Personalized Medicine Pave the Way to a Modern Medicine Era
Shriya Phadnis*
Department of Microbiology, University of Mumbai, Mumbai, India
- *Corresponding Author:
- Shriya Phadnis
Department of Microbiology,
University of Mumbai,
Mumbai,
India,
Tel: 9969152678;
E-mail: shriyaphadnis1@gmail.com
Received Date: June 28, 2021; Accepted Date: July 12, 2021; Published Date: July 19, 2021
Citation: Phadnis S (2021) Personalized Medicine Pave the Way to a Modern Medicine Era. J Mol Biol Biotech Vol.6 No.4:03.
Abstract
The traditional medical treatment model is generalized for all patients. Resulting into a treatment successful for a few patients and not for others. This creates a need to replace the ancient method with an innovative approach in treatment and cure, personalized medicine which has become increasingly important in the future of healthcare. The review article focuses on a modern approach of treatment strategy based on individual biological makeup. Personalized medicine is used to classify, understand, treat, and prevent disease based on the data of individual biological and environmental differences. It applies a datadriven approach seeking knowledge about the genetic factors and enzyme mechanism of each individual making healthcare more effective.
Keywords
Pharmacogenomics; Theranostics; Precision medicine; Next-generation sequencing
Introduction
The advances in the field of human genetics began post completion of the Human Genome Project. This gave an insight to the unique variations and differences in individual human genome. These slight differences in genetic makeup of every individual lead to the individual responses to a drug treatment or causing drug toxicity in some. In modern medicine, using genomic information (RNA, proteins) paves a path for treatment decision making. Genomic medicine that is a part of Pharmacogenomics is a major component of personalized medicine. At the base-pair level human genome is 99.9% but 0.1% variation makes each individual unique. Pharmacogenomics studies how genomic variation affects a patient’s response to a particular medication. Scientists assess gene variants affecting an individual's drug response by identifying the genetic loci associated with known drug responses and then testing individuals whose response is unknown.
Personalized medicine can be broadly described as customisation of healthcare that accommodates differences in all stages from prevention through diagnosis and treatment to post-treatment follow up [1]. Consequently, a long-term vision of a true system-based approach to healthcare would be hindered by personalized medicine using individual genome sequencing.
History
A study over a few decades resulted that variation in drug responses has been administered. This individual response can be genetically determined with various factors like age, epigenetic factors, nutrition, and niche. In order to obtain an individual patient drug therapy, there is a need for accounting different drug response patterns with drug toxicity to drug benign ratio of a population geographically [2].
Scientists observed a greater ratio and a highly variable drug responses that led to the discovery of a new treatment methodology bound to different disciplines of genetics, biochemistry, molecular medicine and pharmacogenomics. Commercialization of this research application now emerged as personalized medicine. PM is considered to be an extension of the traditional treatment with greater precision.
Landscape
A patient’s genetic profile through sequencing can help select drugs or treatment methodology that would minimize the side- effects and ensure successful outcomes. Medical professionals cannot go beyond a generalized treatment for a disease to an individual treatment in order to achieve effective results. PM provides a structural model with a network of electronic health records and a record of genome sequencing of every patient linking the molecular and clinical information [3]. This would assist physicians in making appropriate treatment decisions for individual patients.
Next-generation sequencing
In genetics and biochemistry, sequencing means to determine the primary structure or primary sequence of an un-branched biopolymer. By understanding the sequence of DNA, researchers have been able to elucidate the structure and function of proteins. Next Generation Sequencing (NGS) is a powerful platform that has enabled the sequencing of thousands to millions of DNA molecules simultaneously.
This powerful tool is revolutionizing fields such as personalized medicine, genetic diseases and clinical diagnostics by offering a high throughput option with the capability to sequence multiple individuals at the same time [4]. The advent of capillary instrumentation and the use of fluorescent-based detection methods accompanied by automated analysis have moved traditional DNA Sequencing into ‘Next Generation’ Sequencing (NGS).
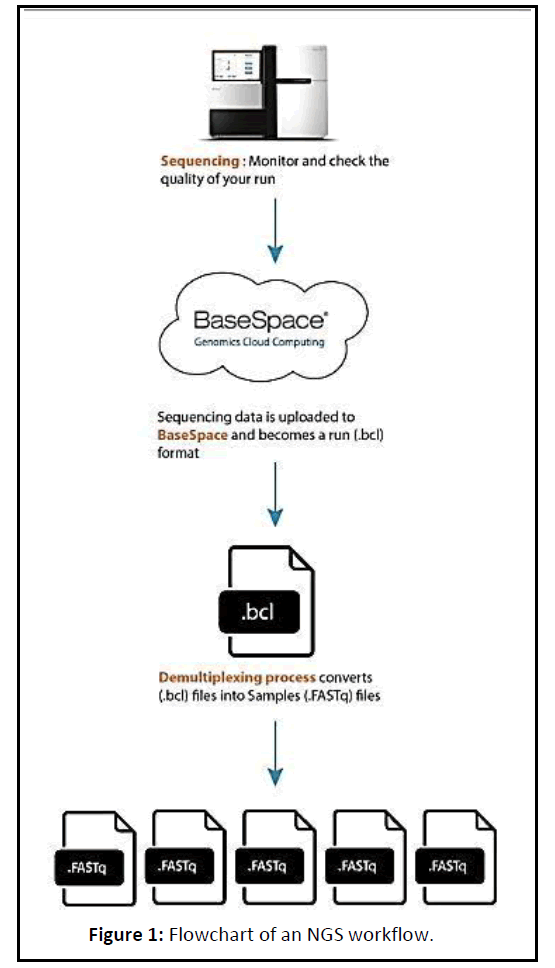
Next Generation sequencing (NGS) data is extremely high throughput, allowing for exponentially higher amounts of data to be generated than the traditional Sanger Sequencing. When performing whole genome sequencing or exome sequencing, it is expected that the result will be highly uniform (as there should be a 1:1 ratio in the starting material) (Figure 1).
Application boosted by NGS technology was the study of protein binding sites in genomic DNA especially that of transcription factor binding sites based on Chromatin ImmunoPrecipitation (ChIP). The application of Next Generation Sequencing (NGS) to ChIP has revealed insights into gene regulation events that play a role in various diseases and biological pathways [5]. A combination of Genome-Wide Association Studies (GWAS) and specific targeting by sequence. Capture of the genomic regions detected is now used extensively trying to identify the variants that functionally link the DNA with the phenotype.
Similarly, genome sequencing can be used as a tool to characterize genetic variation in a specific population. Thus far studies have been mostly performed on the level of cell cultures, whole tissues or sorted cell populations. Although the yield per cell, 30%-70% of all RNA or DNA present, can still be improved recent NGS developments have now made genome-wide single cell analysis feasible. Individual cells turn out to be quite different showing extensive genomic and transcriptomic heterogeneity in both normal development and disease.
Pharmacogenomics
Pharmacogenomics is the branch of pharmacology which deals with the influence of genetic variation on drug response in patients by correlating gene expression or single-nucleotide polymorphisms with a drug’s efficacy or toxicity. It aims to develop rational means to optimize drug therapy, with respect to the patient’s genotype [6].
Such approaches promise the advent of personalized medicine. Pharmacogenomics is the whole genome application of pharmacogenomics, which examines the single gene interactions with drugs. The term comes from the words pharmacology and genomics and is thus the intersection of pharmaceuticals and genetics. Pharmacogenomics combines traditional pharmaceutical sciences such as biochemistry with annotated knowledge of genes, proteins, and single nucleotide polymorphisms. The most common variations in the human genome are called Single Nucleotide Polymorphisms (SNPs). Today, clinical trials researchers use genetic tests for variations in cytochrome P450 genes to screen and monitor patients. In addition, many pharmaceutical companies screen their chemical compounds to see how well they are broken down by variant forms of CYP enzymes. Another enzyme called TPMT (Thiopurine Methyltransferase) plays an important role in the chemotherapy treatment of common childhood leukaemia by breaking down thiopurines. Knowing one’s genetic code will allow a person to make adequate lifestyle and environmental changes at an early age so as to avoid or lessen the severity of a genetic disease.
The main aims of it are; personalized therapy, improvement in efficacy and reduction in adverse drug reactions correlation of genotype with clinical genotype, identification of novel targets for new drugs and pharmacogenetic profiling of patients to predict disease susceptibility and drug response.
Theranostics
Theranostics commonly known as Pharmacodiagnostics is a term derived from a combination of the words therapeutics and diagnostics [7]. New materials and technologies are revolutionizing therapeutic treatments in various domains, such as preventive and diagnostic medicine, management of diseases and implants. The field applying theranostics approach promises to allow the medical professionals to use detailed genotypic information of a patient and simultaneously monitor the individual's therapeutic regimen and track the therapeutic response.
The application of theranostics in cancer
Cancer is a tricky entity, because it is not just one disease, but a heterogeneous group of diseases that is characterized by uncontrolled and rapid cell growth. This is usually due to genetic and/or epigenetic changes in affected patients. Current therapies for cancer include chemotherapy, radiotherapy, immunotherapy and surgery [7].
Chemotherapy in particular has limited usefulness owing to the reduced concentration of drug molecules that reach the tumours which they are intended to treat. Further limitations of chemotherapy include the development of resistance to treatment during therapy, and of course the many side effects associated with the drugs used.
Here Nano medicine with the help of nanoparticles shows great potential in addressing the limitations of conventional treatment. These particles allow molecular targeting to ensure higher concentrations of drug molecules at the tumour site. This has been the focus of extensive studies on drug delivery systems which incorporate nanotechnology, in recent years. These studies investigate the use of Nano medicines in an active or passive manner to trigger site-specific release of drugs. This ensures that the drug bypasses tissues that are off-target, and in doing so improves the therapeutic precision.
Discussion
Artificial intelligence in personalized medicine
Artificial Intelligence is now been used for years in the field of healthcare and biomedicine. In Computer Science, Artificial intelligence (AI) sometimes called machine intelligence mimics cognitive functions that human associate. Personalized medicine is considered to be incomplete without some add-ons like machine learning algorithms. Machine learning is a subfield Artificial Intelligence that provides the ability to automatically improve from experience without being explicitly programmed.
ML specializes in the development of computer programs which can retrieve data and use it to learn for themselves. Most commonly used ML algorithms in medicine includes SVM, deep learning, logistic regression, DA, decision tree, random forest, linear regression, Naïve Bayes, K-Nearest Neighbour (KNN) and Hidden Markov Model (HMM). Three types of machine learning algorithms:
1. Unsupervised (ability to find patterns)
2. Supervised (classification and prediction algorithms based on previous examples)
Reinforcement learning (use of sequences of rewards and punishments to form a strategy for operation in a specific problem space) for a few years now, AI has been used in various medical treatments like neurodevelopmental disorders that include autism spectrum disorder, intellectual disorder. AI can help identify the casual genes and locus using Bioinformatics tools.
Along with this it even assist in Phenotypic and genetic heterogeneity identification, gene to gene interactions, and also in computer aided drug discovery.
One of the promising advances of AI in the field of medicine is the development of Biomarkers. Biomarkers are objective medical signs (as opposed to symptoms reported by the patient) used to measure the presence or progress of disease, or the effects of treatment. Biomarkers can have molecular, histologic, radiographic, or physiological characteristics.
Benefits of personalized medicine
The development of PM could bring benefits to patients in a number of ways; Improved efficacy: patient more likely to receive a medicine delivering a clinical benefit, and treatment targeted at patients who will respond
• Improvements in overall survival
• Reduced adverse events: PM could be targeted at patients who are less likely to have an adverse reaction, reducing safety concerns.
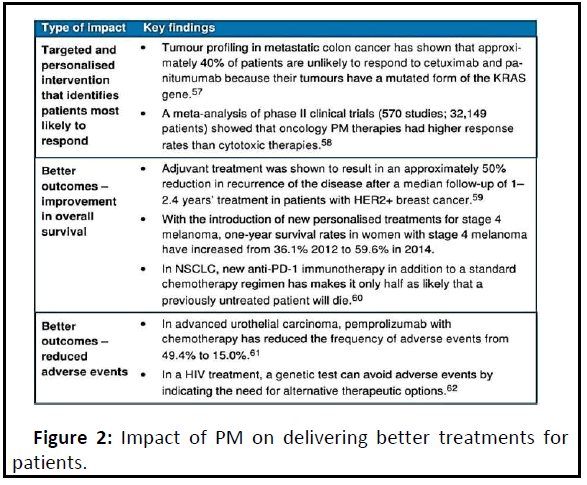
The use of genetic and other forms of molecular screening could help predict the best dosing schedule or combination of medicines for a particular patient. This offers the potential to improve healthcare provision by better matching patient needs and therapeutic benefits, and through a more informed choice of therapy. Genetic information can distinguish between patients who are likely to respond strongly to pharmacologic treatment and those who will receive no benefit. Genetic testing is becoming widely used to evaluate which medicines may work best for cancer treatment (Figure 2).
Conclusion
Technology improves and so does the healthcare system. The blooming field of modern medicine has the potential to bring out the improved health results with assistance of precise drug development. Unlike PM, conventional drug therapy typically considers large patient populations to be relatively homogeneous. Any given drug can be therapeutic in some individuals but ineffective in others, and some individuals experience adverse drug affects whereas others are unaffected. PM also offers an opportunity to enhance the value of currently approved drugs with limited market share because of significant toxicity or limited efficacy, enabling prescribers to identify patients for whom they can be both effective and safe. Such recognition of inter individual differences in drug response is an essential step toward optimizing therapy. Many times we have already seen that the marriage of drug-related diagnostics can provide better utilization parameters for new products as well as improve safety profiles or efficacy of older chemically based medications. In this second decade of the 21st century, health care will be reframed and designed for at least the next decade. PM provides a kinetic energy to foster change in the health care system.
References
- Meiliana A, Dewi NM, Wijaya A (2016) Personalized Medicine: The Future of Health Care. Indones Biomed J 8: 127-146.
- Pérez JH, Furlow JD, Wingfield JC, Ramenofsky M (2019) Regulation of vernal migration in Gambel’s white-crowned sparrows: role of thyroxine and triiodothyronine. Elsevier 84:50- 56.
- Mathur S, Sutton J (2017) Personalized medicine could transform healthcare (Review). Biomedical Reports 7: 3-5.
- Vogenberg FR, Barash CI, Pursel M (2010) “Personalized Medicine, Part 1: Evolution and Development into Theranostics”. P T. 35: 560-562, 565-567, 576.
- Heloisa Thomazi-Kleina (2019) Next-Gen Medicine teaching partners to launch new center for regenerative therapies. Philadelphia: University of Pennsylvania Press. 435 p.
- Vicente AM, Ballensiefen W, Jönsson JI (2020) How personalised medicine will transform healthcare by 2030: the ICPerMed vision. J Transl Med 18: 180.
- Sanzo MD, Cipolloni L, Borro M, Russa RL, Santurro A, et al. (2017) Clinical Applications of Personalized Medicine: A New Paradigm and Challenge. Curr Pharm Biotechnol. 18:194-203.
- Schork NJ (2020) Artificial Intelligence And Personalized Medicine. Cancer Treat Res. 178: 265-283.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences