ISSN : 0976 - 8688
Der Pharmacia Sinica
One Pot Three Component Synthesis of 2,4,5-triaryl-1H-imidazole Using PEG-400 and their Antibacterial Screening
Chandrashekhar G Devkate1*, Khandu D Warad2, Mahendra B Bhalerao2, Digambar D Gaikwad3 and Mohammad Idrees M Siddique4
1Department of Chemistry, Indraraj Arts, Commerce and Science College Sillod, Aurangabad-431112, India
2Department of Chemistry, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad-431004, India
3Department of Chemistry, Govt. College of Arts and Science, Aurangabad-431001, India
4Department of Chemistry, Government of Institute Science, Nagpur-440008, India
Abstract
2,4,5-triarylimidazoles could be obtained in excellent yields by one-pot three component condensation of benzil, aromatic aldehydes and ammonium acetate in presence of polyethylene glycol PEG-400 as medium and catalyst. The synthesis highlights a comparative study of conventional and ultrasound methods. The compound 4c was investigated in vitro against Gram +ve and Gram –ve bacteria at different concentrations and compared with standard drug ciprofloxacin.
Keywords
2,4,5-triarylimidazoles, PEG-400, Conventional, Ultrasound
Introduction
Imidazole derivatives are a very interesting class of heterocyclic compounds because they are found in many natural products and pharmacologically active compounds, such as antiulcerative agent cimetidine [1], the proton pump inhibitor omeprazole [2], and the benzodiazepine antagonist flumazenil [3], are imidazole derivatives. Many of the substituted imidazoles are known as inhibitors of p38 MAP kinase, fungicides, herbicides, plant growth regulators, and therapeutic agents [4-7]. As a new approach to ‘Green Chemistry’ the substituted imidazole ring systems are substantially used in ionic liquids [8].
Triarylimidazole compounds have gained remarkable importance due to their widespread biological activities and their use in synthetic chemistry. Due to their great importance, many synthetic strategies have been developed. In 1882, Radziszewski and Japp reported the first synthesis of the imidazole from 1,2-dicarbonyl compound, various aldehydes and ammonia, to obtain the 2,4,5-triphenyl imidazoles [9-11]. However, these methods require prolonged reaction time and exotic reaction condition. Thus, the development of a new method for the synthesis of 2,4,5-triarylimidazoles derivatives would be highly desirable.
The use of volatile, toxic, and hazardous organic solvents has been replaced by different alternatives. Where Polyethylene Glycols (PEGs) play an important role in organic synthesis it has become a popular reaction media since last decade. PEGs are known as nontoxic, inexpensive, nonflammable, and nonionic liquid reaction media of low volatility [12]. This kind of solvent system fully meets the demands of green chemistry [13] and is found to be useful for various organic transformations [14]. PEG-400 has been found as an accelerator in various synthetic reactions [15,16].
Materials and Methods
Experimental section
General consideration
All reagents and solvents were of LR grade purchased from commercial sources were used as received without further purification. All reactions were carried out in oven-dried glassware and were magnetically stirred. Melting points are determined following open capillary method and are uncorrected. FTIR spectra were taken on F.T. Infra- Red Spectrophotometer Model RZX (Perkin Elmer) and 1H and 13C spectra were taken on bruker AVANCE II 400 MHz spectrometer with TMS as internal standard CDCl3/DMSO as solvent. ESI-Mass spectral data were recorded on Q-TOF Micro Waters (ESI-MS) Spectrometer.
General procedure for the screening of solvents
To achieve suitable conditions for the synthesis of 2,4,5-trisubstituted imidazoles, various reaction conditions have been investigated in the reaction of benzil 1, benzaldehyde 2c and ammonium acetate 3 as a model reaction (Scheme 1). We examined the effect of different solvents such as EtOH, MeOH, THF, CH3CN, H2O, H2O-EtOH, PEG-400 on a model reaction and the reaction carried out using conventional and under ultrasound irradiation (power intensity: 40%) at 50°C. and it was observed that under ultrasound irradiation all the solvents used gives good yield but the use PEG-400 afforded 95% yield (Table 1, entry 7) of the desired product. The results were summarized in Table 2. Therefore PEG- 400 was chosen as solvent for the reaction.
| Entry | Solvent | Conventional Methoda | Ultrasound Methodb | ||
|---|---|---|---|---|---|
| Time (min) | Yieldc (%) | Time (min) | Yieldc (%) | ||
| 1 | Methanol | 73 | 50 | 53 | 63 |
| 2 | Ethanol | 70 | 43 | 50 | 55 |
| 3 | Water | 70 | 40 | 52 | 50 |
| 4 | CH3CN | 70 | 30 | 50 | 38 |
| 5 | THF | 75 | Trace | 50 | 30 |
| 6 | H2O-EtOH | 72 | 42 | 57 | 54 |
| 7 | PEG-400 | 60 | 85 | 15 | 95 |
| aReaction condition: benzil (1) (1.0 mmol) ,benzaldehyde (2) (1.0 mmol), ammonium acetate (3.0 mmol) and PEG-400 (5 mL) under conventional heating. bReaction condition: benzil (1) (1.0 mmol) ,benzaldehyde (2) (1.0 mmol), ammonium acetate (3.0 mmol) and PEG-400 (5 mL) under ultrasound irradiation. cIsolated yields. |
|||||
Table 1: Screening of solvents for the synthesis of 2,4,5-triphenyl-1H-imidazole (4c)
| Cycle | Yielda |
|---|---|
| Fresh | 95 |
| Ist | 90 |
| IInd | 84 |
| IIIrd | 78 |
Table 2: Recyclability and reusability of PEG-400
General procedure for the synthesis of 2,4,5-triarylimidazole
Method A: By conventional method
A mixture of benzil (1) (1.0 mmol), aromatic aldehyde (2) (1 mmol) ammonium acetate (3 mmol) (3) to that PEG-400 (5 ml) was added as medium and as catalyst to it and the reaction mixture was reflux at 80°C for the respective time given in Table 3. Progress of the reaction was monitored with the help of TLC. After completion of the reaction, the reaction mixture was cooled and then extracted by diethyl ether. With the help of vacuum distillation the ether was removed and the obtained compound was crystallized using (1:1) DMF-Ethanol. And the ether layer containing PEG- 400 was washed two to three times with diethyl ether (20 mL) and was separated by separating funnel and the obtained PEG-400 was reused for reaction. The products obtained are reported compounds and they were characterized by comparing with their spectral data (IR, mass, 1H-NMR and 13C-NMR) and physical properties with those reported in literature.
| Comp. | Aldehyde R |
Product | m.p °C |
Conventional Method |
Ultrasound Method |
||
|---|---|---|---|---|---|---|---|
| Time (min) |
Yield (%) |
Time (min) |
Yield (%) |
||||
| 4a | 4-OMe | 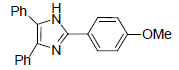 |
228-230 | 55 | 74 | 15 | 95 |
| 4b | 4-Me | 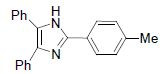 |
230-232 | 57 | 72 | 13 | 93 |
| 4c | H | 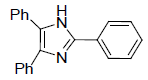 |
276-278 | 60 | 70 | 15 | 95 |
| 4d | 4-F | 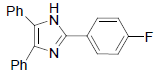 |
188-190 | 60 | 74 | 10 | 92 |
| 4e | 4-NO2 | 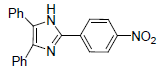 |
232-233 | 80 | 67 | 8 | 87 |
| 4f | 4-OH | 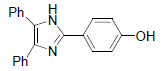 |
269-270 | 53 | 75 | 15 | 94 |
| 4g | 4-Cl | 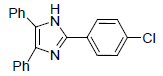 |
260-262 | 58 | 73 | 12 | 92 |
| 4h | 2-Cl | 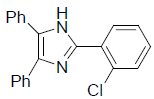 |
195-197 | 64 | 70 | 10 | 88 |
Table 3: One pot synthesis of 2,4,5-triarylimidazoles (4a-h) using PEG-400
Method B: By ultrasound method
A mixture of benzil (1) (1.0 mmol), aromatic aldehyde (2) (1 mmol) ammonium acetate (3) (3 mmol) to that PEG- 400 (5 ml) was added as medium and as catalyst to it and the reaction mixture containing RBF was placed into the ultrasonic water bath, in such a way that the surface of the reactants is slightly lower as compared to the level of the water, and irradiated at 40% of the power of the ultrasonic bath at 50°C for 10-15 min. Progress of the reaction was monitored with the help of TLC. After completion of the reaction the process of recyclation of PEG-400, crystallization and characterization was done as given in (Method A).
Spectral data for representative 2,4,5-triarylimidazoles
Compound 4c: Yield 95%; white solid; mp 276 – 278°C. FTIR Model RZX (Perkin Elmer) cm-1: 3040, 1487, 1462, 1127, 697. 1H-NMR (400 MHz, DMSO-d6, d ppm): 12.60 (brs, 1H, NH), 8.11 (d, 2H, J=7.6 Hz, ArH), 7.28 –7.56 (m,15H, ArH); 13C-NMR (400 MHz, DMSO-d6, d ppm): 125.1,126.3,127.08,128.42,129.5,130.39,131.16,135.1,137. 1,154.5 MS (EI): m/z (%) =297 [M+].
Antibacterial activity
The synthesized compound 4c was screened for in vitro antimicrobial activity against two gram positive bacterial strains, S. aureus and B. subtilis and two gram negative strains, E. coli and P. aeruginosa [17,18]. Ciprofloxacin was used as standard drug. Here agar disc-diffusion method was used.
General procedure: Determination of zone of inhibition by agar disc-diffusion method
Test solutions of concentration of 125-1000 μg/mL were prepared with known weight of compound in DMSO and half diluted suitably to give the resultant. Solution and Whatmann no. 1 sterile filter paper discs (6 mm) were impregnated and allowed to dry at room temperature. By using Mueller Hinton Agar obtained from Himedia Ltd., Mumbai the in vitro antibacterial activity was determined. And then the petri plates were prepared by pouring 20 mL of Mueller Hinton Agar for bacteria containing microbial culture then it was allowed to solidify. 24 h old cultures were used to get 108 suspensions. This suspension of 4 pathogenic cultures was spread on Muller Hinton Agar plates by sterile cotton swabs. The impregnated discs were then kept on these plates and were incubated at 37°C for 24 h (bacteria) and after incubation zone of inhibition was measured in mm as diameter in four directions and expressed as mean. The results were compared against Ciprofloxacin as a standard drug and are reported in Table 4.
| S. No. | Conc. µg/mL |
Zone of inhibition in (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gram +ve Gram-ve | |||||||||
| 4c | |||||||||
| Pathogen–Staphylococcus aureus | Pathogen–Bacillus subtilis | Pathogen–Escherichia coli | Pathogen–Pseudomonas aeruginosa | ||||||
| Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | ||
| 1 | 125 | 12 | 11 | - | - | 8 | 7 | - | - |
| 2 | 250 | 15 | 16 | - | - | 10 | 8 | - | - |
| 3 | 500 | 17 | 19 | - | - | 14 | 11 | - | - |
| 4 | 1000 | 22 | 20 | - | - | 13 | 17 | - | - |
| Standard Ciprofloxacin | |||||||||
| 1 | 125 | 31 | 31 | 27 | 27 | 26 | 26 | 27 | 27 |
| 2 | 250 | 35 | 36 | 29 | 29 | 28 | 28 | 32 | 32 |
| 3 | 500 | 40 | 41 | 30 | 31 | 29 | 31 | 36 | 34 |
| 4 | 1000 | 44 | 45 | 32 | 33 | 30 | 33 | 38 | 39 |
Table 4: Antibacterial activity of 4c
After the antibacterial assessment the obtained data was shown in Table 4, which indicates that the compound 4c activity against Gram positive bacteria is moderate activity against S. aureus no activity against B. subtilis. In case of gram negative bacteria, 4c showed moderate activity against E. coli and it is inactive against P. aeruginosa at all 4 concentrations. Thus on the basis of data it is clear that imidazole based derivatives possesses moderate antibacterial activity.
Results and Discussion
One pot cyclocondensation of Bezil (1), with aromatic aldehydes (2a -h) and ammonium acetate (3) in presence of PEG-400 as a medium and as a catalyst it was carried out under conventional and also ultrasound irradiation which result into the subsequent 2,4,5-triaryl-1H-imidazoles (4a-h) (Table 3).
We have screened different solvents and studied their effect for the synthesis of 4c as a model reaction by reacting benzyl 1c, benzaldehyde 2c, and ammonium acetate 3 using different solvents. We observed that PEG-400 (Table 1, entry 7) have affords the product in good yields as compared to other solvents. The reason is that the intermolecular hydrogen bonding between aldehyde carbonyl with terminal hydroxyl group of PEG-400 which enhancing the electrophilic behavior of carbonyl carbon and give good yield in shorter time. And the PEG-400 is also recycled and reused in the synthesis, the PEG-400 was recycled three times each time there is a loss of 5 and 6% as shown in Table 2. Each time we got good yield and the reaction proceeded cleanly.
Further synthesis of different derivatives of 2,4,5-triaryl-1H-imidazoles was performed by using PEG-400 under conventional and also repeated in ultrasound irradiation here it was noticed that using ultrasound irradiation give good yield in shorter time as compared to conventional method and the obtained result are shown in Table 3.
The synthesized compound 4c was screened for in vitro antimicrobial activity against two gram positive bacterial strains, S. aureus and B. subtilis and two gram negative strains, E. coli and P. aeruginosa. Ciprofloxacin was used as standard drug. Here agar disc-diffusion method was used and the result was shown in Table 4.
Conclusion
In conclusion, we report PEG-400 as medium and catalyst for the synthesis 2,4,5-triarylimidazoles which is eco-friendly and a comparative study of conventional and ultrasound method where we found that using ultrasound method product obtained in good yield in shorter reaction time and it is also a very clean method. Antibacterial screening of 4c compound was found to possess moderate activity against selected strains of bacteria also found inactive for B. subtilis and P. aeruginosa and rest of synthesized will be assessed in future study.
References
- Chung, K.H., et al., Bioorganic & Medicinal Chemistry, 2006. 14(1): p. 5795.
- Tanigawara, Y., et al., Clinical Pharmacology & Therapeutics, 1999. 66(2): p. 528.
- Hunkeler, W., et al., Nature, 1981. 290(1): p. 514.
- Lee, J.C., et al., Nature, 1994. 372(5): p. 739.
- Kirti, S.N., Journal of Heterocyclic Chemistry, 2011. 48(2): p. 742.
- Schmierer, R., Mildenberger, H., Buerstell, H., Chemical Abstracts, 1988. 108(7): p. 37838.
- Heeres, J., et al., Journal of Medicinal Chemistry, 1979. 22(1): p. 1003.
- Shapi, A.S., et al., Tetrahedron, 2005. 61(5): p. 3539-3546.
- Shelke, K.F, et al., Chinese Chemical Letters, 2009. 20(2): p. 283-287.
- Javad, S., Zohre, Z., Ultrasonics Sonochemistry, 2013. 20(6): p. 740-746.
- Deepak, N., et al., Ultrasonics Sonochemistry, 2012. 19(1): p. 94-96.
- Harris, J.M., Plenum Press, NY, USA, 1992.
- Anastas, PT., Warner, J.C., Green Chemistry: Oxford Science Publications: NY, USA, 1998.
- Ballini, R., Luciano, B., Alessandro, P., Green Chemistry Letters and Reviews, 2008. 10(1): p. 1004-1006.
- Kidwai, M., Bhatnagar, D., Mishra, N.K., Green Chemistry Letters and Reviews, 2010. 3(1): p. 55-59.
- Dinesh, L.L., et al., Chemistry & Biology Interface, 2014. 4(5): p. 287-291.
- Fish, DN., American Journal of Health-System Pharmacy, 2002. 59(9): p. 13.
- Belland, R.J., Microbial Cell, 2004. 6(1): p. 117.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences