Occurrence and Distribution of Sulfonamides, Tetracyclines and Quinolones in Livestock Manure in Morogoro Municipality, Tanzania
Mohameda HSA1*, Anders D2, Uswege M3 and Robinson HM4
1Unit Department of Veterinary Physiology, Biochemistry and Pharmacology, Sokoine University of Agriculture, Tanzania
2Department of Veterinary Disease Biology, University of Copenhagen, Denmark
3Tumaini University Dar-es-Salaam College, TUDARCo, Tanzania
4Department of Medicine and Public Health, Sokoine University of Agriculture of Virology, Tanzania
- *Corresponding Author:
- Mohameda HSA
Unit Department of Veterinary Physiology
Biochemistry and Pharmacology
Sokoine University of Agriculture, Tanzania
Tel: +255766661919
E-mail: ally2005mood@yahoo.com; mohamed.ally@suanet.ac.tz
Received Date: Novemeber 23, 2017; Accepted Date: Novemeber 28, 2017; Published Date: Novemeber 30, 2017
Citation: Mohameda HSA, Anders D, Uswege M, Robinson HM (2017) Occurrence and Distribution of Sulfonamides, Tetracyclines and Quinolones in Livestock Manure in Morogoro Municipality, Tanzania. J Zoonotic Dis Public Health 1:5.
Abstract
Antibiotics are deployed in large quantities both in human and veterinary medicine. They have played a major role in improving human health and supporting livestock production. However, the relevance of non-therapeutic and therapeutic applications needs a time-to-time re-evaluation due to its significant relationship to increased emergence of resistant pathogen strains both in humans and animals. This is due to widespread discharge into the aquatic environment from both domestic and agricultural sources of antibiotics which are not completely metabolized (original compounds) or metabolites of which are recycled through drinking water under low doses.
A study was conducted to analyze the presence of sulfonamide, ciprofloxacin and tetracycline in swine, cattle and poultry manure in Morogoro municipality. A total of sixty grab manure samples from poultry, swine and cattle were analyzed. Twenty samples for each type of livestock manure were collected from Morogoro municipality for analysis. The respective antibiotics were analyzed by ELISA. The highest mean concentrations in manure sample were 1320.9967 ± 710.06372 μg/ kg, 2083.2640 ± 826.64583 μg/kg and 1573.7528 ± 222.95739 μg/kg, Sulfonamide, Ciprofloxacin and Tetracycline respectively. In overall, higher concentrations of the antibiotics were detected in poultry and swine manure than in cattle. This indicates heavier usage of veterinary antibiotics in poultry and swine husbandry in the study area as compared to cattle. The findings of this study provide basic information on the risk of environmental antibiotic contamination from animal effluents.
Keywords
Antibiotics; Manure; ELISA; Morogoro; Tanzania
Introduction
The discovery of antibiotics came as a breakthrough in treating bacterial infections worldwide, especially in developing countries where hygiene is sometimes insufficient. Hence they contributed to a better life for general population. However the extended use has resulted in antibiotic pollution of environmental water such as rivers, surface water and ground water [1].
Antibiotics are used extensively for treatment of different infections in humans and animals. With the rapid development of animal husbandry, veterinary antibiotics comprise 70% of world’s antibiotic consumption for the purpose of illness prevention and growth promotion [1,2].
Heavy uses of veterinary antibiotics generate high levels of antibiotics residues in the manure. For instance, chlortetracycline was detected in swine manure with the highest concentrations of 754.4 mg/kg in Shandong Province and concentrations for quinolones such as norfloxacine was 225.45 mg/kg in eight provinces of China [3,4].
More studies have reported detection of antibiotics in manure in different parts of the world. Martinez-Carballo et al. [5] reported the concentrations of oxytetracyclines and tetracycline in 30 pig manure in Austria were up to 46 mg/kg and 23 mg/ kg respectively, whereas the concentrations as high as 225 mg/ kg and 1420 mg/kg of norfloxacin and enrofloxacin respectively were found in chicken manure from China [4].
Depending on type of antibiotics, 70%-90% of the administered antibiotic is excreted in faeces and urine unmetabolized [1]. Manure is used as fertilizers and if antibiotics are present, they can thereby reach the ground water or taken up by crops [6]. In 2008, a study found that local farmers use antibiotics as prophylaxis and treatment of common chicken diseases in Morogoro, Tanzania [7]. This study also found that 65% of the farmers administered the antibiotics without consulting a veterinarian and hundred percent of the eggs investigated contained antibiotic residues. Eggs containing antibiotic residues, sold at market are therefore a way of involuntary exposure of general population to antibiotics.
Most livestock manure is directly or indirectly applied to an agricultural land as fertilizers without treatment, especially in developing countries, which constituent the main source of the dissemination of these chemicals in soil and environments [8]. Hamster et al. [9] detected concentrations of tetracycline up to 300 μg/kg in agricultural field fertilized with liquid manure. Additionally Li et al. [10] found high antibiotic concentrations in vegetable farmlands affiliated with livestock farms and detected maximum concentrations of tatracyclines, sulfonamides and quinolones of 242.6, 321.4 and 1537.4 μg/kg respectively.
Also market demands and economic incentives have resulted in intensified greenhouse vegetable production (GVP), particularly in developing countries; this form of intensive agriculture has expanded worldwide rapidly. However, heavy fertilization strongly increases production and is always adopted under green vegetable production conditions to achieve high vegetable production. In additional, growers of organic vegetables primarily use manure in green vegetable production. Most green vegetables production involves large and repeated amounts of animal manure application and probably increases the risks of antibiotic and antibiotic resistant bacteria. Presently, no regulations address the presence of antibiotics in soil as well as in the manure applied to vegetable production systems. As an emerging class of environmental contaminants, antibiotics have attracted increasing attention due to their adverse effects on non-target organisms and ecological environments.
Current environmental legislations fails to cover these antibiotics release to the environment, hence antibiotics could lead to undesirable effects on ecosystem, as by Li et al. [11]. Furthermore, the high concentrations of antibiotics in manure can be associated with an increased presence of resistant bacteria in environment, which is a major public health concern, due to the increased occurrence of associated clinical infections (Ashbolt et al. and Luo et al.) [12,13].
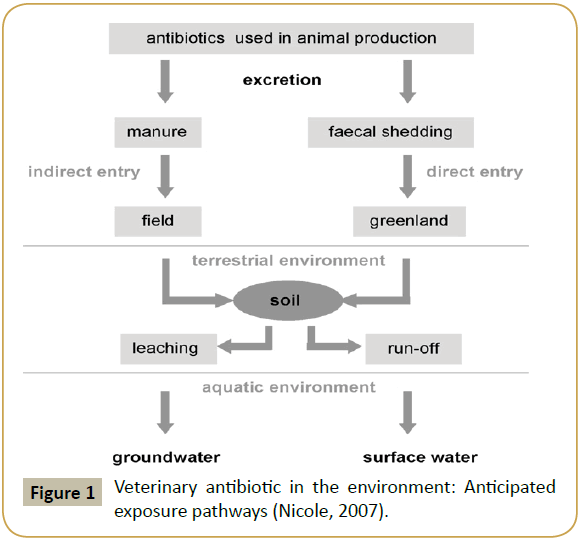
Antibiotics from animal production partly are excreted in urine and faeces as original compounds or metabolites. They can enter the aquatic environment through direct and indirect ways. In indirect way, antibiotics are excreted in manure through urine and faeces. Manure is applied to the field, enter terrestrial environment and through leaching, reaches aquatic environment, which is a ground water. In direct way, antibiotics are excreted in faecal shedding, this is applied to the Greenland in terrestrial and through run-off, antibiotics reach aquatic environment, which is surface water (Figure 1).
Materials and Methods
Study design
A cross-sectional study was conducted in Morogoro municipality, in which sixty grab manure samples were collected from randomly selected poultry, swine and cattle farms. Twenty samples from each type of livestock were collected for antibiotics residues analysis.
Study area and sample collection
Morogoro is a town with approximately 2, 218, 492 inhabitants according to 2012 census. Samples were collected in Morogoro Municipality from identified farms in duplicate. Fresh manure samples approximately 500 grams were collected by using sterile surgical gloves. For poultry only farms with three hundreds chicken were selected for sampling, for swine only farms with fifty grower pigs were selected and for cattle, farms with ten cattle were selected randomly. All farms were managed by intensive system. Within half an hour after sampling samples were taken to laboratory. Faecal samples were picked with a maximum care to avoid contamination. Only upper part of faecal which is not in contact with floor was taken. Samples were preserved in cool box; thermometer was included to monitor temperature and taken to laboratory. Samples were stored at-18°C until processing. Processing was done after two days.
Sample preparation
Solid phase extraction: One gram of manure sample was added to a 50 ml polytetraflouoroethylene (PTFE) centrifuge tube. 10 ml of Na2EDTA-Mc1lvaine buffer(21.00 g of citric acid monohydrate, 17.75 g of Na2HPO4 and 60.50 g of Na2EDTA2H2O in 1.625 l of distilled water, with the pH adjusted to 4.00 ± 0.05 using hydrochloric acid) (pH 4.0) was added. The mixture was vortexed at 2500 rpm for 30s, ultrasonicated for 10 minutes, then centrifuged at 4500 rpm for 15 min. The supernatants were decanted into 50 ml PTFE tubes. The extraction was repeated twice.
The supernatants were combined and then decreased by 10 ml n-hexane, centrifuged at 4500 rpm for ten minutes and then adjusted with deionized sterile water to 250 ml. The resulting solution was filtered twice, first by grade 5 Munktell filter paper with particle retention of 20 μm, then through a grade 120 H filter with particle retention of 1-2 μm and then adjusted to pH 4.0. This was followed by extraction with Oasis hydrophilic-lipophilic balance (Oasis®HLB) cartridges 6 ml/500 mg from Waters (Milford, MA, USA) using a vacuum manifold and pump.
The vacuum manifold was a Vac Master from IST (Sweden) and pump was from ScanVac (Denmark). The drop-rate was adjusted to 1.5 ml/min. Prior to loading cartridges were pre-conditioned with 2 ml MeOH, followed by 2 ml of water. The eluent was fully dried under gentle stream of nitrogen at 33°C. The evaporator was Dionex SE500 (CA, USA). Afterwards, the samples were reconstituted to 100 μl (0.01% formic acid in MeOH) and 900 μl deionized water. The elution and evaporation was done in 12 ml amber tubes. Samples were then transferred to eppendorf tubes ready for analysis.
Chemicals
Pure antibiotics salts of sulfonamides, tetracycline and quinolones (Ciproflaxacin) were purchased from Sigma-Aldrich (Augsburg, Germany). RIDASCREEN kits (Ridascreen sulfonamides (R3004), Ridascreen tetracycline (R3505) and Ridascreen quinolones/ chinolones (R3113) were purchased from R-Biopharm AG, (Darmstadt, Germany). Analytical level methanol was purchased from Sigma-Aldrich (Augsburg, Germany) and HPLC grade water was purchased from Sigma-Aldrich (Augsburg, Germany).
Enzyme-linked immunosorbent assay basis
Sulfonamides: The basis of the test is the antigen-antibody reaction. The micro titter wells are coated with capture antibodies directed against anti-sulfonamide antibodies. Standard or sample sulfonamide conjugate and anti-sulfonamide antibodies are added. Free sulfonamides and sulfonamide conjugate compete for the sulfonamide antibody binding sites (competitive enzyme immunoassay). At the same time, the anti-sulfonamide antibodies are also bound by the immobilized capture antibodies. Any unbound conjugate is then removed in washing step. Substrate/ chromogen is added to the wells and incubated. Bound conjugate converts the chromogen into a blue product. The addition of the stop solution leads to a color change from blue to yellow. The measurement is made photo metrically at 450 nm. The absorption is inversely proportional to the sulfonamide concentration in the sample.
Tetracycline: The basis of the test is the antigen-antibody reaction. The micro titter wells are coated with tetracycline-protein- conjugate. Tetracycline standards or sample solutions and anti-tetracycline antibodies are added. Free tetracycline and immobilized tetracycline compete for tetracycline antibody binding sites (Competitive Enzyme Immunoassay). Any unbound antibody is then removed in washing step and enzyme labeled secondary antibody, which is directed against the antitetracycline antibody, is added. After removing unbound enzyme labeled antibodies by a washing step, substrate/chromogen is added to the wells and incubated. Bound conjugate converts the chromogen into a blue product. The addition of the stop solution leads to a color change from blue to a yellow. The measurement is made photo metrically at 450 nm. The absorption is inversely proportional to the tetracycline concentration in the sample.
Quinolones: The basis of the test is antigen-antibody reaction. The wells are coated with a capture antibodies directed against anti-quinolone antibodies. Standards or sample solutions of ciprofloxacin enzyme conjugate and anti-quinolones antibodies are added. Free quinolones and ciprofloxacin conjugate compete for the quinolone antibody binding sites. (Competitive enzyme immunoassay). At the same time the anti-quinolone antibodies are also bound by the immobilized capture antibodies. Any unbound conjugate is then removed in washing step. Substrate/ chromogen is added to the wells and incubated. Bound conjugate converts the chromogen into a blue product. The addition of stop solution leads to a colour change from blue to yellow. The measurement is made photo metrically at 450 nm; the absorption is inversely proportional to the quinolone concentration in the sample.
Analytical procedure
Standard solutions used to generate standard curves for sulfonamide and quinolones were included in ELISA kits. They were diluted to final concentrations ready for use. The concentrations were 0,1,3,10,30 100 μg/l and 0, 0.5, 1.5, 3, 6 and 18 μg/l sulfonamide and quinolones respectively. Standard solutions for tetracycline were provided as concentrates. In order to produce tetracycline standards ready for use, 50 μl standard concentrate was diluted with 450 μl sample buffer, all were included in kits. Each 50 μl of the following standard concentrate 0, 0.5, 1.5, 3, 6, 18 μg/l were diluted with 450 μl sample buffer to make 0, 0.05, 0.15, 0.3, 0.6 and 1.8 μg/l final concentrations. R2 for the standard curves were 0.994, 0.944 and 0.991 sulfonamide, quinolones and tetracycline respectively.
Sulfonamide: Fifty microliters of each standard and for each prepared sample were added to micro plate wells of ELISA plate in duplicate. Fifty microliters of conjugate was added to each well. Then 50 μl of antibody was added to each well, mixed gently by shaking the plate and incubated for one hour at room temperature. The solution in the wells was the discarded by decanting and the micro plate was tapped three times in blotting paper to ensure complete removal of the fluids. The wells were washed by filling with 250 μl of washing buffer and discarding by decanting and this was repeated three times. One hundred microliters of substrate/chromogen was added to each well, mixed well gently by shaking the plate and incubated for 15 minutes at room temperature in a dark chamber. Then 100 μl of stop solution was added and mixed gently by shaking the plate. Absorbance was read at 450 nm, 30 min after adding the stop solution.
The results were expressed in percentages of the maximum absorbance (B/B0%), using the following equation:
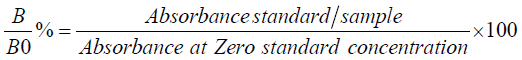
In order to obtain the sulfonamide concentrations in the sample, the B/B0% values were interpolated on the calibration curve built with six sulfonamide standard solutions. Obtained concentrations of sulfonamides were multiplied by dilution factor and expressed as equivalents.
Tetracycline: Fifty microliters for each sample and each of the standard concentration were added in micro plate wells in duplicate. Fifty microliters of anti-tetracycline antibody solution was then added to each well. The plates were incubated at room temperature for one hour after mixing by shaking plates. The solution in the wells was discarded by decanting and the micro plate was tapped three times on blotting paper to ensure a complete removal of the fluids. The wells were washed three times with 250 μl washing buffer. One hundred microliters of enzyme conjugate was then added to each well, mixed gently and incubated at room temperature for 15 minutes. Then one hundred microliters of the stop solution was added to each well, mixed well by gently shaking the plate manually. Absorbance was read at 450 nm, after thirty minutes. The results were expressed as in sulfonamide above.
Quinolones: Fifty microliters of each standard or sample solution were added in each well of micro plate in duplicate. Fifty microliters of conjugate was added to each well. Then fifty microliters of antibody was added to each well of micro plate. Mixed gently by shaking the plate manually and incubated for one hour in refrigerator (2°C -8°C). The solution in wells was discarded and the micro plate was tapped three times on blotting paper to ensure complete removal of solution from wells the wells were filled with 250 μl of washing buffer. The liquid again was poured out and the wash step was repeated three times. One hundred microliters of substrate/chromogen was added to each well and mixed gently by shaking plate, incubated for 15 minutes at room temperature in the dark. Then on hundred microliters of stop solution was added to each well of ELISA micro plate, mixed gently by shaking the plate, absorbance was measured at 450 nm, after thirty minutes. The results were expressed as in sulfonamide above.
Results and Discussion
Results
The traditional chromatographic methods sometimes linked with mass spectrophotometry technologies are most frequently used for manure, soil and water and sediments antibiotics residues analysis. They can be employed for both screening and quantitative analysis. Immunochemical methods such as ELISA are cost-effective and can serve as the tools for both semi-quantitative and screening of antibiotic residues from environmental samples.
Recovery: The recovery rates for spiked samples were 55.13%- 90.3%, 60-115% and 45%-92.5% sulfonamide, tetracycline and quinolones respectively.
Limit of detection and Limit of quantification: The calculated limits of detections (LOD) in this study were 55.01, 26.96 and 78.43 μg/kg quinolone, sulfonamide and tetracycline respectively. Limit of quantifications (LOQ) were 136.07 μg/kg quinolones, 42.57 μg/kg sulfonamide and 206.4 μg/kg tetracycline.
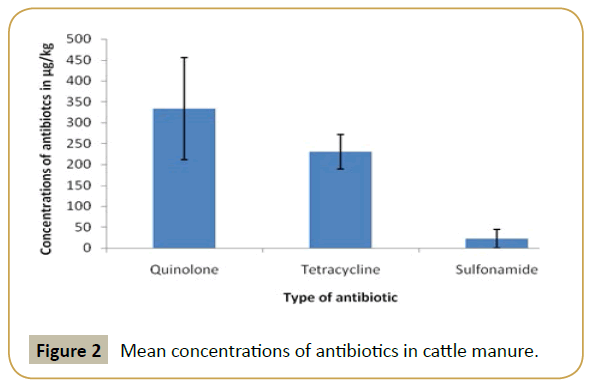
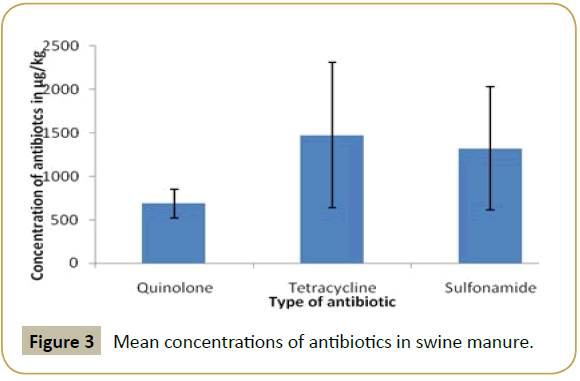
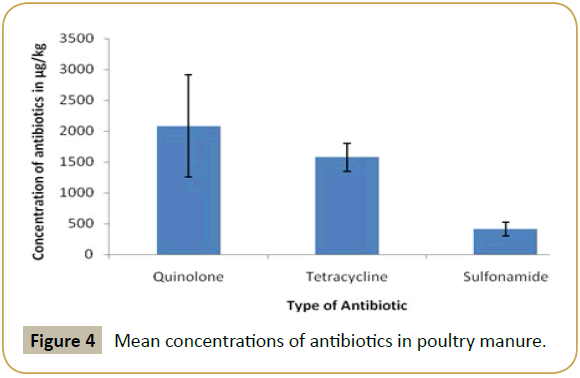
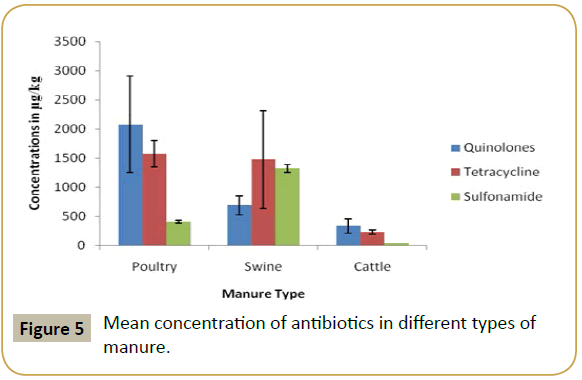
According to results, in the poultry manure the highest mean concentration detected was that of quinolone and the lowest mean concentration was that of sulfonamide (Figure 2). In swine manure, sulfonamide and tetracycline were detected at high level while quinolone was detected at lowest mean concentration (Figure 3). In cattle quinolone was detected at high level followed by tetracycline (Figure 4). In general tetracycline and quinolones usage is higher compare to sulfonamide. Also according to findings antibiotics usage is heavier in poultry and swine than in cattle in study area, this may be the same trend in Tanzania, because swine and poultry keeping has become source of income in urban and peri-urban areas. This shows the risk of environmental contamination by antibiotic residues from livestock effluents (Figure 5).
Discussion
In Tanzania legislations regarding antibiotic drug application in farm animals as well as monitoring and control of their residues are not adequately enforced. The inadequacy has probably led to the reported high rates of antimicrobial residues in livestock products. For instance, chicken eggs tested in Morogoro were found to be 100% positive to antimicrobial residues [7]. In the same region, studies have shown that there is an elevated level of antimicrobial residues in milk, beef and chicken meat [14]. Additionally, 50% of broiler tissues tested were positive to antimicrobials, whereas 70% of chicken farms visited were positive to selected antimicrobial residues [15].
In this study, all three selected antibiotics were detected in faeces of all selected animal species at varying degrees. Farmers in developing countries freely use antibiotics despite their limited knowledge about antibiotics. Economic incentives drove their decision to use antibiotics. Diseases are rarely diagnosed; farmers either use antibiotic that seemed to work previously or switch antibiotics until they find one that seems to cure the disease.
In the past decade many efforts have been concentrated in developing multi residue methods by which residues levels can be monitored [16,17]. However it is necessary taking into account not only the detectable compounds, but also their identity.
The mean concentrations of antibiotics (Sulfonamide, tetracycline and quinolones) are summarized in Table 1. The highly detected analyze in poultry manure was quinolones, followed by tetracycline and the least detected analyzed was sulfonamide. In swine manure sulfonamide was highly detected followed by tetracycline and quinolones respectively. While in cattle manure the detection was in order of quinolones, tetracycline and sulfonamides.
| Manure | Drug | Mean |
|---|---|---|
| Poultry | Quinolones | 2083.2640 ± 826.64583 |
| Tetracyclines | 1573.7528 ± 222.95739 | |
| Sulfonamides | 415.8774 ± 110.48513 | |
| Swine | Quinolones | 685.8652 ± 161.36830 |
| Tetracyclines | 1473.6110 ± 1558.33237 | |
| Sulfonmaides | 1320.9967 ± 710.06372 | |
| Cattle | Quinolones | 333.4177 ± 99.37554 |
| Tetracyclines | 329.2308 ± 70.21343 | |
| Sulfonamides | 22.9391 ± 12.16023 |
Table 1: Mean concentrations of antibiotics in manure.
The highest level of antibiotics detected in this study was in line with other studied conducted previously in the different parts of the world, although they were low, probably this is due to low intensification in the study area. The concentrations of tetracycline ranged from 82 ± 78, 516 μg/kg, quinolones 36 ± 51, 912 μg/kg and 12 ± 5773 μg/kg sulfonamide in poultry manure in Malaysia. Again it was found that the highest concentration of tetracycline in poultry manure in five cities of Khuzestan province in Iran was 5, 360 μg/kg and the lowest concentration was 500 μg/kg, while the highest concentration was 13,770 μg/kg and lowest was 47 μg/kg in cattle manure [18].
In eight provinces in China concentrations of these three antibiotics in poultry, swine and cattle manure were as follows:
In Pig and cattle manure a maximum of 33980 μg/kg and minimum 29000 μg/kg quionolone and 59590 μg/kg for tetracycline. Also, a concentration of 1, 420, 760 μg/kg quinolones and 6, 040 μg/kg sulfonamide in chicken manure.
The impact of antibiotics administered to animals in terrestrial and aquatic environment depends not only on the used amount and the type of administration, but also on animal husbandry practices, metabolism within the animal, manure handling and storage and degradation rates in it. However, knowledge about the extent of occurrence of antibiotics, their transport and fate in the environment is lacking [19].
The main concern of extended use of antibiotics and their shedding into environment is the promotion of resistance in bacteria of human and veterinary importance. Commensally and environmental bacteria can serve as a feasible reservoir of resistance genes for pathogenic bacteria. Antibiotics released in the environment can enhance the formation of single, cross and even multiple resistance in pathogens, commensally and environmental bacteria [20]. There is still a gap of reliable studies on the relationship between antibiotic residues and the occurrence of resistant bacteria [21-30].
Conclusion
This study presents a viable method for analysis of selected antibiotics, quinolones, sulfonamides and tetracycline in pig, chicken and cattle manure samples from intensively managed livestock in study area. Manure from Pig, Poultry and Cattle from Morogoro municipality have significant concentrations of these selected antibiotics. These results suggest regular monitoring of antibiotics pollutants in the environment from manure is required. Some control measures should be designed urgently to prevent the associated risks to humans from the excessive use of antibiotics through water contaminations.
Even if the occurrence, effects and fate of antibiotics have been put in the perspective of scientific interest, still little is known about actual risk to both humans and the environment. Huge gap still exist in the understanding of interaction between residues, metabolites and resistance promotion after excretion. Appropriate use of antimicrobials in livestock production will preserve efficacy of existing antibiotics, support animal health and welfare and limit the risk factors of transferring antibiotic resistance to animals and humans.
Acknowledgements
We gratefully acknowledge financial support through DANIDA through Urban and Peri-Urban Livestock Farming in Tanzania project.
References
- Kummerer K (2009) Antibiotics in aquatic environment: A review part 1. Chemosphere 75: 417-434.
- Hirsch R, Ternes T, Haberer K, Kratz KL (1999) Occurrence of antibiotics in the aquatic environment. Sci Total Environ 225: 109-118.
- Pan X, Qiang Z, Ben W, Chen M (2011) Residual veterinary antibiotics in swine manure from concentrated animal feeding operations in Shandong Province, China. Chemosphere84: 695-700.
- Zhao L, Dang YH, Wang H (2010) Residues of veterinary antibiotics in manures from feedlots livestock in eight provinces of China. Sci Total Environ 408: 1069-1075.
- Martinez-Carballo E, Gonzalez-Barreiro C, Scharf S, Gans O (2007) Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ Pollut 148: 570-579.
- Khachatourians GG (1998) Agricultural use of antibiotics and evaluation and transfer of antibiotic-resistant bacteria. CMAJ 159: 1129-1136.
- Nonga HE, Simon G, Karimoribo ED, Mdegela RH (2010) Assessment of antimicrobial usage and residues in commercial eggs from smallholder poultry keepers in Morogoro Municipality, Tanzania. Zoonoses Public Health 57: 339-344.
- Zhuo LJ, Ying GG, Liu S, Zhang RO, Lai HJ, et al. (2013) Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci Total Envron 444: 183-195.
- Hamster G, Sczesny S, Hoper H, Nau H (2002) Determination of persistent tetracycline residues in soil fertilized with liquid manure by high performance liquid chromatography. Anal Chem 74: 1509-1518.
- Li YW, Wu XL, Mo CH, Tai YP, Huang XP, et al. (2011) Investigation of sulfonamide, tetracycline and quinolone antibiotics in vegetable farmland soil in Pearl River Delta area, Southern China. J Agric Food Chem 59: 7268-7276.
- Li W, Shi Y, Gao Y, Liu J, Cai Y (2012) Investigation of antibiotics in Molluscs from coastal waters in the Bohai Sea of China. Environ Pollt 162: 56-62.
- Ashboltt NJ, Amezquita A, Backhaus T, Borriela P, Brandt KK, et al. (2013) Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ Health Prospect 121: 993-1001.
- Luo Y, Mao D, Rysz M, Zhou Q, Zhang H, et al. (2010) Trends in antibiotics resistance genes occurence in the Hehe River, China. Environ Sci Technol 44: 77220-7225.
- Kurwijila LR, Omore A, Staal S, Mdoe NS (2006) Investigation of risk of exposure to antimicrobial residues present in marketed milk in Tanzania. J Food Prot 69: 2487-2492.
- Nonga H, Simon C, Karimuribo E, Mdegela R (2009) Assesment of antimicrobial usage and antimicrobial residues in broiler chicken in Morogoro, Municipality, Tanzania. Pakistan J Nutri 8: 203-207.
- Zhang XX, Zhang T, Fang HH (2006) Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol 82: 397-414.
- Zhang MK, Fang LP (2007) Effects of tillage and green brick making site. Soil Tillage Res 93: 87-93.
- Cheng D, Liu X, Wang I, Gong W, Liu G, et al. (2014) Seasonal variation and sediment-water exchange of antibiotics in a shallower large lake in Northern China. Sci Total Environ 476-477: 266-275.
- The Council of the European Union (2002) Council Recommendations of 15 November 2001 on the Prudent use of antimicrobial Agents in Human Medicine, Brussels, Belgium.
- Wegner HC, Aarestrup FM, Jensen JB, Hammerum AM, Bager F (1998) The association between the use of antimicrobial growth promoters and development of resistance in pathogenic bacteria towards growth promoting and therapeutic antimicrobials. J Anm Feed Sci 7: 7-14.
- Al-Odaini NA, Zakaria MP, Yaziz MI, Surif S (2010) Multiresidue analytical method for human pharmaceuticals and synthetic hormones in river water and sewage effluents by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatographra A 1217: 6791-6806.
- Arikan OA, Rice C, Codling E (2008) Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination 226: 121-133.
- Blackwell PA, Holten LHC, Halling-Sorensen B, Boxall ABA, Kay P, et al. (2004) Ultrasonic extraction of veterinary antibiotics from solis and pig slurry with SPE clean-up and LC-UV and fluorescence detection. Talanta 64: 1058-1064.
- Giraud E, Douet DG, Le Bris H, Bonju-Albert A, Donnay-Moreno C, et al. (2006) Survey of antibiotic resistance in an integrated marine aquaculture system under oxolinic acid treatment. FEMS Microbiol Ecol 55: 439-448.
- Hao PT, Mnagaki S, Nakada N, Takada H, Shimizu A, et al. (2011) Antibiotic contamination and occurrence of antibiotic resistant bacteria in aquatic environment of Northern Vietnam. Sci Total Environ 409: 2894-2901.
- Huang C, Chen X, Xie W, Feng J, Liu H, et al. (2009) Detection method for simultaneously measuring the residues of tetracyclines (TCs) drugs in royal jelly. European Patent Office, China.
- Karci A, Balcuoglu A (2009) Investigation of the tetracycline, sulfonamide and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci Total Environ 407: 4652-4664.
- Kim S, Aga DS (2007) Potential ecological and human health impacts of antibiotics resistant bacteria from wastewater plants. J Toxicol Environ Health B Crit Rev 10: 559-573.
- Mashiko K, Masaki K, Takashi O (2007) Extraction of liquid for assaying residual veterinary drug. Organization WP, Japan.
- Tusevljak N, Dutil L, Rajic A, Uhland FC, McClure C, et al. (2013) Antimicrobial use and resistance in aquaculture: Findings of a globally administered survey of aquaculture-allied professionals. Zoonoses Public Health 60: 426-436.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences