Phase I Study of CIGB-300 Administered Intravenously in Patients with Relapsed/ Refractory Solid Tumors
1Clinical Oncology Department, Hermanos Ameijeiras Hospital, Havana, Cuba
2CIGB-300 Research and Development Group, Center for Genetic Engineering and Biotechnology (CIGB), Cuba
3Institute of Pharmacy and Food, University of Havana, Havana, Cuba
4Oncology Service, Celestino Hernández Robau Hospital, Santa Clara, Cuba
5Oncology Service, Center for Medical- Surgical Research, Havana, Cuba
6Molecular Oncology Laboratory, National University of Quilmes, Buenos Aires, Argentina
7ELEA Laboratories, Buenos Aires, Argentina
- *Corresponding Author:
- Silvio E Perea-Rodríguez
Laboratory of Molecular Oncology
Biomedical Research Department
Center for Genetic Engineering and Biotechnology
Cubanacán, Playa, Havana 10600, Cuba.
Tel: +46 8 52480000
Fax: +53 (7) 2736008
E-mail: silvio.perea@cigb.edu.cu
Received date: December 26, 2017; Accepted date: January 29, 2018; Published date: February 05, 2018
Citation: Batista-Albuerne N, González-Méndez L, García-García I, Fernández-Sánchez E, García-Diegues R, et al. (2018) Phase I Study of CIGB-300 Administered Intravenously in Patients with Relapsed/Refractory Solid Tumors. J Med Oncol. Vol.1 No.1:4
Abstract
Background: CIGB-300 is an anti-CK2 peptide previously tested by intralesional injections. Here, we explored tolerability, drug accumulation ratio and clinical benefit of intravenous CIGB-300 in patients with relapsed/refractory solid tumors. Methods: A multicenter and open-label phase I study. CIGB-300 administration was performed during 15 min once daily on days 1-5 in alternate weeks until completing three cycles. Stage 1 aimed to determine Maximum Tolerated Dose (MTD) and dose-limiting toxicity (DLT) in dose-escalation (0.2-1.6 mg/kg) while stage 2 explored drug accumulation ratio comparing Cmax and AUC0-12 from first and fifth infusions with 1.6 mg/kg of CIGB-300. Clinical benefit was assumed if patients lived ≥ 6 months. Results: In total, 16 patients were enrolled. Neither MTD nor DLT was observed. The most frequent adverse events were those related to mild allergic syndrome (61.6%) whose magnitude behaved dose-dependently. Drug accumulation ratio between infusions #1 and #5 was 1.0. Twelve patients (75%) lived >6 months and seven patients (43.7%) lived ≥ 12 months after CIGB-300 monotherapy. Four patients died before 6 months. Conclusion: Intravenous administration of CIGB-300 1.6 mg/kg is recommended to be tested in Phase 2-3 trials in patients with either relapsed/refractory NSCLC or breast cancer. Efficacy of once versus twice-daily CIGB-300 administrations can be also compared in upper phase trials.
Keywords
CIGB-300; Refractory tumors; Tolerability; Drug accumulation ratio; Survival
Introduction
Chemoradiotherapy is the widely deemed gold standard care for cancer patients since many years ago albeit a relative limited therapeutic index is observed in solid tumors [1]. Particularly, intrinsic drug resistance and relapse constitute the mayor problem to envision a rationale therapeutic regime [2]. Therefore, the use of the dubbed molecularly targeted drugs combined to standard chemoradiotherapy in cancer patients could be a right path to outreach tumor complexity ensuing better clinical outcome [3,4]. Among the newcomer oncology targets, experimental validation of CK2 has experienced tangible progress by using specific inhibitors comprising small molecules [5-7], antisense oligonucleotides [8] and synthetic peptides [9,10]. As CK2 is responsible about near 24% of the cellular phosphoproteoma and it is linked to physiopathology from many solid and hematopoietic tumors, the CK2 inhibitors could be appraised as promising anticancer drug candidates. However, only two of them, the CX-4945 and CIGB-300 are being tested in the clinical ground in cancer patients.
CX-4945 (Silmitasertib) is an ATP-competitive inhibitor of both CK2α and CK2α’ catalytic subunits [7] whose Maximum Tolerated Dose (MTD) has been investigated in combination with gemcitabine plus cisplatin in a Phase I trial (ClinicalTrials.gov Identifier: NCT02128282). A subsequent Phase II study is currently carried out to investigate the antitumor activity of CX-4945 combined with gemcitabine and cisplatin in cholangiocarcinoma patients at the combination previously determined. Furthermore, CIGB-300 is a synthetic peptide that impairs the CK2-mediated phosphorylation by targeting the substrate phosphoaceptor domain outcompeting the enzyme [9,11] and elicits antitumor activity when tested in preclinical animal models by either intratumor injections [9,12] or systemic delivery [12]. Importantly, inhibition of metastasis and tumor angiogenesis has been also documented in vivo by using systemic administration of CIGB-300 in a mouse model [13]. Molecularly, CIGB-300 chiefly binds and inhibits the B23/nucleophosmin (B23/NPM) phosphorylation with subsequent cellular events indicating nucleolar disassembly [11]. However, recent data in chronic lymphocytic leukemia (CLL) have shown that an inhibition of the CK2-mediated phosphorylation is also observed in substrates other than B23/NPM like AKT, PTEN and GSK-3 proteins [14]. Importantly, synergistic interactions between CIGB-300 and conventional chemotherapeutic drugs like cisplatin and paclitaxel and other cancer targeted therapies like Erlotinib have been documented at the preclinical stage [15,16]. In the clinical ground, CIGB-300 has been tested in Phase I clinical trials in cervical malignancies [17-19] where a thorough optimization of the intralesional delivery allowed the design of an appropriate protocol to be further accomplished in a Phase II trial. Briefly, injection of 70 mg of CIGB-300 in two sites into tumors leads to the highest tumor uptake. Also, a systemic allergic-like syndrome, well-correlated with plasmatic histamine levels, was the most relevant systemic side effect reported after intralesional injection of CIGB-300. However, no previous clinical studies using the intravenous route for the CIGB-300 delivery had been performed so far.
We designed a phase I study of CIGB-300 intravenously administered in patients with relapsed/refractory solid tumors with an Eastern Cooperative Oncology Group (ECOG) performance status of 3-4 and life expectancy ≤ 6 months. The primary objectives of this study were to test safety and tolerability of CIGB-300. Secondary objectives were to explore the drug accumulation ratio in plasma and clinical benefit was defined as any disease stabilization and increase of survival beyond 6 months.
Materials and Methods
Patient selection
Sixteen patients with progressive disease and refractory or relapsed solid tumors (≥ 1 cycle of chemotherapy) were recruited and verified for eligibility in the oncology service from Hermanos Ameijeiras Hospital, Celestino Hernández Hospital and Center for Medical-Surgical Research. Patients were included if they were 18 to 75 years old and gave their written, informed consent to participate and if their relapse was at least 4 weeks after the last chemotherapy treatment. All of recruited patients exhibited ECOG 3-4 with ≤ 6 months of life expectancy. Exclusion criteria were concomitant ingestion of immunosuppressive or immunomodulatory therapy, psychiatric disease, pregnancy, breastfeeding, uncontrolled chronic diseases such as systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, epilepsy, diabetes or hypertension, autoimmune disease, coagulation dysfunction and severe allergy with hives, dermatitis, bronchitis and persistent bronchial asthma. Patients entered into other clinical trials in the last eight weeks were also excluded.
This trial followed the principles of Good Clinical Practices, in accordance with the Declaration of Helsinki and its amendments. The protocol was approved by the Ethic Committees of the involved hospitals and by the Cuban Regulatory Authority, the State Center for the Control of Drugs, Equipment & Medical Devices (CECMED): reference number: 06.027.11.B. Cuban Public Registry of Clinical Trials: RPCEC00000112.
Study design and treatment plan
A randomized, open-label, uncontrolled, dose-escalating, sequential trial was performed. In the stage 1, an escalation protocol for determining the Maximum Tolerated Dose (MTD) was performed by the Continual Reassessment Method, starting from 0.2 mg/kg until 1.6 mg/kg of CIGB-300. Treatment allocation was planned from a dose-toxicity model that sets the subsequent dose to use in terms of the toxicity observed in the previous dose level ensuring that probability of severe toxicity did not exceed the maximum allowable dimension (33%). In the stage 2 of this study, a pharmacokinetic analysis was performed after infusions #1 and #5 during the first CIGB-300 cycle in four patients treated with the highest dose (1.6 mg/kg).
CIGB-300 administration was performed by 15 min intravenous infusions using appropriated pumps once daily on days 1-5 in alternate weeks until completing 3 cycles (15 doses). CIGB- 300 was synthesized and supplied by the Peptide Synthesis Unit, Center for Genetic Engineering and Biotechnology (CIGB), Havana, in 5, 15 or 35 mg vials, as lyophilized powder. Vials were reconstituted in 50 mL of saline solution to provide the appropriated dose in all the cases. Premedication with diphenhydramine and hydrocortisone 30 min prior to each CIGB- 300 administration was performed, in order to mitigate the expected allergic-like events, previously reported by intralesional route [17-19]. If necessary, other interventions were indicated for the management of adverse events according to established clinical practices.
Safety evaluation
Pretreatment evaluation included a detailed history and physical examination. In addition, hematological counts, blood chemistry, coagulation and imagenogical studies were performed. Systemic toxicity was evaluated during 24 h after each CIGB- 300 administration and further followed during 3, 6, 9 and 12 months after completing CIGB-300 treatment. Severity of adverse events was classified according to the Cancer Therapy Evaluation Program, Common Terminology Criteria [20] and they were subsequently classified by the WHO Adverse Reaction Terminology [21]. Causal relationship was classified as very probable (definitive), probable, possible or remote (doubtful) [22]. MTD was defined as the highest dose explored with <33% of severe toxicity. The CIGB-300-induced histamine levels were measured in plasma by a commercially available EIA kit (Labor Diagnostika, GmBH, Oberursel, Germany) before, 15 min and 24 h after the first infusion.
Drug accumulation ratio
To verify whether CIGB-300 accumulates after successive intravenous infusions, blood samples were taken immediately, 5, 15, 30 min and 1, 2, 3, 5, 7 and 12 h after the first and the fifth infusions in the first CIGB-300 cycle. Heparinized plasma was collected and CIGB-300 was quantified by an in-house validated immune competition-based enzyme immunoassay (EIA) [23]. Drug disposition evaluation was performed individually by non-compartmental analysis (NCA) and the pharmacokinetic parameters were calculated using the WinNonlin professional software (Version 5.1, Pharsight Inc., 2005, NC, USA). To calculate the drug accumulation ratio, both Cmax and AUC0-12 values from infusions #1 and #5 were taken into account.
Clinical activity
Signs of CIGB-300 clinical activity were defined as any increase in the patient survival beyond their life expectancy (≤ 6 months). Survival time was followed up since the beginning of CIGB-300 treatment until patients die.
Statistical analysis
Clinical data were double entered and validated on MS InfoPath and then imported into SPSS for Windows (version 15.0, IBM Analytics 2006, Armonk, North Castle, NY, USA) for further analysis. Assumptions of normality and the homogeneity of variance were verified using Shapiro-Wilks test and Levene’s test, respectively. The probability of severe toxicity was estimated in each dose group conditioned to the number of subjects included sequentially. Also the probability of exceed the maximum level of acceptable toxicity (30%) and the upper bound of toxicity were estimated. Pharmacokinetic parameters obtained from plasma profiles after first and fifth infusions were compared by Student's t-test. Correlation between histamine and CIGB-300 levels in plasma and between histamine levels and severity of the allergic reaction were performed by Spearman rank correlation analyses. The survival time was estimated from the Kaplan Meier curve.
Results
Patient characteristics and dosing history
Sixteen enrolled cancer patients, 9 (lung cancer), 4 (breast), 1 (ovary), 1 (thymus sarcoma), 1 (skin squamous cell carcinoma), fulfilled the selection criteria. Baseline characteristics are shown in Table 1. Most of the patients were men and white and mean age was 56 years, ranging from 41 to 73 year-old. Mean weight was 70 kg, ranging from 46 to 108 kg. Fourteen patients completed the whole CIGB-300 treatment and patients HHA-03 and HHA-15 definitively interrupted it. Patient HHA-03 received just eleven administrations of CIGB-300 because of disease progression and patient HHA-15 got infection after the second CIGB-300 administration at the site of implantable system for drug delivery. During stage 1 two individuals were included sequentially in each dose level (0.2, 0.4 and 0.8 mg/kg) and 10 patients were allocated to the highest dose level (1.6 mg/kg) for the stage 2 of this trial.
| Characteristic | Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|---|
| (0.2 mg/kg) | (0.4 mg/kg) | (0.8 mg/kg) | (1.6 mg/kg) | ||
| N=2 | N=2 | N=2 | N=10 | ||
| Male gender | 0 | 1 (50%) | 1 (50%) | 7 (70%) | |
| Skin color | White | 1 (50%) | 1 (50%) | 2 (100%) | 8 (80%) |
| Non-white | 1 (50%) | 1 (50%) | 0 | 2 (20%) | |
| Age (years) | 46.5 ± 5 | 51.5 ± 6 | 56.5 ± 19 | 59 ± 10 | |
| (43-50) | (47-56) | (43-70) | (41-73) | ||
| Weight (kg) | 65.5 ± 1 | 77.5 ± 20 | 73 ± 2 | 70 ± 18 | |
| (65-66) | (63-92) | (71-75) | (46-108) | ||
| Height (cm) | 163 | 164 ± 17 | 151 ± 1 | 167 ± 9 | |
| (152-176) | (150-152) | (151-180) | |||
Data are reported as number of patients (%) or mean ± standard deviation (range)
Table 1: Demographic and baseline characteristics of the cohorts.
Safety and tolerability
During the stage 1, neither MTD nor dose-limiting toxicity (DLT) were observed using this CIGB-300 monotherapy regimen. A total of 294 adverse events were recorded. Among them, mild side effects were the most common (61.6%), moderate (37.1%) and severe (1.4%). For moderate and severe adverse events, a dose-dependent effect was observed in terms of frequency. Otherwise, mild effect rate behave irrespectively to the CIGB-300 dose level. Near all the patients exhibited allergic-like reactions at some extent except patient HHA-07 from the highest dose level group (1.6 mg/kg) who curiously did not experience any adverse event. Particularly, itching (62.5%), allergic reaction (50%) and flushing (25%) were the most common adverse events (Table 2). Duration of all side effects was about 15 min with spontaneous resolution. The only two drug-related severe events (facial edema and itching) were also classified as allergic reactions in two patients allocated to the 1.6 mg/kg CIGB-300 dose. Both patients experienced spontaneous recovery so they did not interrupt the CIGB-300 treatment. However, two patients, CH-09 and HHA- 11, from the same cohort, had a fever episode and a drug nonrelated alimentary intoxication, respectively, which required treatment and temporary interruption of the CIGB-300 delivery. Patient CH-09 discontinued just one day when he started second cycle, whereas patient HHA-11 interrupted it seven days just after the first infusion, first cycle. Overall, rates and severity of adverse events decreased as successive CIGB-300 infusions were delivered during the entire regime (data not shown).
As previously reported for CIGB-300, the adverse events classified as “allergic-like reactions" (18) had a significant correlation (p<0.0001) with plasma histamine levels which in turn did correlate (p<0.0001) with the plasma CIGB-300 levels, 5 min after infusions. Overall, vital signs remained within normal ranges during treatment and it did no modify neither blood counts nor serum chemistry at any dose level (data not shown).
| Group I | Group II | Group III | Group IV | |
|---|---|---|---|---|
| (0.2 mg/kg) | (0.4 mg/kg) | (0.8 mg/kg) | (1.6 mg/kg) | |
| N=2 | N=2 | N=2 | N=10 | |
| Any adverse event | 2 (100%) | 2 (100%) | 2 (100%) | 8 (80%) |
| Number of reports | 41 | 22 | 107 | 124 |
| Mild | 41 (100%) | 13 (59.1%) | 74 (69.2%) | 53 (42.7%) |
| Moderate | 0 | 9 (40.9%) | 33 (30.8%) | 67 (54.0%) |
| Severe* | 0 | 0 | 0 | 4 (3.2%) |
| Most frequent (≥ 10%) adverse events recorded at each dose level | ||||
| Skin and appendages disorder | ||||
| Itching | 1 (50%) | 2 (100 %) | 2 (100%) | 5 (50%) |
| Wheals | 0 | 0 | 1 (50%) | 1 (10%) |
| Dry mouth | 0 | 0 | 1 (50%) | 1 (10%) |
| Erythema | 0 | 0 | 1 (50%) | 1 (10%) |
| General disorders | ||||
| Allergic reaction | 0 | 1 (50%) | 1 (50%) | 6 (60%) |
| Extracardiac vascular disorders | ||||
| Flushing | 1 (50%) | 1 (50%) | 1 (50%) | 1 (10%) |
| Central & peripheral nervous system disorder | ||||
| Headache | 0 | 0 | 1 (50%) | 2 (20%) |
| Tingling | 1 (50%) | 1 (50%) | 1 (50%) | 0 |
* Required or prolonged hospitalization
Table 2: Treatment-emergent adverse events.
Drug accumulation ratio
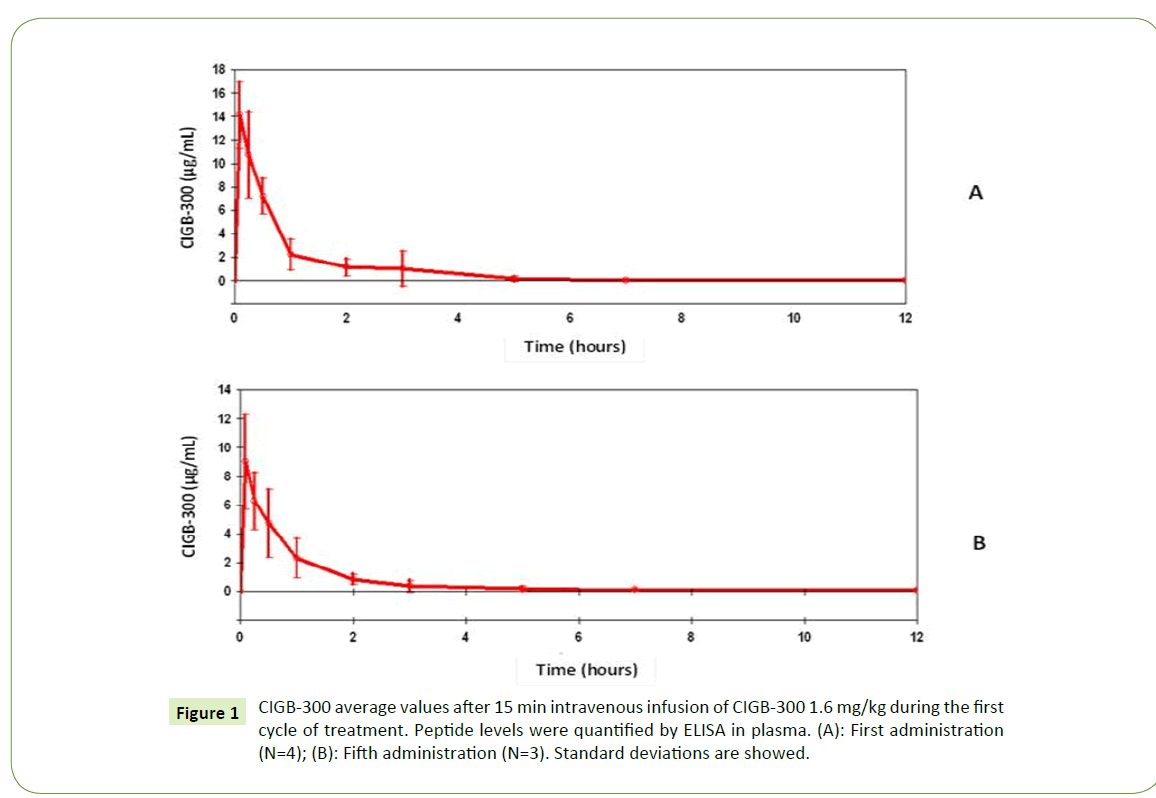
Similar clearance curves were observed on days 1 and 5 during the first cycle of CIGB-300 1.6 mg/kg (Figures 1A and 1B). Pharmacokinetic data summarized in Table 3 indicated no significant differences (p>0.05) respect CIGB-300 infusions from the first and fifth day. Accordingly, the RCmax and RAUC values near to 1.0 indicated no relevant accumulation of successive CIGB-300 i.v. deliveries from day 1 to 5. Furthermore, both t1/2 and total CL values did not experience significant changes among both CIGB- 300 infusions.
Figure 1: CIGB-300 average values after 15 min intravenous infusion of CIGB-300 1.6 mg/kg during the first cycle of treatment. Peptide levels were quantified by ELISA in plasma. (A): First administration (N=4); (B): Fifth administration (N=3). Standard deviations are showed.
| Parameter | 1st dose (N=4) | 5th dose (N=4) | p (student t-test) |
|---|---|---|---|
| Tmax (h) | 0.12 ± 0.09 | 0.083 ± 0.0 | 0.44 |
| Cmax (µg/mL) | 14.8 ± 2.7 | 14.6 ± 3.3 | 0.95 |
| l (h-1) | 0.92 ± 0.17 | 0.92 ± 0.17 | 1 |
| RCmax | -- | 1 | -- |
| t1/2 (h) | 0.75 ± 0.12 | 0.77 ± 0.13 | 0.77 |
| AUC0-12 (µg.h/mL) | 11.4 ± 3.52 | 11.8 ± 4.22 | 0.91 |
| RAUC | -- | 0.9997 ± 0.0006 | -- |
| MRT (h) | 0.92 ± 0.50 | 1.06 ± 0.49 | 0.72 |
| CL (L/h) | 12.4 ± 7.4 | 12.4 ± 9.0 | 1 |
| CL/kg (L/h.kg) | 0.15 ± 0.05 | 0.15 ± 0.06 | 1 |
| Vss (L) | 8.95 ± 1.95 | 10.2 ± 1.32 | 0.38 |
| Vss/kg (L/kg) | 0.13 ± 0.05 | 0.14 ± 0.04 | 0.62 |
Abbreviations: Tmax: Time to Peak; Cmax: Maximum Observed Plasma Concentration; AUC: Area under Plasma Concentration-Time Curve from time zero to the last measurable time point; λ: Elimination Rate Constant; t1/2: Terminal Half-Life; MRT: Mean Residence Time; CL: Total Plasma Clearance; Vss: Volume of Distribution at Steady State; RCmax: Accumulation Ratio of Cmax; RAUC: Accumulation Ratio of AUC
Data are expressed as mean ± standard deviation.
Table 3 :Main pharmacokinetics parameters estimated by NCA using plasmatic concentration data obtained from ELISA after first and fifth infusion of 1.6 mg/kg of CIGB-300, cycle 1.
Clinical response
On the intend-to-treat basis, 75% (12/16) and 43.7% (7/16) of patients were alive after 6 and 12 months respectively (Table 4). Lung cancer patients had a median survival of 11.4 months whereas for those patients who received 1.6 mg/kg survival reached 15.2 months. Interestingly, one patient with Lung Epidermoid Carcinoma lived >39 months post-CIGB-300 treatment with stable disease according to RECIST criteria [24]. Of note, the CIGB-300 regime significantly improved the respiratory symptoms and recurrent pneumonitis in a patient with thymic carcinoma exhibiting lung and pleura baseline metastasis who lived 27.0 months. CIGB-300 long-term toxicity was not observed in any patient (data not shown).
| Patient | Target | Stage | CIGB-300 | Survival after CIGB-300 start (months) |
|---|---|---|---|---|
| Dose level (mg/kg) | ||||
| HHA-01 | Breast lobular carcinoma | IV | 0.2 | 2.67 |
| HHA-02 | Undifferentiated NSCLC | IV | 0.2 | 6.8 |
| HHA-03 | NSCLC | IV | 0.4 | 1.97 |
| HHA-04 | Infiltrating ductal carcinoma of the breast | IV | 0.4 | 12.97 |
| HHA-05 | NSCLC | IV | 0.8 | 8.37 |
| CH-06 | Infiltrating breast lobular carcinoma with lymph node infiltration | IV | 0.8 | 18.53 |
| HHA-07 | NSCLC | IV | 1.6 | 10.47 |
| CH-08 | Skin epidermoid carcinoma | IV | 1.6 | 14.97 |
| CH-09 | Moderately differentiated Lung Epidermoid Carcinoma | IV | 1.6 | 39.3 |
| HHA-10 | Adenocarcinoma of ovary | IV | 1.6 | 5.57 |
| HHA-11 | SCLC | IV | 1.6 | 7.77 |
| HHA-12 | Mucoproductive adenocarcinoma of the lung | IV | 1.6 | 4.7 |
| HHA-13 | Lung Epidermoid Carcinoma | IV | 1.6 | 10.93 |
| HHA-14 | Carcinoma of Thymus with pericardium and lung infiltration | IV | 1.6 | 27 |
| HHA-15 | Lung Epidermoid Carcinoma | IV | 1.6 | 20.23 |
| HHA-16 | Breast lobular carcinoma | IV | 1.6 | 12 |
Abbreviations: NSCLC: Non-Small Cell Lung Carcinoma; SCLC: Small Cell Lung Cancer
Table 4: Therapeutic response: Survival time after CIGB-300 monotherapy.
Discussion
Patients with relapsed/refractory solid tumors are very difficult to treat and have considerable unmet need for novel treatments. This dose-escalation Phase 1 study is the first in describing safety, tolerability, pharmacokinetics, and clinical effect of CIGB-300 using the i.v. route in such a kind of patients. Of note, seven patients (43.7%) experienced survival increase >12 months and two patients lived 39.3 and 27.0 months after CIGB-300 monotherapy, respectively.
Despite the unbalanced amount of patients included in each clinical niche, lung cancer patients trended to have a better clinical response profile to CIGB-300 in terms of survival. Interestingly, in patients included in the expansion phase who received the highest CIGB-300 dose, the median of survival time was about 15 months. Such increase beyond the expected limit (≤ 6 months) is an encouraging finding for these kinds of patients who had already failed prior regimens and exhibited ECOG 3-4. Of note, 3/4 breast cancer patients lived 12 months or more.
Importantly, the safety profile for the CIGB-300 intravenous delivery in both the dose-escalation and expansion phases were manageable up to 1.6 mg/kg and consistent with previous clinical observations reported for this experimental drug by intralesional delivery [17-19]. In both phases of the study the most common adverse events were itching, allergic-like reaction and flushing. Neither DLT nor MTD were observed despite the dose-dependent effect in terms of frequency and magnitude of moderate/severe events (grade ≤ 3). Otherwise, mild effects were irrespective to the CIGB-300 dose level. The previously reported correlation between transient CIGB-300-induced histamine and peptide levels in plasma [18,19] was similarly observed in this study supporting the appearance of the above-mentioned histamine related events. Interestingly, the Cmax and AUC values indicated no relevant accumulation ratio from days 1 to 5 which suggest that CIGB-300 can be also tested in more intensive regimens based on twice daily administrations. As expected for nude peptides, the CIGB-300 exhibited appropriate volume of distribution and a relatively short half-life after intravenous delivery (45 min). However, considering that CIGB-300 internalizes tumor cells as soon as 3 min [25], we would expect that the CIGB-300 intravenously administered can efficiently target circulating tumor cells in blood stream thus preventing implantation and growth of new metastasis in these patients. Supporting this notion, in two different murine metastasis models the i.v. administration of CIGB-300, it significantly reduced the tumor cell dissemination and lung colonization [13]. Likewise, the antiproliferative effect of CIGB-300 has been also confirmed and molecularly characterized on in vitro models [26,27].
Conclusion
In conclusion, the CIGB-300 intravenous delivery as monotherapy was safe, well tolerated and exhibited efficacy signs in patients with relapsed/refractory solid tumors using this clinical protocol described here. Particularly, the 1.6 mg/kg dose of this peptidebased drug merits to be tested in Phase 2-3 trials in NSCLC patients where either once- or twice-daily administrations can be taken into account.
Acknowledgment
The following are members of the clinical trial team: Ivelisse Nuvia-Fleites (“Hermanos Ameijeiras” Hospital), Leovaldo Álvarez- Falcón, Lissandra Méndez-Sánchez (CIGB-300 Research and Development Group), Ubaldo Vásquez-Estévez, Tania Bartomeu Villamil, Luis J López-Carrazana (“Celestino Hernández” Hospital), Mauricio Catalá-Ferrer, Marlén Izquierdo-González (Center for Medical-Surgical Research).
We thank to Drs. Sergio Martínez, Jorge Rodriguez and Luis Castillo and the nurses Yorinko Chacón-Díaz and Ada I Zamora- López, for their valuable contribution in the clinical work. Also we thank to Yunia Delgado, Cimara Bermúdez, Grettel Melo, Ketty Cruz, María A Delgado, Silvia Barcelona, Marisol Cruz, Lourdes Hernández for their logistic assistance and Matilde López, Osvaldo Acosta, Hilda Garay for their contribution in the drug characterization and formulation. This study was supported by HeberBiotec S.A, Havana (product, insurance, reagents), by Biorec and by the Ministry of Public Health of Cuba (hospital facilities and general medical care of the patients).
Competing Interests
Authors LGM, IGG, IRC, CMVS, VRN, YPN, BEAC, VLMG and SEPR are employees of the Center for Genetic Engineering and Biotechnology (CIGB), Havana network, where CIGB-300 peptide was conceived and their formulation developed. DFA and RG are employed by Biorec. SEPR is the main author of the patent WO 03/054002 and US patent # 7374767 that sustain this project. The remaining authors declare no conflict of interests.
References
- Seiwert TY, Salama JK, Vokes EE (2007) The concurrent chemoradiation paradigm - General principles. Nat Clin Pract Oncol 4: 86-100.
- Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, et al. (2014) Drug resistance in cancer: An overview. Cancers 6: 1769-1792.
- Morgan MA, Parsels LA, Maybaum J, Lawrence TS (2014) Improving the efficacy of chemoradiation with targeted agents. Cancer Discov 4: 280-291.
- Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, et al. (2016) Clinical development of new drug–radiotherapy combinations. Nat Rev Clin Oncol 13: 627-642.
- Sarno S, de Moliner E, Ruzzene M, Pagano MA, Battistutta R, et al. (2003) Biochemical and three-dimensional-structural study of the specific inhibition of protein kinase CK2 by (5-oxo-5,6-dihydroindolo-(1,2-a)quinazolin-7-yl)acetic acid (IQA). Biochem J 374: 639-646.
- Pagano MA, Meggio F, Ruzzene M, Andrzejewska M, Kazimierczuk Z, et al. (2004) 2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole: A novel powerful and selective inhibitor of protein kinase CK2. Biochem Biophys Res Commun 321: 1040-1044.
- Pierre F, Chua PC, O'Brien SE, Siddiqui-Jain A, Bourbon P, et al. (2011) Discovery and SAR of 5-(3-chlorophenylamino)benzo(c)(2,6)naphthyridine-8-carboxylic acid (CX-4945), the first clinical stage inhibitor of protein kinase CK2 for the treatment of cancer. J Med Chem 54: 635-654.
- Trembley JH, Unger GM, Tobolt DK, Korman VL, Wang G, et al. (2011) Systemic administration of antisense oligonucleotides simultaneously targeting CK2α and α' subunits reduces orthotopic xenograft prostate tumors in mice. Mol Cell Biochem 356: 21-35.
- Perea SE, Reyes O, Puchades Y, Mendoza O, Vispo NS, et al. (2004) Anti-tumor effect of a novel proapoptotic peptide that impairs the phosphorylation by the Casein Kinase 2 (CK2). Cancer Res 64: 7127-7129.
- Laudet B, Barette C, Dulery V, Renaudet O, Dumy P, et al. (2007) Structure-based design of small peptide inhibitors of protein kinase CK2 subunit interaction. Biochem J 408: 363-373.
- Perera Y, Farina HG, Gil J, Rodríguez A, Castellanos L, et al. (2009) Anticancer peptide CIGB-300 binds to nucleophosmin/B23, impairs its CK2-mediated phosphorylation, and leads to apoptosis through its nucleolar disassembly activity. Mol Cancer Ther 8: 1189-1196.
- Perea SE, Reyes O, Baladron I, Perera Y, Farina H, et al. (2008) CIGB-300, a novel proapoptotic peptide that impairs the CK2 phosphorylation and exhibits anticancer properties both in vitro and in vivo. Mol Cell Biochem 316: 163-167.
- Benavent F, Capobianco CS, Garona J, Cirigliano SM, Perera Y, et al. (2017) CIGB-300, an anti-CK2 peptide, inhibits angiogenesis, tumor cell invasion and metastasis in lung cancer models. Lung Cancer 107: 14-21.
- Martins LR, Perera Y, Lúcio P, Silva MG, Perea SE, et al. (2015) Targeting chronic lymphocytic leukemia using CIGB-300, a clinical-stage CK2-specific cell-permeable peptide inhibitor. Oncotarget 5: 258-263.
- Perera Y, del Toro N, Gorovaya L, Fernandez-de-Cossio F, Farina HG, et al. (2014) Synergistic interactions of the anti-casein kinase 2 CIGB-300 peptide and chemotherapeutic agents in lung and cervical preclinical cancer models. Mol Clin Oncol 2: 935-944.
- Perea SE, Perera Y, Baladrón I, González L, Benavent F, et al. (2015) CIGB-300: A promising anti-casein kinase 2 (CK2) peptide for cancer targeted therapy. In; Khalil A, Issinger OG, Ryszard S (eds.) Protein Kinase CK2 Cellular Function in Normal and Disease States, Advances in Biochemistry in Health and Disease. Springer International Publishing Switzerland pp 281-298.
- Solares AM, Santana A, Baladrón I, Valenzuela C, Diaz J, et al. (2009) Safety and preliminary efficacy data of a novel casein kinase 2 (CK2) peptide inhibitor administered intralesionally at four dose levels in patients with cervical malignancies. BMC Cancer 9: 146.
- Soriano-García JL, López-Díaz A, Solares-Asteasuainzarra M, Baladrón-Castrillo I, Batista-Albuerne N, et al. (2013) Pharmacological and safety evaluation of CIGB-300, a casein kinase 2 inhibitor peptide, administered intralesionally to patients with cervical cancer stage IB2/II. J Cancer Res Ther 1: 163-173.
- Sarduy MR, García I, Coca MA, Perera A, Torres LA, et al. (2015) Optimizing CIGB-300 intralesional delivery in locally advanced cervical cancer. Br J Cancer 112: 1636-1643.
- Garrett-Mayer E (2006) The continual reassessment method for dose-finding studies: A tutorial. Clin Trials 3: 57-71.
- https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40
- https://www.who-umc.org/whodrug/whodrug-portfolio
- Naranjo CA, Shear NH, Busto U (1998) Adverse drug reactions. In: Kalant H, Roschlau WHE (6thed) Principles of Medical Pharmacology, pp: 791-800.
- Reyes V, Perera Y, Díaz E, Rosales I, García G, et al. (2014) Implementation of a competitive ELISA for pharmacokinetics studies of CIGB-300 in human plasma. Biotechnol Appl 31: 232-236.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205-216.
- Perera Y, Costales HC, Diaz Y, Reyes O, Farina HG, et al. (2012) Sensitivity of tumor cells towards CIGB-300 anticancer peptide relies on its nucleolar localization. J Pept Sci 18: 215-223.
- Cirigliano SM, Díaz Bessone MI, Berardi DE, Flumian C, Bal de Kier ED, et al. (2017) The synthetic peptide CIGB-300 modulates CK2-dependent signaling pathways affecting the survival and chemoresistance of non-small cell lung cancer cell lines. Cancer Cell Int 17: 42.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences