ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Pharmacokinetic Study of 2 Formulations of GFH009 Maleate Injection in SD Rats
Fu-Sheng Zhou1*, Jin-Zhu Zhao1, Hong-Can Ren1, Dragan Cicic2, Nicholas J Sarlis2, Jiong Lan1 and Qiang Lu1
1Preclinical R&D, Genfleet Therapeutics, Shanghai, China
2Department of Clinical Development, Sellas Life Sciences Group, New York, USA
- *Corresponding Author:
- Fu-Sheng Zhou
Preclinical R&D, Genfleet Therapeutics, Shanghai,
China,
E-mail: fszhou@genfleet.com
Received date: May 26, 2023, Manuscript No. IPAPP-23-16825; Editor assigned date: May 29, 2023, PreQC No. IPAPP-23-16825 (PQ); Reviewed date: June 09, 2023, QC No. IPAPP-23-16825; Revised date: June 19, 2023, Manuscript No. IPAPP-23-16825 (R); Published date: June 26, 2023, DOI: 10.36648/2471-9633.10.2.151
Citation: Zhou FS, Zhao JZ, Ren HC, Cicic D, Sarlis NJ, et al. (2023) Pharmacokinetic Study of 2 Formulations of GFH009 Maleate Injection in SD Rats. Am J Pharmacol Pharmacother Vol.10 No.2:151.
Abstract
Background: Members of the cyclin-dependent kinase family of proteins have been identified as potential therapeutic targets for suppression of tumor progression due to their role in regulating cell division, proliferation, and growth. Cyclin-Dependent Kinase 9 (CDK9) has been implicated in lymphomas, leukemia and other hematologic malignancies. GFH009, a potent, highly selective inhibitor of CDK9 has been characterized in an acute myelogenous leukemia cell line and demonstrated induced apoptosis.
Methods and Findings: This study assessed the Pharmaco Kinetics (PK) of 2 formulations of GFH009, one at pH 4.0 and another at pH 6.0, in Sprague Dawley rats. The mean plasma concentration of each formulation was similar at all time points, peaked at 5 minutes post dose and decreased gradually over time. The ratios of area under the curve from time 0 to infinity, area under the curve from time 0 to the last quantifiable concentration, drug concentration at time zero, clearance, and half-life between the 2 formulations were 0.890, 0.893, 1.02, 1.11 and 0.963, respectively.
Conclusion: Although the PK profiles of the 2 formulations were comparable, the pH 6.0 formulation is more appropriate for clinical research because it is closer in pH to that of normal human blood.
Keywords
GFH009; CDK9 inhibitor; Hematologic cancer; Pharmacokinetics
Abbreviations
AML: Acute Myelogenous Leukemia; APL: Acute Promyelocytic Leukemia; AUC: Area Under The Curve; BCL2: B-Cell Lymphoma 2; BCL2A1: BCL2-Related Protein A1; C0: Drug Concentration At Time Zero; CDK: Cyclin- Dependent Kinase; CI: Confidence Interval; CL: Clearance; c- MYC: Cellular Myelocytomatosis Proto-Oncogene (Human Homolog of Avian Virus Oncogene); CV: Coefficient of Variation; EDTA: Ethylenediaminetetraacetic Acid; FAB: French-American British (Morphologic Classification of AML); HPLC/MS: High Performance Liquid Chromatography (with Tandem) Mass Spectrometry; iASPP: Inhibitory (Member of the) Apoptosis-Stimulating Protein of p53; MCL-1: Myeloid Cell Leukemia-1; MDM4: Double Minute 4 (Human Homolog of); PK: Pharmacokinetic; P-TEFb: Positive Transcription Elongation Factor b; RD%: Percent Relative Deviation; SD: Sprague Dawley; STK: Serine/Threonine Kinase; T1/2: Half-Life; XIAP: X-Linked Inhibitor of Apoptosis Protein.
Introduction
Cyclin-dependent kinases are a family of proteins that play a crucial role in regulating the cell cycle, working in concert with cyclins to regulate cell division, proliferation, and growth. As such, members of this family are potential therapeutic targets for suppression of tumor progression and other cancer treatment strategies [1]. Cyclin-Dependent Kinase 9 (CDK9) is a Serine/Threonine Kinase (STK) that regulates the expression of short-lived oncogene products, such as c-MYC and MCL-1, both of which control tumor cell proliferation and survival. Dysregulation in CDK9-related pathways is implicated in the development of a variety of human cancers, including lymphomas, leukemia, and other hematologic malignancies [2].
CDK9 is a global transcriptional control protein, primarily involved in the recruitment of key transcription factors to the initiation complex and influencing the expression of genes regulated by super enhancers. This, in turn, drives expression of genes that preserve cell identity, including MYC, a downstream proto-oncogene involved in cell-cycle control, and MCL1, an apoptotic regulator [3]. In normal cells, steady state c-MYC acts as a global transcription enhancer and is tightly regulated by growth factors. However, in tumor cells, deregulation leads to supraphysiological levels of c-MYC, driving pathological expression of tumor-specific oncogenes and leading to uncontrolled cell proliferation [4].
CDK9 is a critical component of the positive Transcription Elongation Factor b (P-TEFb) complex, inducing phosphorylation of the C-terminal domain of RNA polymerase II. Although the P-TEFb complex does not exert direct control on the cell cycle, it does play a role in transcription regulation, enhancing productive elongation of several mRNA transcripts and increasing production of the cognate protein products [1,3,5,6]. Among these are several oncogenic proteins with short halflives, including MCL-1, BCL2A1, Mdm4, iASPP, XIAP, and c-MYC. Intermittent, dynamic-over-time inhibition of CDK9 is sufficient to disrupt the oncogenic effects of gene products with short halflives [1]. More specifically, inhibition blocks CDK9-mediated transcription of c-MYC, inhibiting the survival and growth of cancer cells [3].
GFH009 is a potent, highly selective, small molecule that targets and inhibits the activity of the CDK9/cyclin T1 regulatory complex of P-TEFb. In preclinical studies, the tumor antiproliferation activity of GFH009 has been characterized in the Acute Myelogenous Leukemia (AML) cell line MV-4-11, derived from human biphenotypic B-myelomonocytic leukemia blast cells. These in vitro studies indicated that the anti-proliferation activity of GFH009 is primarily a result of induced apoptosis. Further supporting this finding, GFH009 reduced expression of both c-MYC and MCL-1 in MV-4-11 cell line experiments, as well as the Acute Promyelocytic Leukemia (APL)-derived cell line HL-60. A 4-week toxicity study in Sprague Dawley (SD) rats tested GFH009 maleate at 2 mg/kg, 4 mg/kg and 8 mg/kg, with the 4 mg/kg treatment group approaching the biologically efficacious exposure level (Data on File, Genfleet).
A pH 4.5 formulation was initially developed for preclinical drug development and no infusion or toxic reactions were reported prior to clinical trials. However, it was deemed necessary to develop a formulation with a pH of 6.0 that more closely aligns with the physiological pH of the human body. The change in pH can potentially affect the solubility, stability, and efficacy of the drug and a bioequivalence study is needed to ensure that the Pharmacokinetics (PKs) of the formulations are comparable.
The objective of this study was to characterize the preclinical PK profiles of 2 different formulations of GFH009 maleate (pH 4.5 and 6.0) in SD rats and to determine if the 2 formulations are appropriate for future clinical applications. Should results show that the 2 formulations are bioequivalent; the pH 6.0 formulation should be considered a suitable alternative to the pH 4.5 formulation for clinical use.
Materials and Methods
Subject selection and dosing
SD rats were selected for use in this study as they are an international standard rodent model for long-term preclinical toxicity studies. SD rats were randomized by sex and body weight and placed into 1 of 2 groups. GFH009 maleate was administered to all test subjects at 0.8 mg/mL in a 0.9% sodium chloride solution. Group 1 SD rats received a solution of GFH009 (Lot No. 20190912) at pH 6.0 and Group 2 received a solution of GFH009 (Lot No. 20190416) at pH 4.5.
PKs for this study were examined using a 4 mg/kg dose previously determined to be comparable to a biologically efficacious exposure dosing level. Each animal received a single dose of 1 formulation of the study drug as an Intravenous (IV) bolus over 1 to 2 minutes to the tail vein. All dosed animals were observed daily starting 2 days prior to randomization, including the day of randomization. Observations included, but were not limited to, overall outward physical appearance, breathing, activity, elimination, and behavioral changes.
Sample collection
For PK assessment, 0.3 mL samples of whole blood were collected from the jugular vein of both Group 1 and Group 2 rodents prior to dosing and at 5 minutes, 15 minutes, 30 minutes, and at 1 hour, 2 hours, 3 hours, 4 hours, 7 hours, 10 hours, 12 hours, and 24 hours post dose. Upon collection into tubes containing K2-EDTA, all samples were placed on ice until centrifugation at 2000 g for 10 minutes at 2°C to 8°C for 2 hours. Following centrifugation, plasma was transferred to 2 prelabeled Eppendorf tubes for PK analysis. Plasma samples were stored at -70°C to -90°C within 1 hour of processing until transferred on dry ice to the Department of Bioanalysis I of Shanghai InnoStar Bio-Tech Co, Ltd. (Shanghai, China) for concentration analysis.
Bioanalysis
The plasma concentration of GFH009 in Group 1 and Group 2 samples was determined by validated high-pressure liquid chromatography with tandem mass spectrometry (LC-MS/MS) via an API 4000 mass spectrometer (SCIEX, Framingham, MA, USA) coupled with a Waters Ultra High-Performance liquid chromatography system (ID: EQ201537; Waters Corp., Milford, MA, USA). GFH1170 was used as an internal standard. All plasma samples were protein precipitated and analyzed over a linearity range of 1.000 to 1000.000 ng/mL.
Data analysis
Data processing for the GFH009 study utilized Thermo Fisher Scientific's Analyst V 1.6.3 software, whereas Watson laboratory information management system V 7.4.2 was employed for generating regressions and reports. Both software products were developed in Shanghai, China. Noncompartmental PK analysis was conducted on the plasma concentration of GFH009 in this study using the Phoenix WinNonlin software (version 8.1). To facilitate statistical analysis, GFH009 exposure measures such as the Area Under the Curve (AUC) and drug Concentration at time zero (C0) were converted to a logarithmic scale. For all other PK parameters, statistical analysis was performed directly without conversion.
Results
Groups 1 and 2 consisted of 12 SD rats each (6 female, 6 male, 24 test subjects in total) that were dosed with 1 of 2 pH formulations of GFH009. Female subjects ranged in mass from 198 g to 220 g and males 225 g to 236 g. All subjects were between 6 and 8 weeks of age. Concentrations of GFH009 maleate injection were measured by high-performance LC, and the concentration of study drug formulation was within 90.0% to 110.0% of nominal concentration. Percent Relative Deviation (RD%) were within 5% for both parallel samples (Table 1). The CV was <5.0%.
| Group 1 (pH 6.0) |
Group 2 (pH 4.5) |
|
|---|---|---|
| Nominal Concentration (mg/mL) | 0.8 | 0.8 |
| Mean (mg/mL) | 0.842 | 0.861 |
| RD (%) | 0.2 | 0 |
| Recovery (%) | 105.3 | 107.6 |
Table 1: Results of concentration analysis of dose formulations.
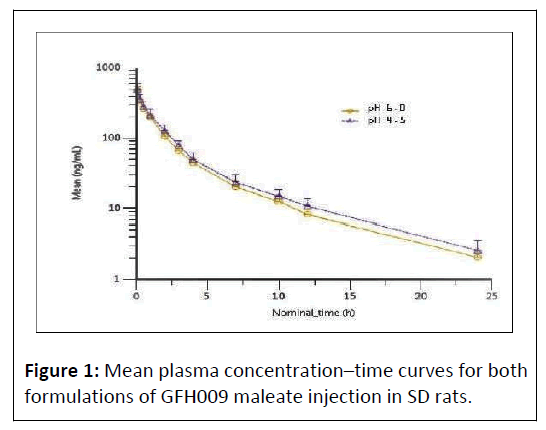
Mean plasma concentration peaked 5 minutes post dose at a value of 479 ng/mL in Group 1 and 486 ng/mL in Group 2. Concentration decreased gradually over time and followed a similar profile for both treatment groups (Figure 1).
Independent sample t-test comparison of AUC and C0 values for male and female animals showed no significant difference in PK parameters for each group (P>.05). No statistical gender differences of key PK parameters were observed and data from male and female animals were pooled for subsequent statistical analysis.
PK parameters following administration of single dose GFH009 maleate at 4 mg/kg in SD rats are summarized in Table 2. In Group 1 (pH 6.0), C0 was 586 ± 109 ng/mL, and the AUC from time 0 to the last quantifiable concentration (AUClast) was 825 ± 73.8 h* ng/mL. AUC from time 0 to infinity (AUCINF) in this group was 842 ± 75.0 h* ng/mL. Half-life (T1/2) approximately 5.45 hours and Clearance (CL) was 4780 ± 429 mL/h/kg. In Group 2 (pH 4.5) C0 was 572 ± 147 ng/mL and AUClast was 924 ± 144 h* ng/mL. The AUCINF within this group was 946 ± 145 h* ng/mL. T1/2 was approximately 5.66 hours with a CL of 4310 ± 607 mL/h/kg.
| Parameter | Group 1 (pH 6.0) | Group 2 (pH 4.5) | Ratioa | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | ||
| AUCinf (h* ng/mL) | 842 | 75 | 767, 917 | 946 | 145 | 801, 1091 | 0.89 |
| AUClast (h* ng/mL) | 825 | 73.8 | 751.2, 898.8 | 924 | 144 | 780, 1068 | 0.893 |
| C0 (ng/mL) | 586 | 109 | 477, 695 | 572 | 147 | 425, 719 | 1.02 |
| CL (mL/h/kg) | 4780 | 429 | 4351, 5209 | 4310 | 607 | 3703, 4917 | 1.11 |
| t1/2 (h) | 5.45 | 0.649 | 4.801, 6.099 | 5.66 | 0.973 | 4.687, 6.633 | 0.963 |
| Note: aRatio=Group 1/Group 2, SD=Standard Deviation | |||||||
Table 2: Average PK parameters, Group 1 (pH 6.0) and Group 2 (pH 4.5).
The ratios of AUCINF, AUClast, C0, CL and T1/2 between the 2 formulations were 0.890, 0.893, 1.02, 1.11, and 0.963, respectively (Table 2). Simultaneous bioequivalence analysis showed that the 90% Confidence Interval (CI) of the AUCINF, AUClast, and C0 ratios were 102.37% to 121.63%, 101.94% to 121.20%, and 82.17% to 112.25%, respectively (Table 3).
| Dependent | Units | Reference | Test | 90% CIa |
|---|---|---|---|---|
| AUCINF | h* ng/mL | Lot 20190912 (pH 6.0, Group 1) |
Lot 20190416 (pH 4.5, Group 2) |
102.37-121.63 |
| AUClast | h* ng/mL | 101.94-121.20 | ||
| C0 | ng/mL | 82.17-112.25 | ||
| Note: 90% confidence interval after double unilateral test | ||||
Table 3: Bioequivalence test of formulations of GFH009.
Discussion
The PK profiles of the 2 tested formulations of GFH009 (pH 6.0 and pH 4.5) were comparable following intravenous administration in SD rats, regardless of pH used for preparation. Simultaneous bioequivalence analysis of the 2 formulations met 90% CI values for AUCINF, AUClast, and C0, with all ratio values falling within the acceptable range of 80% to 125% (Table 3). These data indicate that the total systemic exposure with the 2 formulations was functionally identical when dosed intravenously. Given that drug formulations should have a target pH as close as possible to physiological pH, the GFH009 preparation used in Group 1, i.e., pH 6.0, was deemed the more appropriate (euhydric) for clinical use [7,8].
The sample analysis was performed on a high-performance LC-MS/MS method that has been previously validated. This method has a detection limit of 1 ng/mL and a linear range from 1 ng/mL to 1000 ng/mL. The recovery rate of 95% with a CV of 1.5% after diluting the 15000 ng/mL sample 20-fold suggests that the method can accurately measure GFH009 even after dilution of <20-fold. Selectivity, stability, recovery, intra-assay, and inter-assay sensitivity comply with the necessary criteria for accurate and precise measurement of the target analyte (data will be reported in another paper). Overall, this method is robust and reliable for detecting and analyzing GFH009 in the samples.
Although drugs that interact with the CDK family of proteins would seem ideal for inhibiting tumor growth, targeting therapeutics to specific proteins is critical to limiting adverse events that stem from nonspecific CDK inhibition. First generation CDK-inhibitor treatments, including flavopiridol (also known as alvocidib) and seliciclib (also known as roscovitine) were characterized by relatively burdensome toxicity resulting from off-target inhibition [1]. Targeting treatments specifically to CDK9 has been a goal of recent research and several other CDK9-selective inhibitors are currently in the development pipeline. Among these are KB-0742, a potent selective inhibitor of CDK9, which showed similarly rapid absorption and high bioavailability in PK studies of oral dosing [9]. PK assessment of the CDK9 inhibitor enitociclib (BAY1251152) demonstrated a mean elimination half-life of 3 hours to 9 hours, similar to that of GFH009 (approximately 5.5 hours), with no accumulation over multiple doses. However, to date, phase 1 studies of single-agent enitociclib have not yielded objective responses, despite achieving therapeutic plasma levels and a demonstrated target specificity [10,11]. The PKs and pharmacodynamics of AZD-4573, another selective CDK9 inhibitor that has shown promise against a variety of hematologic cancers, have been determined, and it is in phase 1 studies. A pilot trial of AZD-4573 combined with the Bruton kinase inhibitor acalabrutinib is currently underway in patients with relapsed or refractory diffuse large B-cell lymphoma [12,13].
Conclusion
The PK profiles of both tested GFH009 formulations were comparable and equivalent when administered via intravenous injection at a dose of 4 mg/kg of body mass. The pH 6.0 formulation of GFH009 is more appropriate for clinical research settings, given that it is closer to the normal human blood pH range of 7.35 to 7.45. This formulation is currently being used in an ongoing phase 1 clinical trial (ClinicalTrials.gov Identifier: NCT04588922) examining the use of GFH009 as a monotherapy for patients with relapsed and/or refractory hematologic malignancies, including AML, chronic lymphocytic leukemia, small lymphocytic lymphoma and lymphoma. Going forward, the pH 6.0 formulation of GFH009 will be used for all additional clinical studies to determine dosing, efficacy, and safety of this new drug.
Acknowledgements
Technical, editorial, and medical writing assistance were provided under the direction of the authors by Adrienne Evans Fernandez, MAT, Kim Storvik, PhD, and Amplity Health. Funding for this support was provided by SELLAS Life Sciences Group, Inc., New York, NY, USA.
References
- Borowczak J, Szczerbowski K, Ahmadi N, Szylberg L (2022) CDK9 inhibitors in multiple myeloma: A review of progress and perspectives. Med Oncol 39: 39.
[Crossref], [Google Scholar], [Indexed]
- Romano G (2013) Deregulations in the cyclin-dependent kinase-9-related pathway in cancer: Implications for drug discovery and development. Hindawi 5: 1-14.
[Crossref], [Google Scholar], [Indexed]
- Boffo S, Damato A, Alfano L, Giordano A (2018) CDK9 inhibitors in acute myeloid leukemia. J Exp Clin Cancer Res 37: 36.
[Crossref], [Google Scholar], [Indexed]
- Wolf E, Lin CY, Eilers M, Levens DL (2015) Taming of the beast: Shaping Myc-dependent amplification. Trends Cell Biol 25: 241-8.
[Crossref], [Google Scholar], [Indexed]
- Rahaman MH, Kumarasiri M, Mekonnen LB, Yu M, Diab S, et al. (2016) Targeting CDK9: A promising therapeutic opportunity in prostate cancer. Endocr Relat Cancer 23:211-226.
[Crossref], [Google Scholar], [Indexed]
- Cassandri M, Fioravanti R, Pomella S, Valente S, Rotili D, et al. (2020) CDK9 as a valuable target in cancer: From natural compounds inhibitors to current treatment in pediatric soft tissue sarcomas. Front Pharmacol 11:1230.
[Crossref], [Google Scholar], [Indexed]
- Allen LV (2020) Sterile basics: Intravenous admixture preparation considerations, part 5: pH considerations. Int J Pharm Compd 24: 305-309.
[Google Scholar], [Indexed]
- Samuelsen L, Holm R, Lathuile A, Schönbeck C (2019) Buffer solutions in drug formulation and processing: How pKa values depend on temperature, pressure and ionic strength. Int J Pharm 560: 357-364.
[Crossref], [Google Scholar], [Indexed]
- Day M, Saffran DC, Rioux N, Chen T, Lee C, et al. (2021) Abstract p228: Preclinical pharmacokinetics and pharmacodynamics of KB-0742, a selective, oral CDK9 inhibitor. Mol Cancer Ther 20: 228.
[Crossref], [Google Scholar]
- Byrne M, Frattini MG, Ottmann OG, Mantzaris I, Wermke M, et al. (2018) Phase I study of the PTEFb inhibitor BAY 1251152 in patients with acute myelogenous leukemia. Blood 132: 4055.
[Crossref], [Google scholar]
- Shadman M, Mato AR, Batlevi CL, Flinn IW, Gutierrez M, et al. (2022) Enitociclib (VIP152/formerly BAY1251152) is a selective and active CDK9 inhibitor: Preliminary safety and early signs of efficacy in patients with Non-Hodgkin Lymphoma (NHL) and Chronic Lymphocytic Leukemia (CLL). Blood 140: 9454-9456.
[Crossref], [Google Scholar]
- Rule S, Kater AP, Brümmendorf TH, Fegan C, Kaiser M, et al. (2018) a phase 1, open-label, multicenter, non-randomized study to assess the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of AZD4573, a potent and selective CDK9 inhibitor, in subjects with relapsed or refractory hematological malignancies. J Clin Oncol 36: 1-15.
[Crossref], [Google Scholar]
- Strati P, Kim T, Danilov A, Cheah C, Yoon D, et al. (2022) Phase 1b/2a study of AZD4573 (CDK9i) and acalabrutinib in patients with relapsed/refractory Diffuse Large B-Cell Lymphoma (r/r DLBCL): Results from dose-escalation. Blood 140: 6656-6658.
[Crossref], [Google Scholar]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences