Phosphorus fertilizing Potential of Biochar derived from agricultural residues: A review
Guta Amante*
Department of natural resource management, Jimma University, Jimma, Ethiopia.
- *Corresponding Author:
- Guta AmaDepartment of natural resource management, Jimma University, Jimma, Ethiopia; E-mail: gutiyye@gmail.com
Received Date: Noveber 20, 2020; Accepted date: August 16, 2021; Published date: August 26, 2021
Citation: Guta Ama (2021) Phosphorus fertilizing Potential of Biochar derived from agricultural residues: A review, J Environ Res. Vol.5 No.1.
Abstract
Phosphorus (P) is the second most important nutrient after nitrogen for plant growth. 30-40% of the arable soils are low in P over the world. To guarantee food security for the increasing world population, mineral fertilizers including P fertilizers have been widely supplied to maintain soil nutrients at a high-level favoring crop growth. On the other hand, a high P rate to create a high soil P availability may increase the risk of P loss through soil leaching, runoff, and erosion. In the recent world introduced Biochar as a potential material for making slow-releasing phosphorus (P) fertilizers for the sake of increasing soil P use efficiency and mitigating P losses. Therefore, the recycling of P in agricultural residues is critical for P sustainability in agricultural systems, which is dominated by the route of direct land application. Biochar production from agricultural residues and its subsequent land application has been suggested as solutions for waste biomass disposal, carbon sequestration, soil amendment/remediation, and crop production promotion. Phosphorus in agricultural residues can be retained and transformed into stable forms of P in the resulting biochar. Thus, compared to agricultural residues, biochar provides lower amounts of labile P and releases its P more slowly while providing a long-lasting P source, and the loss potential of P from biochar is reduced by low mobility of its P, indicating that the biochar based P recycling route could substantially promote P recycling by acting as sustainable P source and diminishing the loss of P applied to the soil. It was also reviewed that biochar has a high potential of improving phosphorus availability in P fixing acid soils and its high liming potential to decrease soil acidity. On the other hand physical and activation of biochar produced from agricultural residues by using different single and dual metal oxides was suggested as a novel strategy to adsorb phosphate from wastewater which in turn cause severe environmental pollution.
Introduction
Phosphorus is an essential element needed in all living organisms, and it is a non-renewable resource-dependent exclusively on mined rock phosphates. An input of phosphorus is crucial for food production since all plants need an adequate supply of it for successful growth. A shortfall in phosphorus will result in a reduction of crop yield, where 30-40% of the arable soils are low in P over the world[1]. To guarantee food security for the increasing world population, mineral fertilizers including P fertilizers have been widely supplied to maintain soil nutrients at a high-level favoring crop growth. However, only a few mineral P fertilizers supplied to the soils could be available for plant uptake in a season as P would be quickly constrained by multiple interactions with soil constituents, such as fixation and adsorption[2]. On the other hand, a high P fertilization rate to create a high soil P availability may increase the risk of P loss through soil leaching, runoff, and erosion[3].
Biochar is carbon-rich charcoal that is formed by the pyrolysis (thermal decomposition) of organic biomass or agricultural residues, which is used as a soil amendment[4]. It is composed of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), sulfur (S), and ash in different proportions. It is mainly used to improve soil nutrient content and to sequester carbon from the environment (Lehmann 2009). It is highly porous structure makes it attractive, is usually used as a soil amendment because of its improvements on soil fertility[5], water retention[6], and microorganism diversity [7]as well as its function of mitigating climate change via carbon sequestration.
Biochar is attracting increasing attention in recent years as a potential soil amendment. Numerous researches internationally have now shown that biochar can function as a reservoir of P for soils and that a certain fraction of this P is in a suitable form available for plant uptake. Feedstock type and manufacture conditions of biochar are critical to the amount of total P and available P in biochar.[8]reported that the amount of P in biochar derived from animal bone chips could be 152.0 g kg−1 and that water-extractable P was 6.6 g kg−1. In addition to being a potential P source, some biochar can also adsorb phosphate efficiently from solutions[9,10]suggesting that biochar could play a role in retaining P applied as fertilizer.
Biochar has specific properties that correct soil acidity due to its alkaline nature and high pH buffering capacity. A common consensus has been reached that the addition of biochar increases acid soil pH to some extent, as the pH of biochar in most cases is >7.0, at least 1.5 units higher than acid soil (normally > 5.5). Cations in feedstock form carbonates or oxides during the pyrolysis process. These carbonates and oxides react with the H+ and monomeric Al species in acid soils, and so increase the soil pH and decrease exchangeable acidity[11]. Biochar has been reported to decrease Al bioavailability in acid soils and to alleviate its toxicity to plant growth. In addition to decreasing soil acidity and Al toxicity, biochar can improve the fertility of acid soils by increasing the nutrient availability, and so enhance crop productivity. Lehmann and Joseph (2015) concluded the concentrations of average available macronutrients varied from about 25 to 117 mg kg−1 (available P) and from 1400 to 14,000 mg kg−1 (available K), respectively.
Recently a novel mechanism of phosphate adsorption on biochar has received increasing attention, as biochar could be a low-cost and effective adsorbent for removal of phosphate in water and wastewater. The raw biochar from various agricultural residues biomass has shown limited adsorption capacities for phosphate due to lack of effective interactions with phosphate (Shepherd et al., 2016). On the other hand, the metal oxide-coated lignocellulose- derived biochar such as CaO and MgO-coated biochar revealed high adsorption capacities of phosphate. Thus, the review was aimed at review biochar and its potential as a source of phosphorus fertilizer, acid soil ameliorating ability, improving P availability of biochar, and implying current knowledge on engineered biochar to adsorb P from liquid wastes to sustainably use phosphorus, which is a scarce global resource in our era.
Biochar and its role as a soil amendmentBiochar is a carbon-rich product that results from the pyrolysis (thermal decomposition), in the absence or low oxygen and at relatively low temperatures (<700°C) combustion of carbonaceous biomass such as agricultural residues, stall bedding, sewage sludge, and sawmill wastes, which is a stable form of carbon and can last for thousands of years in the soil[12,13]. The conditions of pyrolysis and the materials used can significantly affect the properties of biochar. The physical properties of biochar contribute to its function as a tool for managing the environment. It has been reported that when biochar is used as a soil amendment, it stimulates soil fertility and improves soil quality by increasing soil pH, increasing the ability to retain moisture, attracting more useful fungi and other microbes, improving the ability of cation exchange, and preserving the nutrients in the soil[14]. Biochar produced from waste biological matter under controlled conditions and subsequently added to agricultural soils is much safer than traditional slash and burn techniques [15], and is much more economically feasible. It is produced for adding to the soil as a means of sequestering carbon and improving soil quality. Biochar reduces soil density and soil hardening, increases soil aeration and cation-exchange capacity, and changes the soil structure and consistency through the changes in physical and chemical properties. It also helps to reclaim degraded soils. It has shown a greater ability to adsorb cations per unit carbon as compared to other soil organic matters because of its greater surface area, negative surface charge, and charge density[16], thereby offering the possibility of improving yields[17]. Samples with a sufficient amount of stable carbon can be added to the soil to be sequestered; a high sorption surface of biochar can characterize it as a soil additive, competent of halting risk elements in the soil.
Studies of these soils have found an abundance of soil organic matter and nutrients essential to soil fertility including N, P, and Ca, stemming from the incomplete combustion of plants, leading to charcoal mixed in with soils. (Glaser et al. 2002
Recent studies have examined the potential of biochar to increase crop yield in a variety of soil conditions. Biochar has been noted to increase the soil fertility, pH in acidic soils, soil cation exchange capacity and improve soil microbial activity and nutrient retention (Qian et al. 2015).
It is composed of carbon (C), hydrogen (H), oxygen (O), nitrogen (N), sulfur (S), and ash in different proportions. It is mainly used to improve soil nutrient content and to sequester carbon from the environment[18]. It is a highly porous structure makes it an attractive option for soil amendment as it improves the water holding capacity of the soil by increasing the total surface area of the soil[19].
Biochar is widely used as a soil amendment in fields. It improves overall soil quality. Biochar improves the water holding capacity of the soil thus helps in water retention for a longer period, which may be attributed to its highly porous structure[20,21]. Thus, it reduces the cost involved in the irrigation by reducing its frequency and intensity. The addition of biochar to acidic soil has shown an increase in soil pH[22]. Thus, biochar possesses a liming effect on soil. [23] suggested that the increase in the soil pH suppresses the activity of an enzyme(s) involved in the conversions of nitrite to nitrous oxide thereby increasing nitrogen availability in the soil. Addition of biochar in soil results in increased CEC, which in turn reduces the loss of nutrients through leaching[24].
Since the biochar possesses high CEC it can hold the nutrient present in the soil, therefore it increases the nutrient use efficiency of the soil which otherwise are washed away due to precipitation. When the biomass is converted to biochar, 50% of carbon present in the biomass is trapped in its structure that is more stable as compared to the biomass, which on degradation releases the carbon back in the atmosphere. Therefore, the production of biochar and its subsequent use in the soil creates a carbon sink
Phosphorus and its current global issuePhosphorus (P) is an essential, irreplaceable element in all living cells; without phosphorus, there would be no living thing on earth. The importance of phosphorus to life has focused attention on the efficient use of P in agriculture in recent years for three main reasons. First, phosphate rock (PR) from which phosphate fertilizers are made is a finite, nonrenewable resource, and it must be used efficiently to maximize its life span. Second, there is a need to maintain and improve the P status of many soils, particularly those in least-developed countries, for the growth of crops for food, fiber, and bioenergy. Third, a major determinant of the adverse effect of eutrophication in surface water bodies can be P transferred from soil to water.
The size of the global phosphorus resource has been a major source of concern recently. Of all the elements required in large amounts by living cells, the global resource of phosphorus is the smallest. Recently it was, suggested that with no change in demand, exploitable reserves of PR, the economically minable reserve of PR would be exhausted in 60–100 years at the then-current rates of extraction[25].Such predictions highlight the need to both use phosphorus efficiently and recycle it to close the cycle of supply and demand[26]. Increasing nutrient concentrations in the water has led to increasing reports of the production of often-toxic algal blooms and excessive aquatic plant growth that impacts adversely on biodiversity, water quality for domestic use, fish stocks, and the recreational use of the water body. Often, these adverse effects are associated with the transfer of P from agriculturally managed soil to water. Much of the P that is transported appears to derive from specific areas (hot spots) within a river catchment [27]and is associated with eroded soil mineral and organic particles or application of excessive amounts of P fertilizer or animal manure applied when soil conditions are not suitable[28,29]. In some cases, this transfer has been linked with the inefficient use of phosphorus in agriculture.
Many agricultural lands throughout the world are suffering P deficiency, especially in tropical and subtropical areas because of high rainfall and high P fixation [30]. Approximately 15 million tons of P fertilizer is globally applied to agricultural land each year To meet plant P requirements[31]. However, the crop takes up only 5–30% of P applied in the year following application [32].
A substantial amount of the applied ortho-phosphate is often lost to the aqueous environment via runoff. [33] reported that natural runoff due to rainfall accounts for approximately 79% of P loss from rice fields. Currently, the majority of P fertilizer is derived from mined rock phosphate, a non-renewable resource. It has been predicted [34] that supplies of rock phosphate may be depleted by the end of this century, as international demand for P further increases over the next 50 years, in response to global population growth.
Thus, there is a pressing need to explore new strategies that can provide P in forms available for plant uptake, which can supplement and enhance or supersede traditional P fertilizer treatments, reducing the loss of plant-available P from soils and thereby overcoming P deficiency in the short term.
Biochar as a promising source of P fertilizerBiochar is a potential material for making slow-releasing phosphorus (P) fertilizers for the sake of increasing soil P use efficiency and mitigating P losses. Biochar, which is the product of biomass (i.e. crop residues, manure, and biosolid) pyrolysis, is usually used as a soil amendment because of its improvements on soil fertility, water retention, and microorganism diversity as well as its function of mitigating climate change via carbon sequestration[35,36,37]. In most cases, the P content of biochar is very low except for the biochar derived from manure[38], which may have high P content; however, biochar could increase its P content through P adsorption because of its porous structure. [39] reported that the biochar derived from sugar cane and Miscanthus had phosphate adsorption at 12.8-15.8 mg PO43- g-1. [40]reported a higher P adsorption at 8.8-13.9 mg Pg-1 for the pine and maize-straw biochar. Even so, biochar P content could not be comparable to that of mineral fertilizers. For instance, the P content of the commercial triple superphosphate (TSP) is over 46% as P2O5. [41] mentioned the maximum P adsorption of biochar could be as high as 100 mg Pg-1. [42] reported that Biochar derived from dead dairy cattle can be used as an amendment for soil acidity, and as a P fertilizer. Where fine-size biochar (< 1 mm) charred at 450°C for 4 h is used, the biochar can give similar results to superphosphate fertilizer for DM production and P uptake by the plant.
The P in biochar is resistant to loss, and, on most occasions, the application of biochar results in a reduction of P loss from soil. The P loss potential of soil can be influenced by modifying its physical, chemical, and biological properties (e.g., pH, CEC, P adsorption capacity, contents of multivalent metal cations, water-holding capacity, bulk density, structure, aggregate stability, dissolved organic matter concentration, microbial structure, and activity) [43,44] reported that biochar has the potential to improve soil P availability through either its direct inputs or its retention of fertilizer P. However, the extent of biochar effects on soil P availability varies greatly with biochar type, pyrolysis temperature, and time.
During pyrolysis of organic material, a complex mixture of P species is formed. This may include amorphous, semi-crystalline, and crystalline constituents, along with organic constituents[45]. However, organic P forms will tend to disappear while inorganic P forms will subsequently be formed, with crystallinity increasing as pyrolysis temperature increases[46] . Phytate has been observed to be converted to inorganic P when pyrolyzing at a temperature ≥of 350 °C [47,48] . Orthophosphate (PO43–) is the only P species observed in biochar produced from manures at temperature≥500°C (Wang et al., 2013), whereas pyrophosphate (P2O74–) can still be found in biochar produced from plant residues at temperatures as high as 650°C[49,50].
In addition to pyrolysis conditions, the availability of P in biochar is related to the amount of P originally present in the feedstocks, and the nature of the form of P. Biochar produced from lignocellulosic residues generally have a low amount of available P (< 1 g kg–1 as estimated using 2% formic acid proposed by[51], whereas considerable amounts of available P can be found in biochar derived from animal and human waste (> 4 g kg–1)[52]. Values of 2% formic acid extractable P > 100 g kg–1 have also been reported by [53]when using meat and bone meal as feedstock, these representing > 90% of total P. The fraction of available P out of total P is also generally smaller in plant residue–derived biochar (< 40%) than in animal–waste-derived biochar (> 65%) [54] detected a dominance of pyrophosphate over orthophosphate in biochar pyrolysis from cottonseed hulls under 350 and 650°C. They suggested that this form of P in this biochar might be stabilized through complexation of P with ash components or with organic C (e.g., electrostatic and H–bonding interactions) rendering it less available.
[55]demonstrated that both high and low-temperature pyrolyzed biochar could be used to lade P and work as P fertilizers. Compared to mineral P fertilizers, P-laden biochar fertilizers have shown some advantages such as slow-releasing the P laden and using fewer P to have a high soil available P level.
Role of biochar in improving Phosphorus availability in acid soilsSoil acidity is a major constraint for crop production on highly weathered tropical soils characterized by high Al toxicity, low cation exchange capacity, and low P availability to plants. In such soils, acidic cations such as Al and Fe predominate, and depending on soil pH, they fix the applied inorganic P. Phosphorus is generally available to crops at a soil pH of 6 to 7. Below this range, P is fixed due to active forms of Al and Fe oxides and hydroxides. Therefore, in acidic soils, application of regular P fertilizers, such as rock phosphates and triple superphosphate, are required to saturate Al and Fe ions to maintain an adequate supply of plant-available P. However, this approach is neither economical nor environmentally friendly. Excessive or unbalanced use of P fertilizers causes water pollution, such as eutrophication.
Biochar increased crop yield through liming and fertilization, of low soil pH, and low fertility, typical of tropical soils. More specifically[56] reported increasing soil moisture, available K, and P by the addition of biochar in a moderately acidic low-input Nepalese soil. Application of pine-chip and poultry litter (50:50) biochar blend significantly increased soil concentrations of K, P, Na, Mg, and Ca by 830%, 669%, 2315%, 687%, and 307%, respectively, relative to the control Biochar amendment can change the soil pH, depending on the type of soil or BC that is applied to the soils. Simply, the application of alkaline BCs to acidic soils can increase soil pH and thus affect the nutrient bioavailability. Therefore, as a soil amendment, BC can induce a liming effect neutralize soil acidity, and enhance soil quality by improving the availability of basic soil nutrients.
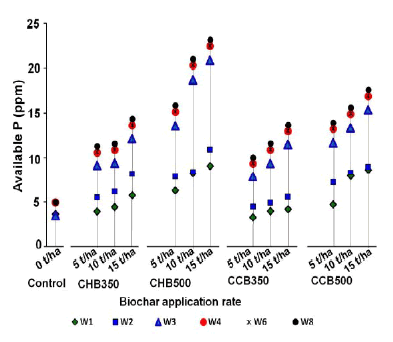
Biochar incorporation into soils was most effective on P availability in acidic soils. In acidic soils, Al3+ and Fe2+ /Fe3+, mainly regulate the plant availability of P by forming Al-and Fe-phosphates. As soon as the result of applying biochar to acid soils, increases soil pH, the P sorption onto Fe and Al oxides decreases. Moreover, biochar itself can be a source of Ca and Mg and those elements can affect P availability. On the other hand, studies hypothesizing that through the application of biochar to acidic soils phosphate bonded with free cations such as Fe3+, Al3+, Ca2+ and Mg2+ dissolved and released plant-available P. After the application of biochar to acidic soil, phosphates bound with free cations such as Ca2+, Mg2+, Fe3+, and Al3+ dissolve, and released P is available for plant uptake again. In addition to being a potential P source, some biochar can also adsorb phosphate efficiently from solutions, suggesting that biochar could play a role in retaining P applied as fertilizer. Most of the biochar produced by different researchers have alkaline property, pH more than 8 so that it could be used as liming material and improve plant-available phosphorus in acidic soils. [57] reported an increase in soil pH and a decrease in exchange acidity and exchangeable Al upon biochar application. They conclude that application of the biochar prepared from coffee husk produced at 500°C and applied at a rate of 15t/ha increased available P by 84.3% in acid soils. The biochar application has also increased the pH and CEC of the soil.
Where CHB- coffee husk biochar, CCB - corncob biochar and both pyrolyzed at 350°C and 500°C
Effects of engineered biochar on phosphorus adsorption from aqueous wastes
Biochar has unique properties, including a porous structure and abundant functional groups, which can offer potentially alternative and environmentally friendly options for the removal of various contaminants (i.e., heavy metal and phosphate) from aqueous solution. Recently, biochar has been used as a potential adsorbent for pollutant recovery due to its beneficial properties including low cost, environmental friendliness, excellent stability, general safety, and high porosity[58]. As a result, research has focused on the application of biochar for phosphate recovery because of its strong sorption affinities and low material cost. However, the surfaces of biochar are predominantly net negatively charged, which confers them only negligible ability to absorb anionic pollutants[59]. In agreement with this finding, reported that the adsorption capacity of conventional biochar has a limited ability to effectively adsorb pollutants due to its limited functionalities. To improve the phosphate adsorption performance of biochar, cation-modified biochar has recently been developed. Recently Results suggest that functionalization and modification of raw biochar could dramatically improve its adsorption ability. Such methods include modification by impregnating with single- (Mg, Al, Fe, and Mn) [60]or dual-metal agents (Ca/Fe) (Agrafioti et al., 2014).
The use of carbon/biochar to remove phosphate from aqueous solutions is an emerging and promising concept with plenty of advantages over traditional wastewater treatment technologies, such as lower cost and/or being more environmentally friendly.
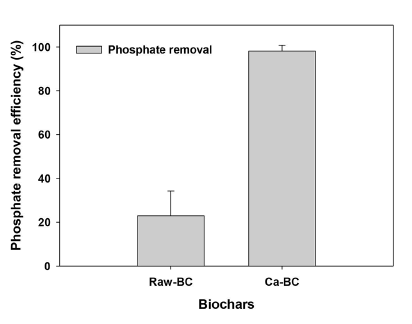
Studies have developed new functional materials by loading cations, such as Fe-modified wheat straw Bi-modified cottonwood and Al-treated biochar [61] which have yielded phosphate removal percentages in the range of 84.65–99.3%.
Calcium and magnesium can be used as metal sources with many benefits because they are involved in chlorophyll formation in plants and are environmentally friendly; besides, the metal salts are easily obtained in nature, and their alkaline character can neutralize acids and provide a suitable pH range for nutrient recovery. prepared mesoporous MgO modified sugarcane residue biochar with a maximum removal capacity of 398 mg/g for phosphate in swine wastewater, and used a calcium-rich adsorbent for phosphate adsorption and achieved high phosphorus removal rates, adopting calcium or magnesium modification individually. Metal oxide deposited on the BC surface becomes more attractive for both anionic and cationic molecules by providing specific affinities. Generally, metal impregnated BC containing positive sites and improved oxidized surface would be attractive for anionic ions. [61] reported that MgO impregnated BC derived from a pair of different biomass feedstock showed excellent removal efficiencies of phosphate in water. They showed that impregnation of Mg caused the formation of nano-sized MgO grains on the BC surface. Steam activation of biochar accelerated nutrient retention and uptake by plants relative to nonactivated biochar. [62] prepared three magnetic biochar by chemical co-precipitation of Fe3+/Fe2+ on the raw material of orange peel powder and pyrolyzed it under different temperature conditions. All these studies showed that biochar needs to be treated for specific purposes to achieve optimal environmental services.
Conclusion
Global Phosphorus reserves are rapidly being depleted. Moreover, agricultural soils especially of tropical and subtropical regions of the world are inherently poor in available P content thus ultimately affecting crop yield. Ensuring phosphorus remains available for food production by future generations and preventing pollution with phosphorus in water systems requires actions in two main areas: reducing phosphorus losses, especially from agricultural lands, and increasing phosphorus recovery and reuse to agricultural lands. Therefore, the recycling of P in agricultural residues is critical for P sustainability in agricultural systems, which is dominated by the route of direct land application. Biochar production from agricultural residues and its subsequent land application has been suggested as solutions for waste biomass disposal, carbon sequestration, soil amendment/remediation, and crop production promotion.
Even though there is a possibility to lose some nutrients like nitrogen during pyrolysis, phosphorus is resistant to high-temperature heating during biochar production, which makes biochar an alternative source of slow-release fertilizer. Many recent experiments concluded that high surface areas of biochar make it to absorb more nutrients from exchangeable sites and improve their availability for plant uptake. It is also investigated that the high pH of biochar produced from different agricultural residues makes its application to acid soil to improve phosphorus availability and decreasing Aluminum toxicity for plant growth. Finally, in this review, recent knowledge on engineered biochar by metal oxides to adsorb phosphate from liquid wastewater was investigated as a novel approach to recycle phosphorus and reduce its environmental impact.
References
- Adnan A, Mavinic DS, Koch FA (2003) Pilot-scale study of phosphorus recovery through struvite crystallization examining the process feasibility Journal of Environmental Engineering and Science 2(5) pp.315-324.
- Agrafioti E, Kalderis D, Diamadopoulos E (2014) Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions Journal of Environmental Management 146 pp.444-450.
- Ajema L (2018) Effects of biochar application on beneficial soil organisms International Journal of Research Studies in Science Engineering and Technology 5(5) pp.9-18.
- Blake L, Mercik S, Koerschens M, Moskal S, Poulton PR, Goulding K W T, Weigel A, Powlson DS (2000) Phosphorus content in soil uptake by plants and balance in three European long-term field experiments Nutrient Cycling in Agroecosystems 56(3) pp.263-275.
- Borchard N, Wolf A, Laabs V, Aeckersberg R, Scherer HW, Moeller A, Amelung W (2012) Physical activation of biochar and its meaning for soil fertility and nutrient leaching–a greenhouse experiment Soil Use and Management 28(2) pp.177-184.
- Brewer CE, Hu YY, Schmidt Rohr K, Loynachan TE, Laird DA Brown RC (2012) Extent of pyrolysis impacts on fast pyrolysis biochar properties Journal of environmental quality 41(4) pp.1115-1122.
- Camps-Arbestain M, Amonette JE, Singh B, Wang T, Schmidt HP (2015) A biochar classification system and associated test methods Biochar for environmental management science technology and implementation pp.165-193.
- Chen B, Chen Z Lv S (2011) A novel magnetic biochar efficiently sorbs organic pollutants and phosphate Bioresource technology 102(2) pp.716-723.
- Chintala R, Mollinedo J, Schumacher T E, Malo D D, Julson JL (2014) Effect of biochar on chemical properties of acidic soil Archives of Agronomy and Soil Science 60(3) pp.393-404.
- Choi YK, Jang HM, Kan E, Wallace AR, Sun W (2019) Adsorption of phosphate in water on a novel calcium hydroxide-coated dairy manure-derived biochar Environmental Engineering Research 24(3) pp.434-442.
- Dume B, Ayele D, Regassa A, Berecha G (2017) Improving available phosphorus in acidic soil using biochar Journal of Soil Science and Environmental Management 8(4) pp.87-94.
- Gburek W J, Sharpley AN, Heathwaite L, Folmar GJ (2000) Phosphorus management at the watershed scale A modification of the phosphorus index Journal of environmental quality 29(1) pp.130-144.
- Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal–a review Biology and fertility of soils 35(4) pp.219-230.
- Guo Y, Tang H, Li G, Xie D (2014) Effects of cow dung biochar amendment on adsorption and leaching of nutrient from an acid yellow soil irrigated with biogas slurry Water Air & Soil Pollution 225(1) p.1820
- Hilton J, Johnston AE and Dawson CJ (2010) The phosphate life-cycle rethinking the options for a finite resource. In Proceedings-International Fertiliser Society International Fertiliser Society.
- Hinsinger P, Brauman A, Devau N, Gérard F, Jourdan C, Laclau JP, Le Cadre E, Jaillard B and Plassard C (2011) Acquisition of phosphorus and other poorly mobile nutrients by roots Where do plant nutrition models fail Plant and Soil 348(1-2) p.29.
- Hou J, Huang L, Yang Z, Zhao Y, Deng C, Chen Y and Li X (2016) Adsorption of ammonium on biochar prepared from giant reed. Environmental Science and Pollution Research 23(19) pp.19107-19115.
- Johnston AE and Dawson CJ (2005) Phosphorus in agriculture and in relation to water quality Peterborough Agricultural Industries Confederation.
- Kirkby EA and Johnston AEJ (2008) Soil and fertilizer phosphorus in relation to crop nutrition In The ecophysiology of plant-phosphorus interactions Springer Dordrecht.
- Laird D, Fleming P, Wang B, Horton R and Karlen D (2010) Biochar impact on nutrient leaching from a Midwestern agricultural soil Geoderma pp.436-442.
- Lehmann J and Joseph S (2015) Biochar for environmental management science technology and implementation Routledge.
- Lehmann J and Joseph (2009) Biochar for environmental management an introduction Biochar for environmental management Science and technology pp.1-12.
- Lehmann J (2007) Bioenergy in the black Frontiers in Ecology and the Environment 5(7) pp.381-387.
- Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC and Crowley D (2011) Biochar effects on soil biota a review Soil biology and biochemistry 43(9) pp.1812-1836.
- Li H, Li Y, Xu Y and Lu X (2020) Biochar phosphorus fertilizer effects on soil phosphorus availability Chemosphere 244 p.125471.
- Li R H, Wang J J, Zhou, B Y, Zhang Z Q, Liu S, Lei S & Xiao R (2017) Simultaneous capture removal of phosphate ammonium and organic substances by MgO impregnated biochar and its potential use in swine wastewater treatment Journal of Cleaner Production 147, 96–107.
- Li R, Wang JJ, Zhou B, Awasthi MK, Ali A, Zhang Z, Lahori AH and Mahar A (2016) Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate-based fertilizer substitute Bioresource technology 215 pp.209-214.
- Liang B, Lehmann J, Solomon D, Kinyangi J, Grossman J, O'neill B, Skjemstad JO, Thies J, Luizão FJ, Petersen J and Neves EG, (2006) Black carbon increases cation exchange capacity in soils Soil Science Society of America Journal 70(5) pp.1719-1730.
- Liang XQ, Tian GM, Li H, Chen YX and Zhu S (2005) Study on characteristic of nitrogen and phosphorus loss from rice field by natural rainfall runoff Journal of Soil Water Conservation.
- Ma YL and Matsunaka T (2013) Biochar derived from dairy cattle carcasses as an alternative source of phosphorus and amendment for soil acidity Soil Science and Plant Nutrition 59(4) pp.628-641.
- Major J, Steiner C, Downie A, Lehmann J and Joseph S (2009) Biochar effects on nutrient leaching Biochar for environmental management Science and technology 271.
- Pandit NR, Mulder J, Hale SE, Martinsen V, Schmidt H P and Cornelissen G (2018) Biochar improves maize growth by alleviation of nutrient stress in a moderately acidic low-input Nepalese soil Science of the Total Environment 625 pp.1380-1389.
- Parvage M.M, Ulén B, Eriksson J, Strock J and Kirchmann H (2013) Phosphorus availability in soils amended with wheat residue char Biology and fertility of soils 49(2) pp.245-250.
- Price, G. ed., 2006. Australian soil fertility manual. CSIRO PUBLISHING.
- Qayyum, M.F., Ashraf, I., Abid, M. and Steffens, D., 2015. Effect of biochar, lime, and compost application on phosphorus adsorption in a Ferralsol. Journal of plant nutrition and soil science, 178(4), pp.576-581.
- Qian, K., Kumar, A., Zhang, H., Bellmer, D. and Huhnke, R., 2015. Recent advances in utilization of biochar. Renewable and Sustainable Energy Reviews, 42, pp.1055-1064.
- Shepherd JG, Sohi SP and Heal KV (2016) Optimising the recovery and re-use of phosphorus from wastewater effluent for sustainable fertiliser development Water Research 94 pp.155-165.
- Sigua GC, Stone KC, Hunt PG, Cantrell KB and Novak JM (2015) Increasing biomass of winter wheat using sorghum biochars Agronomy for Sustainable Development 35(2) pp.739-748.
- Simmonds B, McDowell RW and Condron LM (2017) The effect of soil moisture extremes on the pathways and forms of phosphorus lost in runoff from two contrasting soil types Soil Research 55(1) pp.19-27.
- Streubel JD, Collins HP, Tarara JM and Cochran RL (2012) Biochar produced from anaerobically digested fiber reduces phosphorus in dairy lagoons Journal of environmental quality 41(4) pp.1166-1174.
- Trazzi PA, Leahy JJ, Hayes MH and Kwapinski W (2016) Adsorption and desorption of phosphate on biochars Journal of Environmental Chemical Engineering 4(1) pp.37-46.
- Uchimiya M and Hiradate S (2014) Pyrolysis temperature-dependent changes in dissolved phosphorus speciation of plant and manure biochars Journal of agricultural and food chemistry 62(8) pp.1802-1809.
- Uchimiya M, Hiradate S and Antal Jr MJ (2015) Dissolved phosphorus speciation of flash carbonization slow pyrolysis and fast pyrolysis biochars ACS Sustainable Chemistry & Engineering 3(7) pp.1642-1649.
- Van Kauwenbergh SJ (2010) World phosphate rock reserves and resources (p.48) Muscle Shoals IFDC.
- Wan S, Wang S, Li Y and Gao B (2017) Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions Journal of Industrial and Engineering Chemistry 47 pp.246-253.
- Wang T, Camps-Arbestain M and Hedley M (2014) The fate of phosphorus of ash-rich biochars in a soil-plant system Plant and Soil 375(1-2) pp.61-74.
- Wang T, Camps-Arbestain M, Hedley M and Bishop P (2012) Predicting phosphorus bioavailability from high-ash biochars Plant and Soil 357(1-2) pp.173-187.
- Weber B, Stadlbauer EA, Schlich E, Eichenauer S, Kern J and Steffens D (2014) Phosphorus bioavailability of biochars produced by thermo‐chemical conversion Journal of plant nutrition and soil science 177(1) pp.84-90.
- Xiao X, Chen B and Zhu L (2014) Transformation, morphology and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures Environmental science & technology 48(6) pp.3411-3419.
- Yao Y, Gao B, Chen J and Yang L (2013) Engineered biochar reclaiming phosphate from aqueous solutions mechanisms and potential application as a slow-release fertilizer Environmental science & technology 47(15) pp.8700-8708.
- Yao Y, Gao B, Inyang M, Zimmerman A R, Cao X, Pullammanappallil P and Yang L (2011) Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings Journal of hazardous materials 190(1-3) pp.501-507.
- Yao Y, Gao B, Zhang M, Inyang M and Zimmerman A R (2012) Effect of biochar amendment on sorption and leaching of nitrate ammonium and phosphate in a sandy soil Chemosphere 89(11) pp.1467-1471.
- Yin H and Kong M (2014) Simultaneous removal of ammonium and phosphate from eutrophic waters using natural calcium-rich attapulgite-based versatile adsorbent Desalination 351pp.128-137.
- Zhang H, Chen C, Gray EM, Boyd SE, Yang H and Zhang D (2016) Roles of biochar in improving phosphorus availability in soils a phosphate adsorbent and a source of available phosphorus Geoderma 276 pp.1-6.
- Zhang M and Gao B (2013) Removal of arsenic methylene blue and phosphate by biochar/AlOOH nanocomposite Chemical engineering journal 226 pp.286-292.
- Zhang M, Gao B, Yao Y, Xue Y and Inyang M (2012) Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions Chemical Engineering Journal 210 pp.26-32.
- Zhang R, Zhong Z and Huang Y (2009) Combustion characteristics and kinetics of bio-oil Frontiers of Chemical Engineering in China 3(2) pp.119-124.
- Zhao L, Cao X, Mašek O and Zimmerman A (2013) Heterogeneity of biochar properties as a function of feedstock sources and production temperatures Journal of hazardous materials 256 pp.1-9.
- Zhao S, Wang B, Gao Q, Gao Y and Liu S (2017) Adsorption of phosphorus by different biochars Spectroscopy Letters 50(2) pp.73-80.
- Zhou H, Jiang Z and Wei S (2013) A novel absorbent of nano-Fe loaded biomass char and its enhanced adsorption capacity for phosphate in water Journal of Chemistry.
- Zhu N, Yan T, Qiao J and Cao H, (2016) Adsorption of arsenic phosphorus and chromium by bismuth impregnated biochar Adsorption mechanism and depleted adsorbent utilization Chemosphere 164 pp.32-40.
- Zwetsloot MJ, Lehmann J and Solomon D (2015) Recycling slaughterhouse waste into fertilizer how do pyrolysis temperature and biomass additions affect phosphorus availability and chemistry Journal of the Science of Food and Agriculture 95(2) pp.281-288.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences