve the likelihood of an interaction with a relevant molecular target. In this background I have selected the fruit pulp of one of the important medicinal plant viz.Feronia limonia L. for assessing its hepatoprotective activity against paracetamol as hepatotoxin. From the review it is clear that although the fruit pulp is exhaustively used for various ailments in ethnopharmacology as well as having various important chemical constituents but not well studied scientifically for hepatoprotective activity. In the present study I have investigated the phytochemical analysis of the fruit pulp of Feronia limonia L., predicting the presence of various secondary metabolites presents in it. The results obtained from the experimental bioassay portrays that the damage of liver due to paracetamol over dosage was confirmed by elevated levels of various biochemical parametervs. AST, ALT, ALP, Serum bilirubin, total cholesterol, triglycerides etc. Oral administration of various doses of the extract resulted in gradual normalization of the activities of AST, ALT, ALP, Serum bilirubin, total cholesterol and triglycerides. This evidently suggests the protective effect of the extract in improving the functional integrity of liver cells. It appears from the results that the mode of action of 90% ethanolic extracts of Feronia limonia L. in affording the hepatoprotective activity activity against paracetamol may be due to cell membrane stabilization, hepatic cell regeneration and normalization of serum parameters. Hence the present study justified the traditional uses of Feronia limonia L. in the treatment of liver disorders. Thus in future this herbal extract may be used as strong constituent for the formulation of hepatoprotective herbal drug.
References
- Maxwell AG, Masato Y, Yoko A (1999) Free radical scavenging action of medical herbs from Ghana Thonningia sanginea on experimentally- induced liver injuries. Gen Pharmacol 32:661-667
[Crossref] [Google Scholar] [Indexed]
- Susanta KM, Goutam C, Gupta M, Majumder UK (2006) Invitro antioxidant activity of Diospyros malabarica Kostel bark. Ind J Exp Bio 4:39-44
- Chaterjee TK (2000) Medicinal plants with hepatoprotective properties. Herbal Options. Books & Allied, Calcutta, 155.
- Chattopadhyay RR (2003) Possible mechanism of hepatoprotective activity of Azadirachta indica leaf extract: Part 11. Ethanopharmacol 9:217-219
[Crossref] [Google Scholar] [Indexed]
- Shahani S (1999) Evaluation of hepatoprotective efficacy of APCL-A polyherbal formulation in vivo in rats. Ind Drug 13:79-85
[Crossref] [Google Scholar] [Indexed]
- Ward FM, Daly M (1999) Clinical Pharmacy and Therapeutics. Churchill Livingston, New York, 195-212.
- Karthikeyan A, Shanthi V, Nagasathaya A (2009) Preliminary phytochemical and antibacterial screening of crude extract of of the leaf of Adhatoda vasica. L Int J Green Pharm 3:78-80
[Crossref] [Google Scholar] [Indexed]
- Lozoya M & Lozoya X (1989) Pharmacological properties in vitro of various extracts of Mimosa pudica Linn. Tepescohuite Arch invest Mex 87-93
- Gordon MC, David JN (2001) Natural product drug discovery in the next millennium. Pharm. Biol 39:8-17
[Crossref] [Google Scholar] [Indexed]
- Wink M (1999) Introduction biochemistry, biotechnology of secondary products metabolism. John Wiley & Sons, United States, 2:1-16.
[Crossref]
- Litovitz TL, Klein-Schwartz W, Rodgers GC Jr, Cobaugh DJ, Youniss J, et al. (2002) 2004 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 20:391–452
[Crossref] [Google Scholar] [Indexed]
- Prescott LF (1980) Hepatotoxicity of mild analgesics. Br J Clin Pharmacol 10:373S– 379S
[Crossref] [Google Scholar] [Indexed]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, and Jaeschke H (1999) Inhibition of Fas receptor (CD95)-induced caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol 156:179–186
[Crossref] [Google Scholar] [Indexed]
- Gujral JS, Knight TR, Far hood A, Bajt ML, Jaeschke H (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–328
[Crossref] [Google Scholar] [Indexed]
- Bessems JG, Vermeulen NP (2001) Paracetamol (acetaminophen)-induced toxicity: molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol 31:55-138 [Crossref]
- Nelson SG, Wan Z, and Stan MA (2002) SN2 ring opening of beta-lactones: an alternative to catalytic asymmetric conjugate additions. J Org Chem 67:4680–4683
[Crossref] [Google Scholar] [Indexed]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217
- Dahlin DC, Miwa GT, Lu AY, Nelson SD (1984) N-Acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci USA 81:1327–1331
[Crossref] [Google Scholar] [Indexed]
- Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, et al. (1993) Cytochrome p450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol 6:511–518
[Crossref] [Google Scholar] [Indexed]
- Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT (1993) Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYp3A4. Biochem Pharmacol 45:1563–1569
[Crossref] [Google Scholar] [Indexed]
- Chen W, Koenigs LL, Thompson SJ, Peter RM, Rettie AE, et al. (1998) Oxidation of acetaminophen to its toxic quinone imine and nontoxic catechol metabolites by baculovirus-expressed and purified human cytochromes p450 2E1 and 2A6. Chem Res Toxicol 11:295–301
[Crossref] [Google Scholar] [Indexed]
- Bartolone JB, Birge RB, Sparks K, Cohen SD, Khairallah EA (1988) Immunochemical analysis of acetaminophen covalent binding to proteins: Partial characterization of the major acetaminophen-binding liver proteins. Biochem Pharmacol 37:4763–4774
[Crossref] [Google Scholar] [Indexed]
- Cohen SD and Khairallah EA (1997) Selective protein arylation and acetaminophen-induced hepatotoxicity. Drug Metab Rev 29:59–77
[Crossref] [Google Scholar] [Indexed]
- Roberts DW, Bucci TJ, Benson RW, Warbritton AR, McRae TA, et al. (1991) Immunohis tochemical localization and quantification of the 3-(cystein-S-yl)- acetaminophen protein adduct in acetaminophen hepatotoxicity. Am J Pathol 138:359 –371
- Bartolone JB, Cohen SD, and Khairallah EA (1989) Immunohistochemical localization of acetaminophen-bound liver proteins. Fundam Appl Toxicol 13:859–862
[Crossref] [Google Scholar] [Indexed]
- Pumford NR, Halmes NC, Martin BM, Cook RJ, Wagner C, et al. (1997) Covalent binding of acetaminophen to N-10-formyltetrahydrofolate dehydrogenase in mice. J Pharmacol Exp Ther 280:501–505
- James LP, Farrar HC, Sullivan JE, Givens TG, Kearns GL, et al. (2001) Measurement of acetaminophen-protein adducts in children and adolescents with acetaminophen overdoses. J Clin Pharmacol 41:846–851
[Crossref] [Google Scholar] [Indexed]
- Gupta VC, Hussain SJ, Imam S (1997) Important folk- medicinal plants and traditional knowledge of tribals of Aurangabad & Nasik forest divisions of Maharashtra, India. Hamdard Medicus 40:59-61
- Saima Y, Das AK, Sarkar KK, Sen AK, Sur P (2000) An antitumor pectic polysaccharide from Feronia limonia. Int J Biol Macromol 27:333-335
[Crossref] [Google Scholar] [Indexed]
- Kamble SY, Patil SR, Sawant PS, Sawant S (2010) Studies on plants used in traditional medicine by Bhilla tribe of Maharashtra. Indian J Tradit Knowl 9:591-598
- Agarwal A, Siddique IR, Singh J (1989) Coumarins from the roots of Feronia limonia. Phytochem 28:1229-1231
[Crossref] [Google Scholar] [Indexed]
- Rahuman AA, Gopalakrishnan G, Ghouse BS, Arumugam S, Himalayan B (2000) Effect of Feronia limonia on Mosquito Larvae. Fitoterepia 71:553-555
[Crossref] [Google Scholar] [Indexed]
- Rahman MM, Alexander IG (2002) Antimicrobial constituents from the stem bark of Feronia limonia. Phytochem 59:73-77
[Crossref] [Google Scholar] [Indexed]
- Harborne JB (1984) Phytochemical methods A guide of modern techniques of plant analysis. (2nd edition), Chapman and Hall, London, New York.
- Stock, Rice (1978) Chromatographic methods. Chapman and Hall Publictions London 376
- Marklund S, Marklund G (1974) Involvement of superoxide anion radical in the auto oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47: 469-474
[Crossref] [Google Scholar] [Indexed]
- Edna BP, Bob M, Jacquelyn BA, Leslie HS (1994) Laboratory methods in histology. American Registry of Pathology, Washington, DC.
- Sukumar SK, Micheal JP and Boohan LR (1991) Botanical derivatives in mosquito control. J Am Mosq Control Assoc 7:210-237
- Subramonium A, Pushpangadan P (1999) Development of phytomedicines for liver diseases. Indian J Pharmacol 31:166-175
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences