Organic and Inorganic Nano-Systems Used in Cancer Treatment
1 Department of Polymers, The National Institute for Research & Development in Chemistry and Petrochemistry, Bucharest, Romania
2 Department of Biomaterials and Medical Devices, Faculty of Medical Engineering, University Politehnica of Bucharest, Bucharest, Romania
- *Corresponding Author:
- Madalina Elena Grigore
Department of Polymers
The National Institute for Research
& Development in Chemistry and Petrochemistry
(ICEHIM), Bucharest 060021, Romania
Department of Biomaterials and Medical Devices
Faculty of Medical Engineering
University Politehnica of Bucharest
Bucharest 060042, Romania
Tel: +40752578871
Email: grigore.madalina10@gmail.com
Received Date: January 07, 2017 Accepted Date: January 24, 2017 Published Date: January 31, 2017
Citation: Grigore ME. Organic and Inorganic Nano-Systems Used in Cancer Treatment. J Med Res Health Educ. 2017, 1:1.
Abstract
One of the main causes of death in the world is represented by cancer; it was reported over 8.2 million deaths worldwide. The most common methods to treat cancer refers to conventional anticancer drugs (chemotherapeutics) which display significant shortcomings such as damage of the immune system, low therapeutic indices and other organs or lack of solubility which limit their use in cancer treatment. To solve these limitations of conventional anticancer drugs, nanoparticles were rapidly developed. This study aims to provide an updated survey of the major progress in the flied of cancer therapy, including the limitation of conventional chemotherapy and the advantages or disadvantages of organic and inorganic nanoparticles. Also, this review focuses on the progress made in the field of nanoparticles for drug delivery in cancer therapy over the last years.
Keywords
Cancer therapy; Drug delivery systems; Nanoparticles; Nanotechnology; Chemotherapy
Introduction
Cancer represents the main cause of deaths in the entire world, it was reported over 8.2 million deaths worldwide [1]. One of the most common methods for treat cancer refers to chemotherapy, but this treatment involves toxic side effects which limit the amount of the drug that can be given to a patient. In several cases, it was reported that the tumor tissue should not be exposed to a lethal dose of the drug. As a result of all these problems, the researchers have tried using nanoparticles (NPs) for cancer treatment and it was reported that the use of nanoparticles (organic or inorganic) can improve the pharmacological properties of traditional chemotherapeutics.
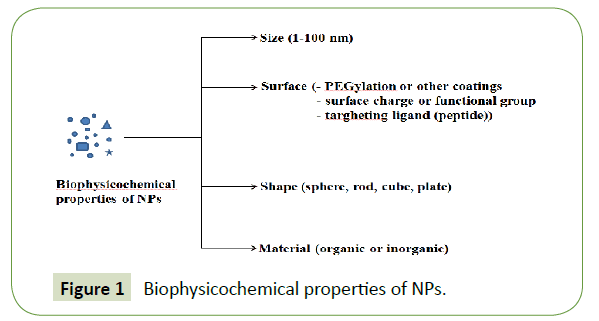
The NPs used for drug delivery can present different biophysicochemical properties (Figure 1) such as different size (1 nm to 100 nm), surface, shape, and materials (can be used soft (organic and polymeric) or hard (inorganic) material) [1-5]. It was reported that the dimension of NPs should be up to 100 nm to reach tumor tissues by passing through the particular vascular structures, for example in the case of the sinusoid in the spleen and fenestra of the Kupffer cells in the liver the dimension varies from 150 nm and 200 nm. Also, it was reported that small NPs with play a vital role in that accumulated inside the tumors by enhanced permeability and retention effect (EPR) [6].
The surface of NPs is also very important because it determines their life span and fate during circulation relating to their capture by macrophages and a solution to escape from macrophage capture is represented by a hydrophilic surface. A hydrophilic surface can be achieved by coating the surface of NPs with a hydrophilic polymer (PEG) [7].
Their small size allows them to pass from circulation through vascular defects present at tumor sites (the phenomenon is called fenestrations) and then they can deliver encapsulated cytotoxic agents to tumor tissue. One advantage of these NPs is that they can deliver a high drug concentration to the targeted cancer cell [8]. It was reported several studies where drugs such as doxorubicin or paclitaxel were used for the treatment of various type of cancer (prostate, liver, lung, breast cancer) [9-14].
Limitations of Conventional Chemotherapy
Chemotherapy refers to effective drugs treatment designed to destroy cancer cells or to slow the spread or growth of these cells. Despite this advantage, the effect of conventional chemotherapy produces also several disadvantages. The conventional chemotherapeutic agents don't destroy only the cancer cells, they damage also the healthy cells causing organ dysfunction, myelosuppression (occurs the reduced production of white blood cells), alopecia (hair loss), mucositis (inflammation of the mucous membranes lining the digestive tract) etc. [15,16]. Other disadvantages of these chemotherapeutics are that they remain in the circulation for a very short time and cannot interact with the cancerous cells and also the poor solubility of the drugs represent a problem because making them unable to penetrate the biological membranes [17].
In several studies, it was reported that a problem for the administered drugs is represented by the surface of the cancerous cells because the surface is covered with a multidrug resistance protein (P-glycoprotein) acting like a reflux pump which prevents the drug accumulation in the tumor. Because of their numerous disadvantages, the researchers tried to replace the conventional chemotherapeutic agents with nanoparticles [18-20].
Nano-Systems in Cancer Treatment
The main problem of conventional chemotherapeutic agents refers to the high volumes who affecting both normal cells and tumor cells [21]. Given that in the 21st century the complete treatment for cancerous disease remained undiscovered, the researchers concluded that the optimal treatment for cancer consists of using NPs (organic or inorganic NPs) [22,23].
The NPs utilization presents a lot of advantages, such as targeted release, stability, and solubility of the drug. Another advantage very important in the cancer treatment is that the NPs present a high permeability at tumor vessels allowing the entry of tumor agents, compared with healthy vessels [24]. In Table 1 is presented some complex used in the cancer therapy.
| Product | NPs | Applications | Phase of development | References |
|---|---|---|---|---|
| Combidex | Iron oxide nanoparticle | Tumor imaging | No available | [25] |
| NK105 | Micellar NPs | Breast cancer | Phase II | [26] |
| CYT-6091 | Gold NPs | Pancreatic cancer, breast cancer | Phase I/II | [27] |
| AuroLase | Gold NPs | Lung cancer and neck cancer | Phase I | [28] |
| Docetaxel-PNP | Polymeric NPs | Advanced solid malignancies | Phase I | [29] |
| C-dots | PEG-coated SiO2 | Melanoma | Phase I | [30] |
Table 1: Nanoparticle-based drugs used in various stages of cancer.
In the cancer treatment, researcher been developed organic systems and each of them present advantages and disadvantages related in Table 2.
| Type of NPs | Size (nm) | Advantages | Disadvantages | Applications |
|---|---|---|---|---|
| Polymers | 10-1000 | Biodegradability, drug release | Low efficiency of delivery | Delivery of components |
| Quantum dots | <10 | Surface modification | Unstable at UV | Detection of cancer |
| Dendrimers | 43952 | High drugs carriage | Cytotoxic | Target delivery |
| Liposomes | 50-100 | Biodegradability | Inflammation | Gene delivery |
Table 2: Types of organic nanoparticles for cancer therapy [31].
Polymeric nanoparticles: The main advantages of these materials are their properties such as biocompatibility, biodegradability,non-toxicity, and hydrophilicity. These systems related below are successfully used in cancer treatment [32,33].
Chitosan NPs: Besides the good properties of chitosan (biological and physicochemical characteristics), the biggest advantage that makes it suitable for targeted release applications refers to the fact that it can form polyelectrolyte complexes with DNA [33,34]. In various studies, it was reported that chitosan NPs can be used for both cancer imaging and therapy [35].
Arya, et al. [36] tested a system composed from herceptin, gemcitabine and chitosan NPs prepared by ionic gelation method against Mia Paca 2, PANC 1 and HEK293 cells. The system was tested in vitro and it was observed that the cells suffered from apoptosis and the efficiency was higher due to the presence of gemcitabine [36]. In another study, Ekinci, et al. [37] used a more complex system composed from methotrexate and chitosan NPs prepared by ionic gelation. After that, the system was radiolabeled with Technetium-99m for in vitro evaluation for breast cancer diagnosis. It was reported that Technetium-99mmethotrexate- chitosan NPs present a high absorption in human breast cancer (MCF-7) cell line and in the case of the normal cells it was observed that this system produces minimum negative effects [37].
Ferreira, et al. [38] created a system composed from chitosan NPs and aminolevulinic acid derivatives which were used in combination with Photodynamic Therapy to evaluate the synergistic effect against melanoma cancer. It was obtained promising results [38]. Due to their properties, chitosan NPs are widely used to deliver anticancer drugs and inhibit tumor growth without systemic toxicity. Currently, the researchers are trying to use this material as a functional biopolymer for encapsulation of small interfering RNA. In a recent study, carboxymethyl dextran (CMD) chitosan NPs were used to encapsulate snail siRNA and doxorubicin (DOX) and this system was tested against epithelial mesenchymal transition (EMT) gene expression of HCT-116 cell lines. It was reported that the treatment led to significant changes of EMT genes, apoptosis cell death and migration inhibition in HCT-116 cells [39].
PLGA and PLA NPs: Poly (Lactide-co-Glycolide) (PLGA) is a copolymer of lactic acid and glycolic acid which can be synthesized by direct polycondensation. This polymer presents good properties such as biocompatibility, biodegradability, and ideal mechanical properties, but the most importantly advantage of this polymer is represented by the fact that was approved by FDA. Because of these properties, PLGA has been extensively studied for the development of controlled delivery devices (proteins or small molecule drugs) [33,40,41]. In a study, were prepared aptamer-labeled paclitaxel-loaded PLGA NPs to study the cytotoxicity against normal human mammary epithelial cells (HMEC cells) and human glial cancer cells (GI-1 cells). It was observed a good retention of NPs inside the cells which causes apoptosis [42]. In another study, it was used a system formed from bicalutamide loaded PLGA NPs in prostate cancer. The authors have used LNCaP and C4-2 cancer cells and it was observed that the system significantly inhibit colony formation in the two cell lines and the cell apoptosis occurs [43]. In another study, it was investigated the in vitro anticancer activity of cisplatin-loaded PLGA-mPEG NPs and control (cisplatin free) on human prostate cancer LNCaP cells. It was reported an anticancer activity against LNCaP human prostate cancer cells when it was used the system formed from cisplatin-loaded PLGA-mPEG NPs [44].
Poly (Lactic Acid) (PLA) is a polymer of lactic acid approved by FDA. It was used in biomedical applications for more than 20 years because of their properties like biocompatibility, biodegradability, and non-toxicity [33]. In a study, it was used PLA NPs loaded with docetaxel (DTX) prepared by emulsion solvent evaporation method. In vivo tests have been performed to study their antimetastatic efficacy in a nude mouse model of liver metastasis. It was observed that the treatment significantly decreased the metastatic tumor area in the nude mouse liver [45]. In another study, it was reported that the action of temozolomide against C6 glioma is sustained only when the temozolomide is loaded into NPs [46].
Both PLGA and PLA NPs present good results when they are loaded with anticancer drugs. At the moment, research has focused on study the best administration route (intra-venous, intra-tumoral and intra-peritoneal) for these systems [47,48].
Polymeric micelles: This category is represented by amphiphilic block copolymers which form a micelle with a hydrophilic shell such as PEG and poly (vinyl alcohol) and a hydrophobic core such as L-lysine, propylene oxide, aspartic acid and D, L-lactic acid [7,49]. The hydrophilic shell serves to the stabilization of the hydrophobic core, while the hydrophobic core serves as a reservoir for hydrophobic drugs. The drugs can be loaded into a polymeric micelle by chemical covalent attachment or physical encapsulation [50,51]. Cytotoxic drugs delivery to the cancer cells using PEG polymeric micelles has been reported in several studies [52]. One of the first studies developed refers to a polymeric micelle carrier system for doxorubicin consists of polyethylene glycol and conjugated DOX-poly aspartic acid. It was reported that this system presents a much stronger activity than the free DOX [53].
Yoo and Park prepared a di-block copolymer of PLGA and PEG. The DOX was chemically conjugated to a terminal end of PLGA and folate was separately conjugated to a terminal end of PEG. It was reported that a DOX moiety at the PLGA end of DOX-PLGAmPEG is buried in the core, while a folate moiety at the PEG end of PLGA-PEG-FOL is expected to be oriented outside. For in vivo tests were used female athymic nude mice and were subcutaneously implanted with a human epidermal carcinoma xenograft cell line (KB cells) and after 21 days the implantation occurs. It was reported that the system presents a significantly lower amount of DOX detected in the heart compared to control (DOX free) at 24 h. These results are promising since the cardiac toxicity is one of the leading side-effects of DOX chemotherapies [54]. Another study was conducted on 17 patients with advanced solid tumor types and NC-6004 (cisplatin-incorporated micellar formulation) was administered intravenously every 3 weeks. It was reported that the recommended dose was 90 mg m-2 and the maximum tolerated dose was 120 mg m-2; and NC-6004 presented a low toxicity than cisplatin free [55].
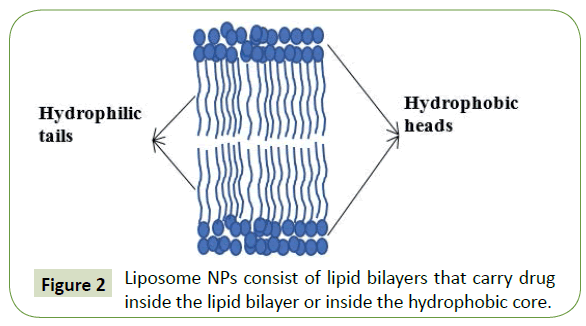
Liposomes NPs: Liposomes are composed of lipid bilayers where the core can be either hydrophilic or hydrophobic and have a spherical shape like phospholipids and cholesterol (Figure 2). Liposomes with a single lipid bilayer contain an aqueous core for encapsulating water soluble drugs, while liposomes with more than a single bilayer entrap lipid soluble drugs. For the treatment of metastatic breast cancer, it was approved liposomal formulations of the anthracyclines doxorubicin and daunorubicin such as Doxil®, Myocet® or DaunoXome® [56-61]. Also, it was reported that liposomes are ideal for the encapsulation of plasmid DNA and siRNA in their hydrophilic core [62].
Doxil® is a PEGylated liposomal doxorubicin which treats metastatic breast cancer, ovarian cancer, and AIDS-related Kaposi’s sarcoma. Several studies were reported on the effectiveness of Doxil®. For example, in a study (a phase III trial) it was compared the efficacy of PEGylated liposomal DOX with conventional DOX and it was observed that patients responded well to the both systems, nevertheless the cardiotoxicity, vomiting and other symptoms were decreased in the groups treated with Doxil®[63]. In another study, the patients (taxane-resistant breast cancer) were treated with PEGylated liposomal DOX and it was observed increased survival results compared to those treated with vinorelbine or mitomycin C plus vinblastine [64]. Also, Sriraman, et al. [65] reported that the in vitro studies in HeLa showed liposomes targeted to transferrin and folic acid had higher cell penetration and efficacy of delivering DOX compared to other systems [65].
The next generation of liposomal drugs refers to the immunoliposomes, which selectively deliver the drug to the desired sites of action. In a study was reported a mechanism of monoclonal antibody (MAb)-directed nanoparticle (immunoliposome) targeting to solid tumors in vivo. The immunoliposomes targeted to HER2 were prepared by the conjugation of anti-HER2 MAb to liposome-grafted PEG chains and it was observed by in vivo tests that this system strongly increased the uptake of the NPs in HER2-expressing breast tumors. Also, it was tested the efficiency of the system without the anti-HER2 antibody and it was observed that is reducing the availability to the tumors [66]. The current research is based on the development of safe liposomes that can be used as the potential contrasting agents for magnetic resonance imaging (MRI) [67].
Polymeric Nanogels: Nanogels are fabricated by cross-linking polymer chains to create an inner porous space that can accommodate a large amount of drug [68]. An ideal nanogel drug delivery carrier must present several characteristics such as biocompatibility, biodegradability, the dimension of the particles must be between 10 nm and 200 nm, prolonged blood circulation time, protection of molecules from the immune system of the body and higher amount of drug or enzyme loading.
Comparing the nature of the polymer source, natural materials are appropriate for pathogens but evoke inflammatory responses due to high positive charge on the particles surface, while synthetic materials offer well-defined morphologies that can be customized to gel networks with biocompatible and degradable properties [69]. The properties of nanogels can be achieved by chemical functional groups, altering the cross-linking density, stimuli-responsive constituents or surface-active [69-71].
For example, physical cross-linked nanogel formation occurs via non-covalent attractive forces like hydrophobic-hydrophobic, hydrophilic-hydrophilic, hydrogen bonding or ionic interactions. The stability of these systems depends on polymer composition, ionic strength of the medium, temperature, concentrations of the polymer and of the crosslinking agent. In the case of chemically cross-linked nanogels (constructed with several cross-linking points throughout a backbone of polymeric chains), the crosslinking (PEG, diallyl phthalate, divinylbenzene) have a vital role in tailoring the swelling or in the morphology/ pore size of the gel macromolecules [69]. Soni, et al. [72] reported that in the case of cancer treatment the PEGylated nanogels improves the circulation time and delivers their drug load into tumors following intravenous injection [72]. For example, Shimoda, et al. [73] developed Polysaccharide-PEG hybrid nanogels (CHPOA-PEGSH) crosslinked by both physical interactions and covalent ester bonds by the reaction of a thiol-modified poly (ethylene glycol) with acryloyl-modified cholesterol-bearing pullulan (CHPOA). The systems were injected intravenously in mice to study their blood clearance. It was observed a significantly longer circulation time (at 24 h approximately 20% to 30% of the nanoparticles remained in the blood) in the case of the CHPOA-PEGSH nanogels, while the control (nanogels formed by cholesteryl groups) was eliminated from the blood within 6 h [73]. Moreover, Nukolova, et al. [74] used diblock copolymer poly (ethylene oxide)-b-poly (methacrylic acid) (PEO-b-PMA) to form nanogels conjugated to folic acid and loaded with different types of drugs such as cisplatin or DOX for targeted therapy of ovarian cancer. It was reported a tumorspecific delivery and superior antitumor effect in vivo [74].
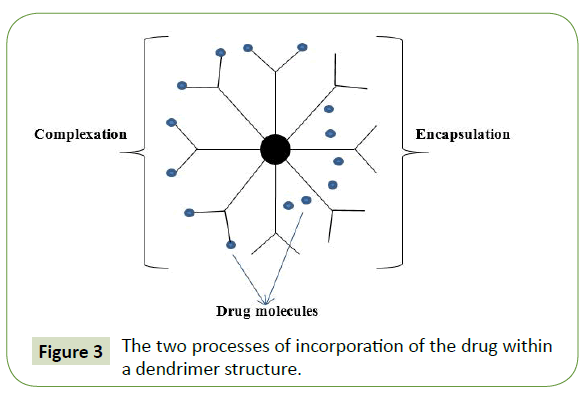
Dendrimers: Dendrimers are composed of multiple highly branched monomers that emerge radially from the central core and their size and shape can be precisely controlled. The main properties that make then suitable for drug delivery applications are a well-defined structure, stability, surface functionalization capability and monodispersity of size. Dendrimers are synthesized from either natural or synthetic elements like sugars, amino acids, and nucleotides. They can incorporate both hydrophobic and hydrophilic molecules via two processes: encapsulation (entrapment inside dendrimer core) or complexation (covalent attachment to end groups) (Figure 3). Dendrimer-based drug delivery systems have been developed to improve the biodistribution of the drugs in the body and to enable the controlled release of the drugs at its target zone [3-5,7,75,76].
Morgan, et al. studied the cytotoxicity of a dendrimer (composed of glycerol and succinic acid) encapsulated camptothecins (10-hydroxy-camptothecin and 7-butyl-10-aminocamptothecin) against four different human cancer cell lines: human breast adenocarcinoma (MCF-7), non-small cell lung carcinoma (NCI-H460), colorectal adenocarcinoma (HT-29) and glioblastoma (SF-268). It was reported that in the case of in MCF-7 breast carcinoma a decrease in the IC50 compared with free drug in DMSO for all of the cell lines, the concentrations were of 16-fold higher than the free drug after 2 h of treatment incubation and also the retention time of the drug was longer compared with the free drug in solution [77]. Papagiannaros, et al. [78] developed a complex formed from a PAMAM dendrimer (G4) loaded with DOX and incorporated into liposomes and this complex was tested against MCF-7 human breast carcinomas and DU145 human prostate carcinomas. It was reported that this complex presents the advantage of modulating the release and in vivo stability of DOX and also a slower release of the drug is beneficial in order to increase its therapeutic index and reduce its side effects on healthy cells [78].
Inorganic NPs
The majority of NPs can be divided in two categories: organic (NPs composed of organic materials) and inorganic (presents physicochemical properties that can be attributed to their inorganic components like metals). The subject “inorganic NPs” is relatively recent, because this category was developed at the end of the last century, so their biomedical applications are recent. The inorganic NPs are formed from two parts: core (contain metals (gold, quantum dots and iron oxide)) and shell (organic polymers or metals that protect the core from chemical interactions or serves as a substrate for conjugation with biomolecules) [79].
Gold NPs: Gold NPs have been used in the fabrication of cancertargeting multimodal drug delivery systems and in tumor imaging, because of their properties (electric and optical), the ease of synthesis, low toxicity and because of the fact that these NPs present negative reactive groups on the surface they can be easily modified [80,81]. These NPs consist of a core of gold atoms and they can be functionalized by the addition of a monolayer of moieties (ligands for active targeting of the gold NPs). It was reported that gold NPs are non-toxic at the cellular level for some of human cell lines and in several studies, it was reported that gold NPs are potentially biodegradable in vivo [1,82-84]. Also, ultra-small gold NPs (their diameter can be controlled by variation of different chemical and physical parameters) exhibits uniform distribution within the tumor tissues due to their ability to diffuse through tissues, but the uptake is poor [6,85].
For example, in a study were synthesized gold NPs with size between 5 nm and 35 nm and tested against human cervical carcinoma cells (HeLa). It was reported that the NPs exhibit an effective in vitro anticancer activity against HeLa cells by induction of DNA damage and cell cycle arrest at G2/M phase [86]. In another study, it was reported that gold NPs loaded with DOX exhibited stronger anticancer activity on HeLa compared to free DOX [87]. Also, these NPs have been used in a growing tumor in mice as drug delivery vectors of tumor necrosis factor and it was observed that this complex can be successfully delivered to destroy the tumor cells in animals [88,89]. It was reported that the future application of gold NPs will be as CT contrast agents and antibacterial agents because of their heir low-cytotoxicity and high CT attenuation efficacy [90].
Quantum dots NPs: QD are semiconductor NPs with unique optical properties, due to their quantum effect and size effect [5]. An ideal QD for drug delivery applications should present the following properties:
- high drug loading capacity
- no reaction with drugs
- good biocompatibility and low toxicity
- longer residence time in vivo
- suitable particle size and shape
- stability and certain mechanical strength [91].
Quantum dots are represented by inorganic NPs such as CdS, CdTe, ZnS and PbS, but the most commonly used QD system is the inner semiconductor core of CdSe coated with the outer shell of ZnS [92]. Due to their unique properties, such as resistance to photobleaching, intense and stable fluorescence for a longer time, highly sensitive detection, QDs are the new class of novel biosensors used for cancer diagnosis [93]. Voura, et al. [94] used QDs and emission spectrum scanning multiphoton microscopy to develop a means to follow tumor cell extravasation in a living animal and the cells labeled with QDs were intravenously injected into mice and followed as they extravasated into lung tissue. It was reported that the QDs and spectral imaging allowed the simultaneous identification of five different populations of cells using multiphoton laser excitation [94]. In another study, QDs were linked to streptavidin and immunoglobulin G to label the breast cancer marker Her2 on the surface of fixed and live cancer cells, to stain actin and microtubule fibers in the cytoplasm and to detect nuclear antigens inside the nucleus. It was reported that by using QDs with different emission spectra conjugated to streptavidin and IgG it was detected two cellular targets with one excitation wavelength [95].
Conclusions and Perspectives
Nanoparticles (organic or inorganic) are highly promising candidates for the development of drug delivery systems for cancer therapy and their success was already demonstrated in clinical applications. Nanoparticles-based drug delivery systems are superior to the conventional anticancer drugs because they can reduce the systemic side effects that patients must endure under traditional chemotherapy by ensuring that the cytotoxic levels of the drugs are only present at the tumor sites.
The future perspectives in cancer treatment refer to the obtaining of a multifunctional system able to apply for simultaneous treatment of cancers and in vivo imaging (CT or MRI contrast agents). These nanoparticles would be able to carry: one or more drugs, a specific targeting moiety, a cell-penetrating agent, a stabilizing polymer for biocompatibility, a stimulus-sensitive element for controlled release of drugs and an imaging agent. At the moment, several types of nanoparticles including polymeric nanoparticles [96], polymeric micelles [97], dendrimers [98] or quantum dots [99] have been evaluated for their suitability as multifunctional nanoparticles and the researchers are trying to find the best administration route (intra-venous, intra-tumoral and intra-peritoneal) for these systems.
References
- Sun T, Zhang YS, Pang B, Hyun DC, Yang M, et al. (2014) Engineered nanoparticles for drug delivery in cancer therapy. Chem Int Ed 53: 12320-12364.
- Khan DR (2011) The use of nanocarriers for drug delivery in cancer therapy. J Cancer Sci Töherapy 2: 058-062.
- Patel DJ, Mistri PA, Prajapati JJ (2012) Treatment of cancer by using nanoparticles as a drug delivery. Int J Drug Develop Res 4: 14-27.
- Sutradhar KB, Amin ML (2014) Nanotechnology in cancer drug delivery and selective targeting. ISRN Nanotechnology 2014: 1-13.
- Srinivasan M, Rajabi M, Mousa SA (2015) Multifunctional nanomaterials and their applications in drug delivery and cancer therapy. Nanomaterials 5: 1690-1703.
- Rahul J (2015) Importance of nanoparticles in targeted drug delivery system for treatment of cancer: A brief review. J Pharma Nanotechnol 5.
- Cho K, Wang X, Nie S, Shin DM (2008) Therapeutic nanoparticles for drug delivery in cancer. Clin cancer res 14: 1310-1316.
- Haley B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol 26: 57-64.
- Sahoo SK, Ma W, Labhasetwar V (2004) Efficacy of transferrinâ€ÂÂconjugated paclitaxelâ€ÂÂloaded nanoparticles in a murine model of prostate cancer. Int J cancer 112: 335-340.
- Liang HF, Chen CT, Chen SC, Kulkarni AR, Chiu YL, et al. (2006) Paclitaxel-loaded poly (γ-glutamic acid)-poly (lactide) nanoparticles as a targeted drug delivery system for the treatment of liver cancer. Biomaterials 27: 2051-2059.
- Zhang C, Wang W, Liu T, Wu Y, Guo H, et al. (2012) Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials 33: 2187-96.
- Qi WW, Yu HY, Guo H, Lou J, Wang ZM, et al. (2015) Doxorubicin-loaded glycyrrhetinic acid modified recombinant human serum albumin nanoparticles for targeting liver tumor chemotherapy. Mol pharm 12: 675-683.
- Alibolandi M, Sadeghi F, Abnous K, Atyabi F, Ramezani M, et al. (2015) The chemotherapeutic potential of doxorubicin-loaded PEG-b-PLGA nanopolymersomes in mouse breast cancer model. Eur J Pharm Biopharm 94: 521-531.
- Guo Y, Wang L, Lv P, Zhang P (2015) Transferrin-conjugated doxorubicin-loaded lipid-coated nanoparticles for the targeting and therapy of lung cancer. Oncol Lett 9: 1065-1072.
- Zhao G, Rodriguez BL (2013) Molecular targeting of liposomal nanoparticles to tumor microenvironment. Int J nanomed 8: 61.
- Gutteridge WE (1985) Existing chemotherapy and its limitations. Br Med Bull 41: 162-168.
- Mousa SA, Bharali DJ (2011) Nanotechnology-based detection and targeted therapy in cancer: Nano-bio paradigms and applications. Cancers 3: 2888-2903.
- Krishna R, Mayer LD (2000) Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 11: 265-283.
- Brown R, Links M (1999) Clinical relevance of the molecular mechanisms of resistance to anti-cancer drugs. Expert Rev Mol Med 1: 1-21.
- Davis ME, Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat rev Drug Discov 7: 771-782.
- Brigger I, Dubernet C, Couvreur P (2002) Nanoparticles in cancer therapy and diagnosis. Adv drug deliv rev 54: 631-651.
- Krishnan SR, George SK (2014) Nanotherapeutics in cancer prevention, diagnosis, and treatment. Pharmacology and Therapeutics. In: Thatha SJ (Ed). Gowder, ISBN, pp. 978-953.
- Ahn S, Seo E, Kim K, Lee SJ (2013) Controlled cellular uptake and drug efficacy of nanotherapeutics. Sci Rep 3: 1997.
- Lytton-Jean AK, Kauffman KJ, Kaczmarek JC, Langer R (2015) Cancer nanotherapeutics in clinical trials. Cancer Treat Res 166: 293-322.
- Bhattacharyya S, Kudgus RA, Bhattacharya R, Mukherjee P (2011) Inorganic nanoparticles in cancer therapy. Pharm res 28: 237-259.
- Kato K, Chin K, Yoshikawa T, Yamaguchi K, Tsuji Y, et al. (2012) Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer. Inves new drugs 30: 1621-1627.
- Libutti SK, Paciotti GF, Byrnes AA, Alexander HR, Gannon WE, et al. (2010) Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res 16: 6139-6149.
- Egusquiaguirre SP, Igartua M, Hernández RM, Pedraz JL (2012) Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin Transl Oncol 14: 83-93.
- Jung KH, Kim KP, Yoon DH, Hong YS, Choi CM, et al. (2012) A phase I trial to determine the maximum tolerated dose and evaluate the safety and pharmacokinetics (PK) of docetaxel-PNP, polymeric nanoparticle formulation of docetaxel, in subjects with advanced solid malignancies. ASCO Annual Meeting Proceedings.
- Friedman R (2011) Nano dot technology enters clinical trials. J Natl Cancer Inst Monogr 103: 1428-1429.
- Bakhtiary Z, Saei AA, Hajipour MJ, Raoufi M, Vermesh O, et al. (2016) Targeted superparamagnetic iron oxide nanoparticles for early detection of cancer: Possibilities and challenges. Nanomedicine 12: 287-307.
- Masood F (2016) Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater Sci Eng C 60: 569-578.
- Parveen S, Sahoo SK (2008) Polymeric nanoparticles for cancer therapy. J drug target 16: 108-123.
- Lee SJ, Min HS, Ku SH, Son S, Kwon IC, et al. (2014) Tumor-targeting glycol chitosan nanoparticles as a platform delivery carrier in cancer diagnosis and therapy. Nanomedicine 9: 1697-1713.
- Kwon IC (2008) Chitosan-based nanoparticles for cancer therapy; tumor specificity and enhanced therapeutic efficacy in tumor-bearing mice. J Control Release 132: e69-e70.
- Arya G, Vandana M, Acharya S, Sahoo SK (2011) Enhanced antiproliferative activity of Herceptin (HER2)-conjugated gemcitabine-loaded chitosan nanoparticle in pancreatic cancer therapy. Nanomedicine 7: 859-870.
- Ekinci M, Ilem-Ozdemir D, Gundogdu E, Asikoglu M (2015) Methotrexate loaded chitosan nanoparticles: Preparation, radiolabeling and in vitro evaluation for breast cancer diagnosis. J Drug Deliv Sci Technol 30: 107-113.
- M Ferreira D, Y Saga Y, Aluicio-Sarduy E, Tedesco A (2013) Chitosan nanoparticles for melanoma cancer treatment by photodynamic therapy and electrochemotherapy using aminolevulinic acid derivatives. Cur med chem 20: 1904-1911.
- Sadreddini S, Reza S, Behzad B, Leili AM, Mohammad AHF, et al. (2017) Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol Lett 181: 79-86.
- Makadia HK, Siegel SJ (2011) Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3: 1377-1397.
- Alimohammadi YH, Joo SW (2014) PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev 15: 517-535.
- Aravind A, Varghese SH, Veeranarayanan S, Mathew A, Nagaoka Y, et al. (2012) Aptamer-labeled PLGA nanoparticles for targeting cancer cells. Cancer nanotechnol 3: 1-12.
- Guo J, Wu SH, Ren WG, Wang XL, Yang AQ (2015) Anticancer activity of bicalutamide-loaded PLGA nanoparticles in prostate cancers. Exp Ther Med 10: 2305-2310.
- Gryparis EC, Hatziapostolou M, Papadimitriou E, Avgoustakis K (2007) Anticancer activity of cisplatin-loaded PLGA-mPEG nanoparticles on LNCaP prostate cancer cells. Eur J Pharm Biopharm 67: 1-8.
- Yang N, Jiang Y, Zhang H, Sun B, Hou C, et al. (2014) Active targeting docetaxel-PLA nanoparticles eradicate circulating lung cancer stem-like cells and inhibit liver metastasis. Mol pharm 12: 232-239.
- Jain DS, Athawale RB, Bajaj AN, Shrikhande SS, Goel PN, et al. (2013) Poly lactic acid (PLA) nanoparticles sustain the cytotoxic action of temozolomide in C6 Glioma cells. Biomed Aging Pathol 3: 201-208.
- Fasehee H, Zarrinrad G, Tavangar SM, Ghaffari SH, Faghihi S (2016) The inhibitory effect of disulfiram encapsulated PLGA NPs on tumor growth: Different administration routes. Mater Sci Eng: C 63: 587-595.
- Cornier J, Owen A, Kwade A, Van de Voorde M (2016) Pharmaceutical Nanotechnology: Innovation and Production, 2 Volumes: John Wiley & Sons.
- Felber AE, Dufresne MH, Leroux JC (2012) pH-sensitive vesicles, polymeric micelles, and nanospheres prepared with polycarboxylates. Adv Drug Deliv Rev 64: 979-992.
- Adams ML, Lavasanifar A, Kwon GS (2003) Amphiphilic block copolymers for drug delivery. J pharm sci 92: 1343-1355.
- Raavé R, de Vries RB, Massuger LF, van Kuppevelt TH, Daamen WF (2015) Drug delivery systems for ovarian cancer treatment: A systematic review and meta-analysis of animal studies. Peer J 3: e1489.
- Yokoyama M, Miyauchi M, Yamada N, Okano T, Sakurai Y, et al. (1990) Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly (ethylene glycol)-poly (aspartic acid) block copolymer. Cancer Res 50: 1693-1700.
- Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, et al. (2001) Development of the polymer micelle carrier system for doxorubicin. J Control Release 74: 295-302.
- Yoo HS, Park TG (2004) Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release 96: 273-283.
- Plummer R, Wilson R, Calvert H, Boddy A, Griffin M, et al. (2011) A Phase I clinical study of cisplatin-incorporated polymeric micelles (NC-6004) in patients with solid tumours. Br J cancer 104: 593-598.
- Anajwala CC, Jani GK, Swamy SV (2010) Current trends of nanotechnology for cancer therapy. Int J Pharm Sci Nanotechnol 3: 1043-1056.
- Markman, M, Pegylated (2006) liposomal doxorubicin in the treatment of cancers of the breast and ovary. Expert Opin Pharmacother 7: 1469-1474.
- Hofheinz RD, Gnad-Vogt SU, Beyer U, Hochhaus A (2005) Liposomal encapsulated anti-cancer drugs. Anti-cancer drugs 16: 691-707.
- Malam Y, Loizidou M, Seifalian AM (2009) Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends Pharmacol Sci 30: 592-599.
- Movahedi F, Hu RG, Becker DL, Xu C (2015) Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomedicine 11: 1575-1584.
- Saengkrit N, Saesoo S, Srinuanchai W, Phunpee S, Ruktanonchai UR (2014) Influence of curcumin-loaded cationic liposome on anticancer activity for cervical cancer therapy. Colloids Surfaces B 114: 349-356.
- Lin YY, Kao HW, Li JJ, Hwang JJ, Tseng YL, et al. (2013) Tumor burden talks in cancer treatment with PEGylated liposomal drugs. PloS one 8: e63078.
- O’brien M, Wigler N, Inbar M, Rosso R, Grischke E, et al. (2004) Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann oncol 15: 440-449.
- Keller AM, Mennel RG, Georgoulias VA, Nabholtz JM, Erazo A, et al. (2004) Randomized phase III trial of pegylated liposomal doxorubicin versus vinorelbine or mitomycin C plus vinblastine in women with taxane-refractory advanced breast cancer. J Clin Oncol 22: 3893-3901.
- Sriraman SK, Salzano G, Sarisozen C, Torchilin V (2016) Anti-Cancer Activity of Doxorubicin-Loaded Liposomes Co-Modified with Transferrin and Folic Acid. Eur J Pharm Biopharma 105: 40-49.
- Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, et al. (2006) Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer res 66: 6732-6740.
- German S, Navolokin N, Kuznetsova N, Zuev V, Inozemtseva O, et al. (2015) Liposomes loaded with hydrophilic magnetite nanoparticles: preparation and application as contrast agents for magnetic resonance imaging. Colloids Surfaces B 135: 109-115.
- Chacko RT, Ventura J, Zhuang J, Thayumanavan S (2012) Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev 64: 836-851.
- Yallapu MM, Jaggi M, Chauhan SC (2011) Design and engineering of nanogels for cancer treatment. Drug Discov Today 16: 457-463.
- Wang Y, Xu H, Ma L (2015) Recent advances of thermally responsive nanogels for cancer therapy. Ther deliv 6: 1157-1169.
- Sivaram AJ, Rajitha P, Maya S, Jayakumar R, Sabitha M (2015) Nanogels for delivery, imaging and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol 7: 509-533.
- Soni G, Yadav KS (2016) Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. saudi Pharm J 24: 133-139.
- Shimoda A, Sawada S, Kano A, Maruyama A, Moquin A, et al. (2012) Dual crosslinked hydrogel nanoparticles by nanogel bottom-up method for sustained-release delivery. Colloids Surfaces B 99: 38-44.
- Nukolova NV, Oberoi HS, Cohen SM, Kabanov AV, Bronich TK (2011) Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 32: 5417-5426.
- Cheng Y, Zhao L, Li Y, Xu T (2011) Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives. Chem Soci Rev 40: 2673-2703.
- Somani S, Dufès C (2014) Applications of dendrimers for brain delivery and cancer therapy. Nanomedicine 9: 2403-2414.
- Morgan MT, Nakanishi Y, Kroll DJ, Griset AP, Carnahan MA, et al. (2006) Dendrimer-encapsulated camptothecins: increased solubility, cellular uptake, and cellular retention affords enhanced anticancer activity in vitro. Cancer res 66: 11913-11921.
- Papagiannaros A, Dimas K, Papaioannou GT, Demetzos C (2005) Doxorubicin-PAMAM dendrimer complex attached to liposomes: Cytotoxic studies against human cancer cell lines. Int J Pharm 302: 29-38.
- Yezhelyev M, Yacoub R, O'Regan R (2009) Inorganic nanoparticles for predictive oncology of breast cancer. Nanomedicine 4: 83-103.
- Kim D, Jon S (2012) Gold nanoparticles in image-guided cancer therapy. Inorg Chim Acta 393: 154-164.
- Patra CR, Bhattacharya R, Mukhopadhyay D, Mukherjee P (2012) Fabrication of gold nanoparticles for targeted therapy in pancreatic cancer. Adv Drug Deliv Rev 62:346-361.
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD (2005) Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1: 325-327.
- Abadeer NS, Murphy CJ (2016) Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J Phys Chem C 120: 4691-4716.
- Bhowmik T, Saha PP, Sarkar A, Gomes A (2017) Evaluation of cytotoxicity of a purified venom protein from Naja kaouthia (NKCT1) using gold nanoparticles for targeted delivery to cancer cell. Chem Biol Int 261: 35-49.
- Pang B, Yang X, Xia Y (2016) Putting gold nanocages to work for optical imaging, controlled release, and cancer theranostics. Nanomedicine 11: 1715-1728.
- Jeyaraj M, Arun R, Sathishkumar G, MubarakAli D, Rajesh M, et al. (2014) An evidence on G2/M arrest, DNA damage and caspase mediated apoptotic effect of biosynthesized gold nanoparticles on human cervical carcinoma cells (HeLa). Mater Res Bull 52: 15-24.
- Tomoaia G, Horovitz O, Mocanu A, Nita A, Avram A, et al. (2015) Effects of doxorubicin mediated by gold nanoparticles and resveratrol in two human cervical tumor cell lines. Colloids surfaces B 135: 726-734.
- Miller G (2003) Colloid gold nanoparticles deliver cancer-fighting drugs. Pharm Technol 7: 17-19.
- Mahmood M, Karmakar A, Fejleh A, Mocan T, Iancu C, et al. (2009) Synergistic enhancement of cancer therapy using a combination of carbon nanotubes and anti-tumor drug. Nanomedicine 4: 883-893.
- Huo D, He J, Li H, Yu H, Shi T, et al. (2014) Fabrication of Au@Ag core-shell NPs as enhanced CT contrast agents with broad antibacterial properties. Colloids Surfaces B 117: 29-35.
- Zhao MX, Zhu BJ (2016) The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy. Nanoscale Res Lett 11: 1.
- Vashist SK, Tewari R, Bajpai RP, Bharadwaj LM, Raiteri R (2006) Review of quantum dot technologies for cancer detection and treatment. AZojono J Nanotechnol Online 2: 1-14.
- Malik P, Gulia S, Kakkar R (2013) Quantum dots for diagnosis of cancers. Adv Mat Lett 4: 811-822.
- Voura EB, Jaiswal JK, Mattoussi H, Simon SM (2004) Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med 10: 993-998.
- Wu X, Liu H, Liu J, Haley KN, Treadway JA, et al. (2003) Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat biotechnol 21: 41-46.
- Cho YW, Park SA, Han TH, Park JS, Oh SJ, et al. (2007) In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: mechanisms, key factors, and their implications. Biomaterials 28: 1236-1247.
- Nasongkla N, Bey E, Ren J, Ai H, Khemtong C, et al. (2006) Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano lett 6: 2427-2430.
- Kukowska-Latallo JF, Candido KA, Cao Z, Nigavekar SS, Majoros IJ, et al. (2005) Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer res 65: 5317-5324.
- Gao X, Cui Y, Levenson RM, Chung LW, Nie S (2004) In vivo cancer targeting and imaging with semiconductor quantum dots. Nat biotechnol 22: 969-976.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences