ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
Nontuberculous Mycobacterium during Pregnancy: A Case Report
Zipori Y1*, Geary M1, Wong H2 and Berger H1
1Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, St. Michael's Hospital, University of Toronto, Ontario, Canada
2Division of Respirology, Department of Medicine, St. Michael's Hospital, University of Toronto, Ontario, Canada
- *Corresponding Author:
- Zipori Y
Division of Maternal-Fetal Medicine
Department of Obstetrics and Gynecology
St. Michael's Hospital, University of Toronto
Ontario, Canada
Tel: 1-416-864-6060
E-mail: zipori74@hotmail.com
Received Date: December 20, 2016; Accepted Date: December 26, 2016; Published Date: January 07, 2017
Citation: Zipori Y, Geary M, Wong H, et al. Nontuberculous Mycobacterium during Pregnancy: A Case Report. J Transm Dis Immun. 2017, 1:1.
Abstract
Background: The purpose of the present case report was to describe an exacerbation of maternal respiratory condition during pregnancy and its association with severe fetal growth restriction. Case: A 29-year old woman, gravida 1, para 0, at 22 weeks’ gestation was admitted to due to poorly controlled asthma and nontuberculous mycobacterium pulmonary infection, namely mycobacterium kansasii. With anti-tuberculous therapy, systemic steroids, in addition to supportive care, gradual clinical improvement was noticed. An uncomplicated cesarean section was performed at 37 weeks’ gestation due to minimal interval growth in a severely growth restricted fetus. Conclusion: Early symmetrical fetal growth restriction should be anticipated with underlying, uncontrolled respiratory pathology, necessitating close monitoring in order to facilitate the optimal timing of delivery.
Introduction
Nontuberculous mycobacteria (NTM) are mycobacterial species different from the classic mycobacterium tuberculosis complex. They are ubiquitously distributed in the environment (soil and water) with worldwide isolation rates that vary between 1-1.8 cases per 100,000 persons. Currently, over 150 species of NTM have been identified based on their 16S rRNA gene sequence differences, with the most common species being the slowly growing mycobacterium avium complex (MAC). Human disease is suspected to be acquired from environmental exposure and thus far, there has been no documentation of human-to-human transmission. Predisposing factors for acquiring NTM include past history of mycobacterial disease, underlying chronic lung disease, HIV, malignancy and alcoholism. NTM manifestations may range from asymptomatic infection to symptomatic disease in humans, with a spectrum of pulmonary, skin and soft tissue infection, superficial lymphadenitis, and a disseminated form [1].
Intrauterine growth restriction is associated with an increased risk of perinatal morbidity and mortality compared with their appropriately grown counterparts, and much attention has been dedicated in recent years to prediction, early recognition, improved surveillance, as well as to timely planned pulmonary in order to minimize some of the adverse pregnancy outcomes [2]. The literature on mycobacteria during pregnancy is limited to the mycobacterium tuberculosis complex. Here, however, we present the first case of a less common, slowly growing NTM pathogen, namely mycobacterium kansasii, which was associated with severe early intrauterine growth restriction during pregnancy.
Case Report
A 29-year old woman, a recent immigrant to Canada from India, gravida 1, para 0, at 22 weeks’ gestation was admitted to the respirology department due to symptoms of poorly controlled asthma and NTM pulmonary infection. She had significant dyspnea, ongoing productive cough, pleuritic chest pain and wheezing despite maintenance therapy of symbicort two inhalations twice daily (combination of budesonide and formoterol), four days of prednisone 20 mg daily, and two weeks of outpatient triple therapy with isoniazid 300 mg daily, rifampin 600 mg daily and ethambutol 800 mg daily for her confirmed NTM mycobacterium kansasii infection. Aside from recent 3 kg weight loss, she had an uneventful pregnancy, including low risk for aneuploidy and normal 20 weeks’ morphology ultrasound.
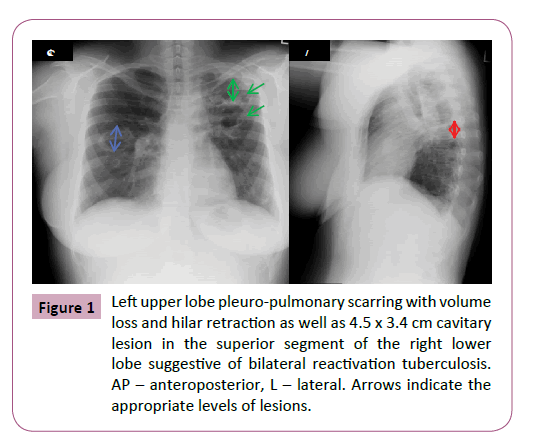
Her past medical history was significant for treatment of tuberculosis 11 years earlier, for which the patient could not specify which drugs were used or be definitive about the duration of therapy. Bronchoscopy and sputum cultures as part of a work up for tuberculosis prior to her immigration were negative. A chest X-ray upon her arrival in Canada described marked left upper lobe pleuro-pulmonary scarring with volume loss and hilar retraction, as well as 4.5 x 3.4 cm cavitary lesion in the superior segment of the right lower lobe suggestive of bilateral reactivation tuberculosis (past images were not available for comparison). Repeated (induced) sputum was 3+ positive for acid- fast bacilli. Thereafter, her culture grew mycobacterium kansasii. Treatment was not commenced at that time as the patient was asymptomatic. Several subsequent spontaneous sputum samples also grew mycobacterium kansasii, and she was finally started on triple therapy as an outpatient when she developed worsening cough, sputum production, and dyspnea despite aggressive treatment of her asthma (Figure 1).
Figure 1: Left upper lobe pleuro-pulmonary scarring with volume loss and hilar retraction as well as 4.5 x 3.4 cm cavitary lesion in the superior segment of the right lower lobe suggestive of bilateral reactivation tuberculosis. AP – anteroposterior, L – lateral. Arrows indicate the appropriate levels of lesions.
On admission, maternal vital signs revealed blood pressure of 130/80 mmHg, tachycardia of 116 bpm, she was afebrile, and had a respiratory rate of 20 per minute with oxygen saturation of 95% on room air. Laboratory analysis was remarkable for hemoglobin of 105 g/L and elevated liver transaminases (AST 60 U/L, ALT 120 U/L). Her arterial blood gases comprised of pH 7.48, pCO2 34 mmHg, pO2 76 mmHg, and bicarbonate 26 mmol/L, suggestive of mild respiratory alkalosis.
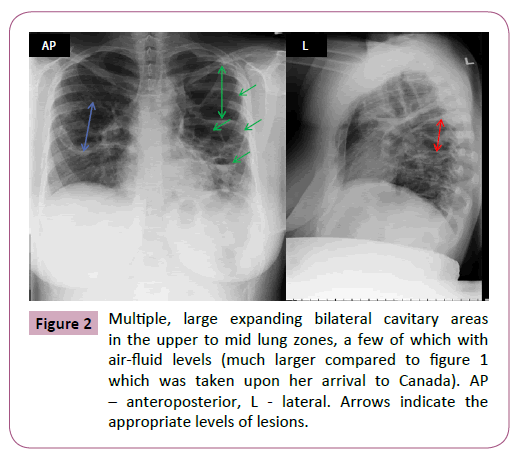
A repeat chest X-ray showed multiple, large expanding bilateral cavitary areas in the upper to mid lung zones, a few of which with air-fluid levels, that were much larger compared to the previous imaging (Figure 2). Maternal upper abdominal ultrasound was normal. Pulmonary function tests were all markedly reduced, with forced expiratory volume in one second (FEV1) of 0.98 L (normal 2.7-3.8 L), forced vital capacity (FVC) 1.59 L (normal 3.1-4.5 L) and FEV1:FVC ratio of 62% (normal 76-94%). Due to the elevated liver enzymes, isoniazid was discontinued and replaced with clarithromycin 500 mg twice daily. With the addition of supportive care, including chest physiotherapy and continued systemic steroids, gradual clinical improvement was noticed within few days, with normalization of the liver function tests. Fetal ultrasound confirmed reassuring fetal status with an estimated fetal weight (EFW) on the 10th percentile. She was discharged after 14 days for continued outpatient management. Rifampin and ethambutol were both discontinued due to resistance once sensitivity results for her NTM were available. She was continued on a suboptimal therapy of rifabutin 150 mg daily and clarithromycin 500 mg twice daily for her NTM infection, as she did not wish to start a fluoroquinolone or linezolid due to the fear of potential fetal complications and cost of medications. Fortunately, she remained well clinically throughout the remainder of the pregnancy. Close fetal surveillance was initiated due to the suspected fetal growth restriction (FGR). Subsequent fetal ultrasounds showed the following; EFW at 28 weeks, 10th percentile; at 32 weeks, 5th percentile; at 34 weeks, <3rd percentile; at 36 weeks, <3rd percentile, however, with normal biophysical profile and fetal dopplers throughout, including cerebro-placental ratio (CPR) twice weekly from 34 weeks’ gestation. The patient had previously declined invasive testing for karyotype and microarray. TORCH screen was negative. A decision was made to deliver at 37 weeks’ gestation due to minimal interval growth in a severely growth restricted fetus. An uncomplicated elective caesarean section was performed under regional anesthesia due to breech presentation. A female infant was delivered weighing 1,872 g (<3rd percentile) with Apgar scores of 9 and 9 at one and five minutes, respectively. The postpartum course was uneventful. Placental histopathology showed villous hypoplasia, acute vasculitis, calcifications, and a total weight of 290 g (<3rd percentile), keeping with severe growth restriction. The anti NTM therapy was continued for a 12 months’ duration after negative sputum culture, and long term follow-up will be continued.
Figure 2: Multiple, large expanding bilateral cavitary areas in the upper to mid lung zones, a few of which with air-fluid levels (much larger compared to figure 1 which was taken upon her arrival to Canada). AP – anteroposterior, L - lateral. Arrows indicate the appropriate levels of lesions.
Discussion
Normal pregnancy, mainly due to progesterone effect on the respiratory centre, triggers a state of chronic mild hyperventilation, leading to almost 50% rise in the minute ventilation at term. This is primarily due to a large increase (up to 40%) in tidal volume rather than the unchanged respiratory rate. The increased minute ventilation leads to a higher PaO2 (104-108 mmHg) and lower PaCO2 (27-32 mmHg) in the maternal circulation, resulting in a state of chronic respiratory alkalosis (pH 7.4-7.45) which is compensated for by an increase in renal excretion of bicarbonate (18-21 mmol/L). A simultaneous increase in oxygen consumption (20-40% above non-pregnant levels) occurs in order to fulfill the maternal, fetal and placental requirements [1-3].
Patients with pulmonary involvement due to mycobacterium kansasii and other NTM species usually present with chest pain, cough, hemoptysis, fever, and night sweats, while their chest radiograph abnormalities are characterized by cavitary infiltrates as well as nodular and bronchiectatic changes. As the clinical features and radiographic appearance are very similar to tuberculosis, and because most of the currently identified NTM are not associated with human disease, the addition of species-specific microbiology isolation is essential to confirm the diagnosis of NTM infection [1]. Even if NTM diagnostic criteria are met, initiation of therapy requires a careful risk -benefit analysis (in our case, 9 months elapsed from diagnosis-to-therapy as the patient was asymptomatic). Treatment of mycobacterium kansasii in pregnancy should not differ from the non-pregnant state, and a triple regimen of daily isoniazid (300 mg/day) coadministered with pyridoxine (25 mg/day), rifampin (600 mg/ day), and ethambutol (15 mg/kg/day) is considered acceptable for use in pregnancy [4]. Nevertheless, the undesired risk of hepatitis and peripheral neurotoxicity associated with isoniazid use, might limit its use during pregnancy. A small prospective study of 18 patients has shown that the macrolide clarithromycin (500 mg twice daily) may be a reasonable alternative for patients unable to tolerate isoniazid, as done in our case [5]. A prospective trial of 28 patients treated with the above regimen found no benefit for prolongation of therapy beyond 12 months’ duration in culture negative patients [6]. Rare cases of fetal abnormalities and hemorrhagic disease have been reported with rifampin use, however the maternal benefit likely justifies the potential risk to the fetus. Sensitivity testing can be helpful to tailor the treatment regimen, especially in those who fail to respond to conventional triple therapy, as in the case of our patient. Although surgical therapy (lobectomy in patients with localized disease, pneumonectomy with multiple cavitary lesions) may play a role in some pulmonary NTM infection (failed medical therapy, patients who cannot tolerate medical therapy), many aspects of adjunctive surgical therapy have not yet clearly defined. Additional therapy directed towards the underlying disease states (asthma in our case), is recommended, including bronchodilators, prednisone use and airway clearance techniques, and these measures may be equally or more important than treatment for the NTM infection.
The effects of chronic lung disease on perinatal outcomes are variable, and are largely dependent on the specific underlying condition and its severity. Inadequately treated respiratory conditions can have a detrimental effect on maternal status, with worsening of symptoms and increased risk of respiratory compromise. Despite known in utero adaptations to chronic hypoxia such as circulatory redistribution, increased tissue oxygen extraction and decreased gross body movements, a common fetal response to chronic oxygen deprivation is deferment of growth needs in favor of vital functions, resulting over time in FGR [7,8]. Currently, there is insufficient evidence to evaluate the benefits and risks of other interventions, specifically maternal oxygen therapy, for suspected impaired fetal growth in women with chronic respiratory disease.
When FGR is suspected, prenatal assessment should strive to clarify the underlying etiology (maternal, fetal or placental) and its severity while initiating close fetal surveillance of interval growth and well-being. This surveillance will guide decision making with regards to the optimal time and route of delivery. Our case clearly suggested a maternal underlying pulmonary condition as a cause of the FGR, supported by early symmetrical FGR appearance only few weeks after initial maternal symptoms. Despite persistent fetal growth below the third centile, normal biophysical profile and doppler studies allowed continuation of outpatient expectant management until 37 weeks of gestation. Interestingly, maternal clinical improvement in later gestation did not alter the FGR status, and even worsened it, suggesting that early pregnancy oxygen deprivation is a major insult that cannot be simply overcome by improving maternal status.
In conclusion, we have described a unique, rare pulmonary pathogen, mycobacterium kansasii, that manifested clinically during pregnancy in a previously asymptomatic patient. A multidisciplinary team effort is essential to optimize maternal outcome, and one year of anti-NTM triple therapy is recommended. Alternatively, initiation of therapy should be considered upon diagnosis even in asymptomatic patients, especially in reproductive age women who are contemplating future pregnancy. Early symmetrical fetal growth restriction should be anticipated with underlying, uncontrolled respiratory pathology, necessitating close monitoring in order to facilitate the optimal timing of a safe delivery.
Disclosure
The authors report no conflict of interest.
References
- Griffith DE, Aksamit T, Brown-Elliott BA (2007) ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416.
- Sharma D, Shastri S, Sharma P (2016) Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin Med Insights Pediatr 10:67-83.
- LoMauro A, Aliverti A (2015)Respiratory physiology of pregnancy: Physiology masterclass. Breathe (Sheff) 11: 297-301.
- Philley JV, Griffith DE (2013)Management of nontuberculous mycobacterial (NTM) lung disease. Semin Respir Crit Care Med 34: 135-42.
- Griffith DE, Brown-Elliott BA, Wallace RJ (2003) Thrice-weekly clarithromycin-containing regimen for treatment of Mycobacterium kansasii lung disease: results of a preliminary study. Clin Infect Dis 37: 1178-82.
- Sauret J, Hernández-Flix S, Castro E,Hernández L, Ausina V, et al.(1995) Treatment of pulmonary disease caused by Mycobacterium kansasii: results of 18 vs 12 months' chemotherapy. Tuber Lung Dis 76: 104-8.
- Richardson BS, Bocking AD (1998) Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A MolIntegr Physiol 119: 717-23.
- Mehta N, Chen K, Hardy E, Powrie R (2015) Respiratory disease in pregnancy. Best Pract Res Clin Obstet Gynaecol 29: 598-611.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences