Phase 2 Randomized Clinical Trial Evaluating the Use of the Vascular Electrical Stimulation Therapy by Device Diavein in Severe Painful Vaso-occlusive Crisis of Sickle Cell Disease: Reduction of Crisis Exit Time and Hospital Stay Duration
Paul Boma Muteb1,2*, Charles Matungulu3, Stephanie Ngimbi Luntadila2,4,5, Daniel Mwamba6, Simon Kakuru Kambale1, Jean-Jean Israel Wela7, Abel Ntambue Mukengeshayi3 and Jules Panda Mulefu1,8
1Department of Research in Health Sciences, University of Lubumbashi, DR Congo
2Department of Health Sciences, Université of Catholique de Bukavu, DR Congo
3Department of Health, University of Lubumbashi, DR Congo
4Department of Pediatrics, University of Lubumbashi, DR Congo
5Department of Pediatrics, Jason Sendwe Provincial General Hospital, Lubumbashi, DR Congo
6Department of Sciences, Centre for Infectious Diseases Research in Zambia (CIRDZ), University of Lubumbashi Lusaka, Zambia
7Department of Health Sciences, University of Kinshasa, DR Congo
8Department of Surgery, University of Lubumbashi, DR Congo
- *Corresponding Author:
- Paul Boma Muteb
Department of Health Sciences, University of Lubumbashi, DR Congo
Tel: 00260978625288
E-mail: pauliboma@hotmail.com
Received Date:March 14, 2022, Manuscript No. IPJCMT-22-12371; Editor assigned date: March 17, 2022, PreQC No. IPJCMT-22-12371 (PQ); Reviewed date: March 31, 2022, QC No. IPJCMT-22-12371; Revised date: May 11, 2022, Manuscript No. IPJCMT-22-12371 (R); Published date: May 18, 2022, DOI: 10.36648/IPJCMT/7.5.001
Citation: Muteb PB, Matungulu c, Luntadila N, Mwamba D, Kamble KS, et al. (2022) Phase 2 Randomized Clinical Trial Evaluating the Use of the Vascular Electrical Stimulation Therapy by Device "Diavein®" in Severe Painful Vaso-occlusive Crisis of Sickle Cell Disease: Reduction of Crisis Exit Time and Hospital Stay Duration. J Clin Med Ther Vol:7 No:5
Abstract
Background
Painful Vaso-Occlusive Crisis (VOCs) remains the main reason for medical consultation and hospitalization of sickle cell patients. These crises can be life-threatening when management is delayed and significantly contribute to the heavy socio-economic burden of sickle cell disease. Various adjuvants are added to the analgesic treatment, these include Vascular Electrical Stimulation Therapy (VEST).
Objective
To assess the efficacy and safety of VEST in severe sickle cell VOCs in Lubumbashi.
Methods
Single-blind, multicenter randomized clinical trial involving 60 sickle cell patients aged 4 years and above in severe VOC (VAS ≥ 6), divided into three arms of witch 2 intervention arms using the Diavein® VEST Device (one arm with analgesics and the other with analgesics coupled with a non-steroidal anti-inflammatory drug) and one control arm. The primary outcome was crisis exit time (at least 6 hours spent at VAS ≤ 2 without recurrence within 5 days). The data was coded on Excel 2010 and analyzed on Epi Info 7 and R version 3.4.4. Appropriate descriptive statistical tests were conducted. A variance test (ANOVA) was used to compare means with P value threshold at 5%.
Results
Sixty-seven patients with similar baseline characteristics were enrolled and randomised between 12/19/2017 and 22/02/2019. With mean seizure exit times of 65.8 ± 23.9 h for the control group and 33.9 ± 21.5 h for the analgesic intervention group, 30.4 ± 21.5 h for the intervention group with analgesics and non-steroidal anti-inflammatory drugs. The VOCs were relieved rapidly in the intervention group with a statistically significant difference (p<0.001). The VEST was well tolerated.
Conclusion
VEST by Diavein®, as an adjuvant to the analgesic treatment of sickle cell VOCs, is effective and well tolerated.
Keywords
Painful vaso-occlusive crises; Vascular electrical stimulation therapy; Diavein®; Efficacy; Tolerance
Introduction
Sickle cell disease is the leading genetic disease in the world with annual births of up to 5476000 heterozygous AS newborns and 312000 homozygous SS. It is therefore a major world public health concern, particularly in Africa where 75% of these births are found [1]. The Democratic Republic of Congo (DR Congo), with its 39700 sickle cell births per year, is one of the most affected countries along with Nigeria and India [2]. In this context, the implementation of treatment and preventive strategies is vital to alleviate the ever-increasing number of people affected and the severity of clinical manifestation [3,4]. Indeed, in DR Congo, clinical manifestation of sickle cell disease is very heterogenous due to environmental and genetic factors resulting in coexistence of highly morbid cases and less severe forms [5]. Although multiple and variable, clinical manifestations of sickle cell disease are essentially determined by a state of chronic anemia, Vaso-Occlusive Crisis (VOC) and extreme susceptibility to infections [6]. This triad makes sickle cell disease the crossroads of several diseases. However, Painful vaso-occlusive crisis is the main reason for consultations from sickle cell patients as well as the main cause of hospitalizations and admissions to emergency departments [7-8]. These crises follow a complex mechanism caused by the polymerization of deoxy-Hemoglobin S, dehydration of sickle cell red blood cells and their adhesion to the vascular endothelium, the inflammatory reaction and activation of cells present in the vessel and finally abnormalities in vascular texture and nitrogen monoxide metabolism [9]. The VOC have a heavy negative impact on the patient’s finances. In DR Congo where the Gross Domestic Product (GDP) was estimated at 300 US dollars per year [10]. This cost is way above the income of most patients’ families and estimated at an average of 183.6 US dollars per crisis or 550.8 US dollars annually for patients who had an average of 3 crises in a year [11]. Painful VOC seriously impact on the quality of life of patients and their survival because they are associated with the risk of occurrence of dangerous severe complications [12-15]. Crisis recurrences are a source of anxiety and depressive syndrome thus making subsequent crisis even worse [16-17]. Therefore, each hyperalgic VOC requires adequate management that involves a rapid pain control with morphine, and the alleviation of all triggering factors [13]. The latter consists of using the various adjuvants such as rehydration, alkalization, oxygen therapy and appropriate treatment of associated and underlying conditions including infections [13-18]. In this therapeutic arsenal against hyperalgic VOC, several approaches have been evaluated for years in order to improve the efficacy of pain control therefore reducing morphine quantity and associated side effects [19-23]. Adjuvants non pharmaceutical approaches that aims at enhancing the efficacy of drug molecules have been suggested, this is where the Vascular Electrical Stimulation Therapy (VEST) device by "Diavein®” intervene.
Methods & Materials
Study Design
This was a prospective, randomized, controlled, multicenter, blinded patient phase 2 study evaluating the efficacy and safety of vascular electrical stimulation therapy in the treatment of severe painful VOC in sickle cell patients. The primary outcome was crisis exit time defined as the time after which the patient spends 6 hours with pain intensity at ≤ 2 assessed by Visual Analogue Scale (VAS) without recurrence within 5 days (no pain greater than 2). Secondary outcomes were time spent in acute phase (VAS/EVA>4), length of hospital stay, amount of morphine administered, change in haemoglobin levels measured at 72h and the occurrence or not of an iatrogenic effect.
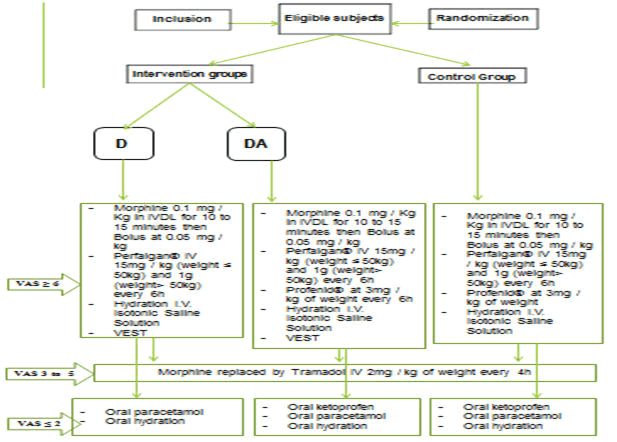
The sample size of 60 patients was determined using the "R Package" method as described by Friede [23]. Based on the available data and depending on the primary outcome (patient discharge time) which could be shortened by 20% in patients on VEST without Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and by 40% for patients on VEST and NSAIDs compared to the control group, with an overall alpha risk of 2.5% in unilateral configuration to achieve >80% power, the inclusion of 20 patients in each arm of the study was sufficient to reject the null hypothesis and conclude on the efficacy of a particular arm. Three arms were formed. One arm of patients subjected to the TVSE medical device in addition to the analgesic protocol with NSAIDs (DA), one arm with patients subjected to the VEST medical device associated with the analgesic protocol without NSAIDs (D) and the control arm subjected to the analgesic protocol with a non-therapeutic stimulation of VEST (C). The allocation of subjects in all three arms was random, balanced by alternating allocation on a list.
Eight sites participated in the study. Two centers specialized in management of sickle cell patients of all ages, the Lubumbashi Sickle Cell Reference Center, a medical unit of the Institute for Research in Health Sciences, and the Polyclinic des Oliviers host site of the non-governmental organization for the fight against sickle cell disease (SS Assistance), have been selected as evaluation sites of VEST's medical device. The other six that contributed to patient recruitment were: Lubumbashi University Clinics, Galen Polyclinic, Kenya General Reference Hospital, Ruashi Military Reference Hospital, and the medical offices of the non-governmental organizations fighting against sickle cell disease, «Matumahini Children of Hope» and the «Center for the Care of Sickle Cell Disease, CPCD»
The study was covered by insurance policy n°01005345–14047 of HDI Global SE and approved by the Medical Ethics Committee of the University of Lubumbashi under the number UNILU/CEM/094/2017 of 04/12/2017.
Patients
The inclusion criteria were: being aged 4 years and above, with a presumptive diagnosis of sickle cell disease confirmed at time of hospital admission by a Sickle Scan rapid test® positive and admitted for a VOC with pain intensity ≥ 6 on a VAS scale graduated from 0 to 10 and obtaining written informed consent. The exclusion criteria were determined by the presence of severe acute anaemia (signs of decompensation and a haemoglobin level ≤ 6 g/d determined on admission using a HemoCue® system), hyperthermia with a temperature ≥ 38°C, the presence of signs of respiratory distress, pregnancy, wearing a pacemaker or a known history of heart failure and history of a blood transfusion within thirty days prior to recruitment.
The study protocol was modified twice to improve recruitment by allowing the inclusion of patients admitted to hospital facilities other than the two sites initially selected in the study and the treatment protocol was also adjusted. Patients received for painful VOC at any of the six secondary recruitment sites were referred to one of treatment site for a study inclusion procedure. Those who were not eligible were treated, stabilized and sent back to the referring center after improvement of their condition. We also expanded the list of laboratory tests by including a blood slide and Widal's test to identify patients with possible Plasmodium falciparum or Salmonella infection in order to adapt treatment. We also decided to stop measuring peripheral velocimetry because the device used could not measure cardiac output simultaneously. We referred to the existing literature publication to confirm to those who asked the question during recruitment that the microcirculatory changes measured in the stimulated territories wereattributedtotheVESTbecause they were not accompanied by the increase in cardiac output [24].
Intervention mechanism and expected effect
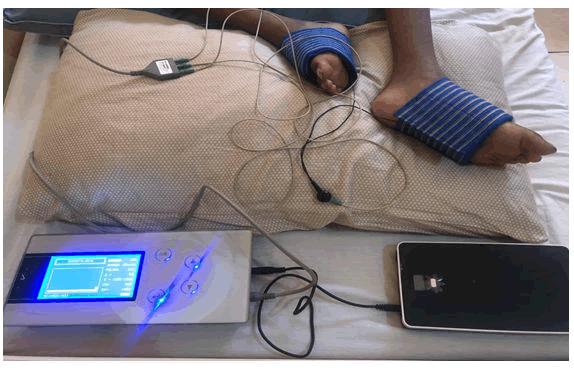
The medical device under study, the Diavein® Device (Figure 1) was administered using 2 or 4 electrodes (depending on the pain points) placed in contact with the patient's skin to deliver a low-voltage electrical current to the smooth muscles of the blood vessels and endothelium via the adrenergic endings. At the start of the treatment, the intensity of the current to be delivered is adjusted and gradually increased from the energy level E0 to a voltage of 0V until to a level considered as the clinically sufficient and marked by the appearance of tremulations near the areas of contact with the electrodes and the sensation of tingling in the stimulated territories. Thus, the therapeutic dose is the electrical voltage at which the patient manifests tremulations and/or perceives paresthesia without an unpleasant or painful sensation. Once this threshold is reached the stopwatch of the device is then set for 4 hours. Since the cable allowing this current passage was black, the patients in the control group were connected with a white cable lacking the transmission properties of electrical energy. For patients with pain of intensity evaluated at ≥ 4 after 24 and 48 hours of treatment, additional sessions of 2 hours of VEST were administered.
Regimen
All patients, regardless of their randomization arms, received analgesic treatment following the treatment protocol for severe VOC validated in both care settings in this study. This protocol included the initial administration of morphine at the loading dose of 0.1 mg/Kg in slowly and intravenously for 10 to 15 minutes followed possibly by a bolus at 0.05 mg/kg until obtaining a VAS score ≤ 5 with relay by intravenous tramadol (Contramal®) at a dose of 2 mg/kg weight every 4 hours as long as the pain intensity score was at 2 or more. These analgesics were systematically combined with paracetamol (Perfalgan®) in infusion at doses of 15 mg/kg for patients with a body weight ≤ 50 kg and 1g for those with a weight >50 kg every 6 hours and then every 8 hours after obtaining an EVA<4. Profenid® at a dose of 3 mg/kg weight was administered in patients in the control group and in the VEST group with NSAIDs. Naloxone (Narcan®), which is permanently available, was not used throughout the study as it was not required. The relay per oral was provided with tramadol effervescent tablet of 50mg (Tramadol denk®) and paracetamol (Doliprane®) as soon as the VAS was ≤ 2. Intravenous hydration using normal saline was systematic. Antibiotics and antimalarials were used, when necessary, in patients with results of the initial assessment justifying their indication. Blood transfusion or even manual exchange transfusion were used in two included patients, and they were therefore excluded from the study (Figure 2).
Efficacy and tolerance
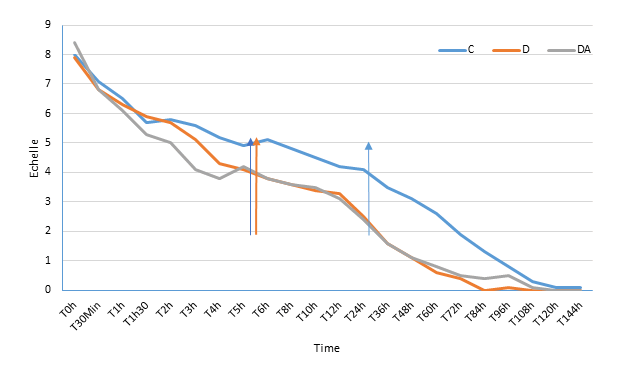
The main endpoint of the effectiveness of the Diavein device® was crisis exit time defined as the time after which the patient spends 6 hours with a pain intensity ≤ 2 assessed by the VAS and without recurrence within 5 days (no pain greater than 2). Subsidiary, the time spent in the acute phase (VAS>4), the duration of hospitalization, the amount of morphine administered completed this evaluation. The scores of pain intensity assessed by the VAS were recorded on arrival of the patient and, while the subjects were awake, at 30 minutes, 1 h, 1 h 30 m, 2 h, 3 h, 4 h, 5 h, 6 h, 8 h, 10 h, 12 h, 18 h, 24 h, 36 h, 48 h, 60 h, 72 h, 84 h, 96 h, 108 h, 120 h, 144 h of the start of treatment.
Tolerance was assessed by monitoring the general condition, some haematological parameters in the laboratory and the occurrence or non-occurrence of adverse events. In accordance with international recommendations, the following definitions have been applied to the adverse reaction form [25].
Adverse event: any harmful event occurring in a person who lends himself to research involving human subjects whether or not that event is related to the research or to a research related product.
Adverse reaction of an investigational drug: any harmful and unwanted reaction to an investigational drug regardless of the dose administered.
Serious event/adverse reaction: any adverse event or effect that results in death, endangers the life of the person who lends himself or herself to research, requires hospitalization or prolonged hospitalization, causes a significant or lasting disability or handicap, or results in a congenital anomaly or malformation; and in the case of the drug; regardless of the dose administered.
Therefore, measurements of body temperature, blood pressure, heart rate, respiratory rate and peripheral oxygen saturation (SpO2) were performed at admission, at 12 hrs, 24 hrs, 48 hrs and 72 hrs. In addition, anemia was monitored at 24 h, 48 h and 72 h by measuring the hemoglobin level and the levels of red blood cells, platelets, Lactate Dehydrogenase (LDH) and white blood cells at admission and at 72 h. Thus, the anticipated adverse events are worsening of anemia (decrease of 2 units in hemoglobin), dyspnea (increase in respiratory rate), high blood pressure or blood pressure drop, hyperthermia, cardiac rhythm disorders, headache as well as nausea and vomiting. The occurrence of hyperthermia (increase of 1.5 to 3°C from the initial temperature) not accompanied by infection markers like Erythrocytes Sedimentation Rate (ESR), C-reactive protein, a positive malaria blood slide test and Widal test on admission and at 72 h, was also sought.
Data management and analysis
The data was encoded on Excel 2010. They were analyzed using Epi Info 7 and R Software version 3.4.4. Simple descriptive statistical calculations were made the comparison of means was made using the Analysis of Variance Test (ANOVA) with a P value at 5% significance.
Results
Characteristics of patients and treatments
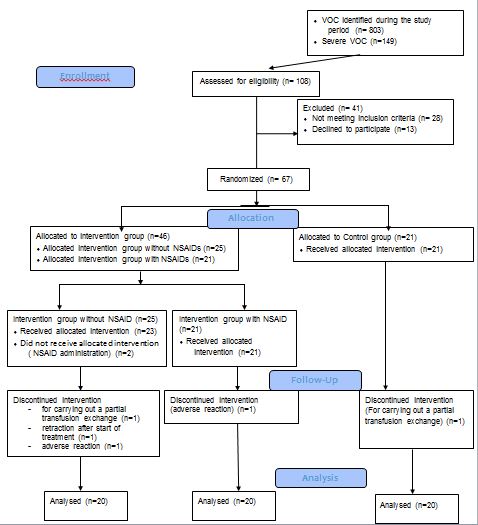
A total of 67 patients were enrolled in the study after consenting and then randomized and treated according to the study protocols revised between 19/12/2017 and 22/02/2019. Seven participants were secondarily removed from the study, for non-compliance with the planned protocol (n=2) worsening of the condition that necessitated blood transfusions (n=2) occurrence of adverse reactions (n=2) and refusal to continue the VEST (n=1) (Figure 3). The basic characteristics were similar between the two intervention groups, treated with painkillers alone and the one with NSAIDs, and the control group (Table 1).
| Variables | C | D | DA | |||
|---|---|---|---|---|---|---|
| n (20) | % | n (20) | % | n (20) | % | |
| Age (Years) | ||||||
| 4_ 9 | 5 | 25,0 | 6 | 30,0 | 5 | 25,0 |
| 10_ 19 | 9 | 45,0 | 7 | 35,0 | 5 | 25,0 |
| 20 and over | 6 | 30,0 | 7 | 35,0 | 10 | 50,0 |
| Min | 4 | 5 | 4 | |||
| Max | 40 | 38 | 29 | |||
| Mean | 16,1±10,3 | 17,5 ± 10,7 | 17,6 ± 7,6 | |||
| ANOVA (p) | 0,675 | 0,590 | ||||
| Sex | ||||||
| Female | 16 | 80,0 | 15 | 75,0 | 9 | 45,0 |
| Male | 4 | 20,0 | 5 | 25,0 | 11 | 55,0 |
| Crisis start time (time between start of the VOC and the time of the consultation) | ||||||
| 0_12 | 1 | 5,0 | 3 | 15,0 | 6 | 30,0 |
| 12_24 | 5 | 25,0 | 5 | 25,0 | 2 | 10,0 |
| 25_48 | 6 | 30,0 | 6 | 30,0 | 3 | 15,0 |
| 49 et + | 8 | 40,0 | 6 | 30,0 | 9 | 45,0 |
| Mean | 124,625 | 43,175 | 79,475 | |||
| Type Hb | ||||||
| SS | 20 | 100,0 | 20 | 100,0 | 19 | 95,0 |
| S-βthal | 0 | 0,0 | 0 | 0,0 | 1 | 5,0 |
| Annual frequency of severe VOCs | ||||||
| 0 | 8 | 40,0 | 7 | 35,0 | 5 | 25,0 |
| 1 | 7 | 35,0 | 10 | 50,0 | 8 | 40,0 |
| 2 | 4 | 20,0 | 1 | 5,0 | 5 | 25,0 |
| 3 | 1 | 5,0 | 1 | 5,0 | 2 | 10,0 |
| 4 | 0,0 | 1 | 5,0 | 0,0 | ||
| Last Severe CVO (in months) | ||||||
| 0_6 | 5 | 25,0 | 2 | 10,0 | 8 | 40,0 |
| 7_12 | 6 | 30,0 | 4 | 20,0 | 6 | 30,0 |
| 13_18 | 0 | 0,0 | 7 | 35,0 | 3 | 15,0 |
| 19_24 | 3 | 15,0 | 3 | 15,0 | 1 | 5,0 |
| 25_29 | 2 | 10,0 | 2 | 10,0 | 0 | 0,0 |
| 31_36 | 2 | 10,0 | 1 | 5,0 | 0 | 0,0 |
| 37 and over | 2 | 10,0 | 1 | 5,0 | 2 | 10,0 |
| Mean | 18,3 | 17,4 | 11,8 | |||
| C=Control group; D=Intervention group with VEST device+analgesics alone; DA= Intervention group with TVSE device+analgesics and non-steroidal anti-inflammatory drug. | ||||||
Table 1: characteristics of patients and treatment groups.
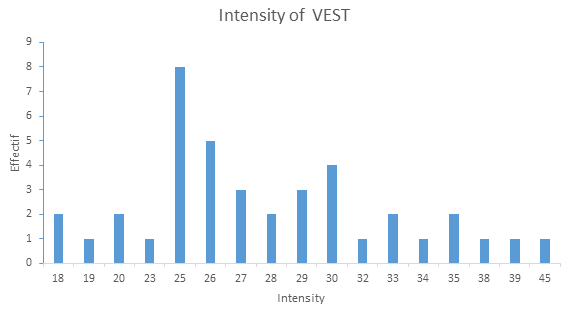
Forty patients received VEST (17 adults, 12 adolescents and 11 children; 24 male and 16 female) and 20 were treated according to the conventional protocol for the management of VOCs while being connected using a cable that did not deliver electrical current (Figure 1). Since the interquartile range is between 25 and 30 volts (Q1=25 and Q3=30), 75% of patients were treated with an intensity between 25 and 30 volts. The average intensity was 27.9 ± 5.6 volts (Figure 4, Figure 5).
Exit time from the crisis
All trial participants were discharged from their severe VOCs. We noted a significantly shorter exit time in the intervention groups than in the control group. 33.9 ± 21.5 h vs. 65.8 ± 23.9h (p<0.0001) for the intervention group with VEST device associated with analgesics alone; 30.4 ± 21.5h vs. 65.8 ± 23.9h (p<0.0001) for the intervention group with VEST device associated with analgesics and NSAIDs (Table 2).
| Variables | C | D | DA | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Crisis exit time (hours) | ||||||
| <10 | 0,0 | 0,0 | 1 | 5,0 | ||
| 10_19 | 1 | 5,0 | 6 | 30,0 | 7 | 35,0 |
| 20_29 | 1 | 5,0 | 3 | 15,0 | 4 | 20,0 |
| 30_39 | 0,0 | 5 | 25,0 | 2 | 10,0 | |
| 40_49 | 2 | 10,0 | 3 | 15,0 | 3 | 15,0 |
| 50_72 | 8 | 40,0 | 1 | 5,0 | 1 | 5,0 |
| >72 | 8 | 40,0 | 2 | 10,0 | 2 | 10,0 |
| Min | 18 | 10 | 7 | |||
| Max | 105 | 88 | 82 | |||
| Mean ± SD | 65,8 ± 23,9 | 33,9 ± 21,5 | 30,4 ± 21,5 | |||
| ANOVA | <0,0001 | <0,0001 | ||||
| Duration of hospitalization (hours) | ||||||
| ≤ 24 | 2 | 10,0 | 4 | 20,0 | 6 | 30,0 |
| 25_48 | 1 | 5,0 | 8 | 40,0 | 7 | 35,0 |
| 49_72 | 5 | 25,0 | 4 | 20,0 | 2 | 10,0 |
| >72 | 12 | 60,0 | 4 | 20,0 | 5 | 25,0 |
| Min | 16 | 12 | 7 | |||
| Max | 125 | 106 | 108 | |||
| Mean ± SD | 74,4 ± 28,1 | 46,2 ± 26,6 | 44,8 ± 29,7 | |||
| ANOVA | <0,01 | <0,01 | ||||
| Acute crisis duration (hours) | ||||||
| >6 | 13 | 65,0 | 4 | 20,0 | 5 | 25,0 |
| ≤ 6 | 7 | 35,0 | 16 | 80,0 | 15 | 75,0 |
| Min | 1 | 1 | 1 | |||
| Max | 37 | 16 | 27 | |||
| Mean ± SD | 15,9 ± 11,8 | 5,2 ± 3,7 | 5,9 ± 6,4 | |||
| ANOVA | <0,001 | <0,01 | ||||
| Relapse | ||||||
| No | 16 | 80,0 | 19 | 95,0 | 17 | 85,0 |
| Yes | 4 | 20,0 | 1 | 5,0 | 3 | 15,0 |
Table 2: treatment effectiveness.
Tolerance
Overall, the VEST was well tolerated. Indeed, it was not found to be particularly associated with worsening of anemia (Table 3) neither the occurrence of hyperthermia (Table 4). No significant difference was noted in these two parameters between the stimulated groups and the control group. However, two patients experienced paresthesia within hours of their treatment with VEST resulting in their discharge from the study.
| Hb | p | ||
|---|---|---|---|
| Groups | <6 g/dL | ≥ 6 g/dL | |
| C | 1 | 19 | |
| D |
3 |
17 | 0,302 |
| DA | 2 | 18 | 0,500 |
| TOTAL | 6 | 54 |
Table 3: difference between the hemoglobin levels at the 72nd hour and admission according to the groups.
| Groups | hyperthermia | ||
|---|---|---|---|
| No | Yes | p | |
| C | 19 | 1 | |
| D | 17 | 3 | 0,592 |
| DA | 20 | 0 | |
| TOTAL | 56 | 4 |
Table 4: frequency of occurrence of hyperthermia in the three groups.
Secondary efficacy endpoints and additional analyses
Significant differences were also noted between the intervention and control groups on secondary outcomes, The average length of hospitalization was significantly reduced in patients treated with the device without an NSAID (46.2 ± 26.6 h vs. 74.4 ± 23.9 h; p< 0.01) as well as in those treated with the device in combination with NSAIDs (44.8 ± 29.7 h vs. 74.4 ± 23.9 h; p<0.01) (Table 2). As it appears on Table 2, patients put on the device with analgesics alone had an average duration of the acute attack less than that of the control group (5.2 ± 3.7 h vs. 15.9 ± 11.8 h; p<0.001), as well as those who benefited from NSAIDs in addition to the device and analgesics (5.9 ± 6.4 h vs. 15.9 ± 11.8; p<0.01). Similarly, patients stimulated with the Diavein® device required less pain killers than those in the control group (Table 6). Morphine analgesics were significantly less consumed in the NSAID stimulated group than in the control group (p=0.015 for morphine; p=0.00437 for tramadol). The same was noted for paracetamol infusion (Perfalgan®). Ketoprofen was significantly more often consumed in the control group than in the VEST stimulated subgroup (p=0.00081). We did not observe a statistically significant difference in analgesic consumption between the two intervention subgroups (p>0.05) (Table 5).
| Variables | D | DA | ||
|---|---|---|---|---|
| n | % | n | % | |
| Duration of hospitalization (hours) | ||||
| ≤ 24 | 4 | 20,0 | 6 | 30,0 |
| 25_48 | 8 | 40,0 | 7 | 35,0 |
| 49_72 | 4 | 20,0 | 2 | 10,0 |
| >72 | 4 | 20,0 | 5 | 25,0 |
| Min | 12 | 7 | ||
| Max | 106 | 108 | ||
| Mean ± SD | 46,2 ± 26,6 | 44,8 ± 29,7 | ||
| ANOVA | 0,88 | |||
| Acute crisis duration (hours) | ||||
| > 6 | 4 | 20,0 | 5 | 25,0 |
| ≤ 6 | 16 | 80,0 | 15 | 75,0 |
| Min | 1 | 1 | ||
| Max | 16 | 27 | ||
| Mean ± SD | 5,2 ± 3,7 | 5,9 ± 6,4 | ||
| ANOVA (p) | 0,63 | |||
| Crisis exit time (hours) | ||||
| <10 | 0,0 | 1 | 5,0 | |
| 10-19 | 6 | 30,0 | 7 | 35,0 |
| 20-29 | 3 | 15,0 | 4 | 20,0 |
| 30-39 | 5 | 25,0 | 2 | 10,0 |
| 40-49 | 3 | 15,0 | 3 | 15,0 |
| 50-72 | 1 | 5,0 | 1 | 5,0 |
| >72 | 2 | 10,0 | 2 | 10,0 |
| Min | 10 | 7 | ||
| Max | 88 | 82 | ||
| Mean ± SD | 33,9 ± 21,5 | 30,4 ± 21,5 | ||
| ANOVA (p) | 0,619 | |||
Table 5: comparative study of the effectiveness of the treatment in the 2 intervention groups.
| Analgesic and NSAID quantity | C | D | DA | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Morphine injection (ampoules) | ||||||
| 1-2 | 15 | 75,0 | 18 | 90,0 | 20 | 100,0 |
| 3-4 | 5 | 25,0 | 2 | 10,0 | 0,0 | |
| Mean | 1,9 ± 1,2 | 1,7 ± 1,8 | 1,2 ± 0,4 | |||
| ANOVA | 0,68341 | 0,01552 | ||||
| Tramadol injection (Contramal®) (ampoules) | ||||||
| 1-5 | 4 | 20,0 | 10 | 50,0 | 10 | 50,0 |
| 6-10 | 8 | 40,0 | 8 | 40,0 | 8 | 40,0 |
| 11-15 | 4 | 20,0 | 2 | 10,0 | 2 | 10,0 |
| 16 and over | 4 | 20,0 | 0,0 | 0,0 | ||
| Mean | 11 ± 7 | 6 ± 3 | 6 ± 3 | |||
| ANOVA | 0,00437 | 0,00437 | ||||
| Paracetamol infusion (Perfalgan®) (vials) | ||||||
| 1-5 | 4 | 20,0 | 12 | 60,0 | 14 | 70,0 |
| 6-10 | 12 | 60,0 | 8 | 40,0 | 6 | 30,0 |
| 11-15 | 4 | 20,0 | 0,0 | 0,0 | ||
| Moyenne | 8 ± 4 | 5 ± 2 | 5 ± 2 | |||
| ANOVA | 0,00086 | 0,00060 | ||||
| Ketoprofen injection (Profenid®) (ampoules) | ||||||
| 0 | 0,0 | 20 | 100% | 0,0 | ||
| 1-5 | 3 | 15,0 | 0,0 | 7 | 35,0 | |
| 6-10 | 4 | 20,0 | 0,0 | 12 | 60,0 | |
| 11-15 | 7 | 35,0 | 0,0 | 1 | 5,0 | |
| 16 and over | 6 | 30,0 | 0,0 | 0,0 | ||
| Mean | 12,6 ± 7,7 | 0 | 6 ± 2,3 | |||
| ANOVA | 0,0000 | 0,00081 | ||||
Table 6: average amounts of analgesics and injectable NSAIDs consumed.
Discussion
Results significance
This multicenter randomized phase II clinical trial aimed to evaluate the efficacy and safety of the Diavein® electrical stimulation vascular therapy device as an adjuvant in the treatment of severe painful VOC in sickle cell patients in Lubumbashi, Democratic Republic of Congo. The three trial groups were comparable in their baseline characteristics, namely hemoglobin sociodemographic characteristics and presence or absence of ongoing hydroxyurea treatment. This study shows that the use of VEST by Diavein® device in sickle cell patients for the management of hyperalgic VOC, result in a shorter crisis time (p<0.0001), a shorter hospital stay (p<0.01) and a shorter average acute attack duration (p<0.001) than in patients not subjected to this therapy. This correlate with the clinical observations made during the study. It was noted that patients treated with Diavein® had a better clinical response to treatment compared to those in the control group. In some cases, this rapid recovery from the painful VOC has allowed identification of underlying conditions therefore facilitating appropriate management. The above primary as well as the secondary endpoints findings leads to the conclusion that the Diavein device® is effective in the treatment of painful VOC in sickle cell disease.
In a phase II single-center, single-blind, study evaluating the efficacy and safety of the same VEST device in Ivory Coast, Botti report a significant shortening of the resolution time of hyperalgic VOCs, a shorter crisis time and a lower tramadol consumption in the Diavein® groups than in the control group [26-27]. Similarly, to Botti; our results could be explained by a potential effect of the Diavein® VEST device on the acceleration of blood circulatory velocity in stimulated areas and on platelet aggregation thus promoting an increase in blood flow and a decrease in cell adhesion phenomena on capillaries endothelial wall as well as a gain in vascular tone [28-29]. The reduction in the consumption of major analgesics observed during these studies is a key finding to consider because it implies a reduction in the cost of the management of severe sickle cell VOC therefore a reduction in the economic burden of sickle cell disease. Our results therefore encourage a medico-economic evaluation of the use of this Diavein® VEST device in reducing the costs of managing sickle cell VOC particularly in countries with limited resources. We did not observe significant differences between the two intervention groups stimulated using the VEST device for the primary outcome. This observation gives free rein to all possible hypotheses, the most striking of which would be that NSAIDs have no place in the treatment of painful VOCs or that VEST, because of its anti-inflammatory potential, would bring the two intervention groups closer. In this study we observed a decrease in hemoglobin levels in all trial volunteers with no statistically significant difference between the intervention groups and the control group, nor was the occurrence of hyperthermia, more common in one group than in the other. Two cases of post-stimulation paresthesia with the Diavein® device were observed, their spontaneous remission under observation and without therapeutic intervention, testifies their benignity; no other cause of paresthesia was found. It is possible that these cases of paresthesia are an extension of the effects of the stimulation when the device had already been disconnected. Botti et al reported one case in their study [30].
Strengths and limitations of the study
The results obtained during this trial show a statistically significant clinical efficacy of the VEST on the main outcomes. However, we noted that the balanced randomization by block of permutation on a list and the simple blind scheme make it possible to anticipate the treatment of patients. The methodological set-up of subsequent studies should take this into account so that this does not influence the provider’s attitude in the selection of patients. Difficulties in assessing pain in children less than 10 years of age and in those with low levels of education were also noted. However, considering that the development of new therapeutic approaches applicable in countries with a high prevalence of sickle cell disease that can minimize the use of analgesic drugs is an absolute necessity [9-11], it is crucial that the Specialized Centers for the Management of Sickle Cell Disease open up to this technique for which we believe that the results obtained allow entry into phase III.
Conclusion
This study suggests that the VEST by the Diavein® device is an adjuvant to be taken into account in the management of painful VOC of the sickle cell and encourages the conduct of phase III studies on the device.
References
- Piel FB (2013) Geographical distribution of sickle cell disease in 2010. Med Sci 29:965– 967
- Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN (2013) Global Burden of Sickle Cell Anaemia in Children under Five, 2010–2050: Modelling Based on Demographics, Excess Mortality, and Interventions. PLoS Med 10:e1001484
[Crossref] [Google scholar] [Indexed]
- Tshilolo L, Aissi LM, Lukusa D, Kinsiama C, Wembonyama S, et al. (2009) Neonatal screening for sickle cell anaemia in the Democratic Republic of the Congo: experience from a pioneer project on 31 204 newborns. J Clin Pathol 62:35–38
[Crossref] [Google scholar] [Indexed]
- Diallo D, Tchernia G (2002) Sickle cell disease in Africa. Curr Opin Hematol 9:111-116
[Crossref] [Google scholar] [Indexed]
- Mikobi TM, Lukusa TP, Aloni MN, Akilimali PZ, Mvumbi-Lelo G, et al. (2017) Clinical phenotypes and the biological parameters of Congolese patients suffering from sickle cell anemia: A first report from Central Africa. J Clin Lab Anal 31:e22140
[Crossref] [Google scholar] [Indexed]
- Montalembert M, Brousse V, Marchetti TM, Allali S (2020) Sickle cell disease in children and adolescents. Elsevier Masson Paris 248
- Bopp T, Stephan C, Samii K, Stirnemann J (2018) Acute chest syndrome: a serious complication of sickle cell anemia. Rev Med Suisse14:1844-1848
[Indexed]
- Ballas SK, Gupta K, Adams-Graves P (2012) Sickle cell pain: a critical reappraisal. Blood 120: 3647-3656
[Crossref] [Google scholar] [Indexed]
- Elion J, Laurance S, Lapouméroulie C (2010) Pathophysiology of sickle cell anemia. Med Trop 70: 454-458
[Indexed]
- Kibala KJ (2020) Poverty and unemployment in the Democratic Republic of Congo: inventory, analyzes and perspectives. Hal 02909695f
- Nsiala BK, Wete GB, Androzo RM, Aitikalema GE, Nkunga RK et al. (2012) Cost analysis of sickle cell treatment by parents at the Yolo Center for Mixed Medicine and Sickle cell Anemia. Annales de l’IRSS 2:1-5
- Argueta DA , Aich A, Muqolli F , Cherukury H , Sagi V, et al. (2020) Considerations for Cannabis Use to Treat Pain in Sickle Cell Disease. J Clin Med 9:3902
[Crossref] [Google scholar] [Indexed]
- Khellaf M (2015) Vaso-occlusive crisis in sickle cell anemia. Urgences 5:1-8
- Darbari DS, Wang Z, Kwak M, Hildesheim M, Nichols J, et al. (2013) Severe Painful Vaso-Occlusive Crises and Mortality in a Contemporary Adult Sickle Cell Anemia Cohort Study. PLoS ONE 8: e79923
[Crossref] [Google scholar] [Indexed]
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, et al. (1991) Pain in Sickle Cell Disease : Rates and Risk Factors. N Engl J Med 325:11-16
[Crossref] [Google scholar] [Indexed]
- Devalois B (2018) Management of pain in emergency of sickle cell crisis. Proceedings of the symposium of listeners of the FMOS sickle cell university diploma 3:52-63
- Galactéros F, Bartolucci P, Habibi A (2011) Medical emergencies in adults with sickle cell disease. Urgences 64:733-743
- Luntadila SN, Mukeba VN, Mwamba D, Mbuyi ST, Muteb PB, et al. (2020) Limitations of Analgesic Treatment in a Severe Sickle Cell Painful Vaso-Occlusive Crisis Complicated or Associated With Focal Involvement - About Two Cases. Med Case Rep 5:161
- Telen MJ, Wun T, McCavit TL, De Castro LM, Krishnamurti L, et al. (2015) Randomized phase 2 study of GMI-1070 in SCD: Reduction in time to resolution of vaso-occlusive events and decreased opioid use. Blood 125:2656-2664
[Crossref] [Google scholar] [Indexed]
- Elander J, Lusher J, Bevan D, Telfer P, Burton B (2004) Understanding the Causes of Problematic Pain Management in Sickle Cell Disease: Evidence That Pseudoaddiction Plays a More Important Role Than Genuine Analgesic Dependence. J Pain Symptom Manage 27:156–169
[Crossref] [Google scholar] [Indexed]
- Merighi S, Gessi S, Varani K, Fazzi D, Stefanelli A et al. (2013) Morphine mediates a proinflammatory phenotype via µ-opioid receptor-PKC?-Akt-ERK1/2 signaling pathway in activated microglial cells. Biochem Pharmacol 86:487-496
[Crossref] [Google scholar] [Indexed]
- Chen C, Farooqui M, Gupta K (2006) Morphine stimulates vascular endothelial growth factor-like signaling in mouse retinal endothelial cells. Curr Neurovasc Res 3:171-180
[Crossref] [Google scholar] [Indexed]
- Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, et al. (2002) Morphine Stimulates Angiogenesis by Activating Proangiogenic and Survival-promoting Signaling and Promotes Breast Tumor Growth. Cancer Res 62:4491-4498
- Friede T, Parsons N, Stallard N, Todd S, Valdes Marquez E, et al. (2011) Designing a seamless phase II/III clinical trial using early outcomes for treatment selection: an application in multiple sclerosis. Stat Med 30:1528–15240
[Crossref] [Google scholar] [Indexed]
- Gersbach P, Ferrari E, Segesser LK von, Gardaz JP, Haesler E (2011) Indications, prospects and limits of epidural electrical stimulation in peripheral or coronary arterial disease. Rev Med Suisse 4:797-804
- National Agency for the Safety of Medicines and Health Products (France). Notice to sponsors of clinical trials of medicinal products including advanced therapy medicinal products. Volume 2: Vigilance of clinical trials Surv_Vig_Doc080 V01 – janvier 2020.Disponible en ligne a l
- Botti RP, Bokoum SS, L’Hermite E, Silue DA, Kouakou B, et al. (2021) Efficacy and tolerance of vascular electrical stimulation therapy in the management of vaso-occlusive crises in patients with sickle cell disease: a phase II single-centre randomized study in Ivory Coast. Adv Hematol 2021:1373754
[Crossref] [Google scholar] [Indexed]
- Aguejouf O, Doutremepuich F, Doutremepuich Ch (2006) Effects of External Electric Stimulation (TVT©) on Laser-Beam-Induced Experimental Thrombosis. Pathophysiology. Pathophysiol Haemost Thromb 35:364-369
[Crossref] [Google scholar] [Indexed]
- Michaut-Paterno F, Van Dreden P, Girard P, Samama MM (1994) Effects of external electrical stimulation on fibrinolysis. Annales Françaises d’Anesthésie Réanimation 13:243
- Bartolucci P, El Murr T, Roudot-Thoraval F, Habibi A., Santin A, et al. (2009) A Randomized, controlled clinical trial of ketoprofen for sickle cell disease vaso-occlusive crises in adults. Blood 118:3742–3747
[Crossref] [Google scholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences