ISSN : 2347-5447
British Biomedical Bulletin
Newer Substituted Indolyl-Pyrazoline Derivatives as Anti-Inflammatory Agents
Minakshi Shroff1* and Daharwal SJ2
1Department of Pharmaceutical Sciences, Uttarakhand Technical University, Dehradun, Uttarakhand, India
2Pandit Ravi Shankar Shukla University, Raipur, Chhattisgarh, India
- *Corresponding Author:
- Minakshi Shroff
Department of Pharmaceutical Sciences, Uttarakhand Technical University
Dehradun, Uttarakhand, India
Tel: +919691611971
Email: mahishroff@gmail.com
Received date: December 21, 2016; Accepted date: January 28, 2017; Published date: January 30, 2017
Citation: Shroff M, Daharwal SJ (2017) Epigenetic Mode of Bacterial Drug Resistance. Br Biomed Bull, 5:298.
Copyright: © 2017 Shroff M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A novel series of nine indolyl pyrazoline pharmacophore were synthesized by the reaction of hydrazine hydrate derivatives with the synthesized chalcones (C4,C5,C6). The chalcones were synthesized by the Claisen Schimdt condensation method. These pyrazoline derivatives were evaluated for anti-inflammatory activity using Carrageenan induced paw oedema method. The activity profile reveals that the unsubstituted N1 of these indolyl pyrazoline was found to give better activity than other N1- sustituted indolyl pyrazolines. The reference drug for the inflammatory activity was Indomethacine.
Keywords
Antiinflammatory; Claisen schimdt reaction; Chalcones; Pyrazoli ne; Indolyl pyrazoline
Introduction
The pyrazolines are the five membered heterocyclic compounds which has attracted the attentions of organic chemists in the past decades due to their immense biological applications. These compounds are generally prepared from the reactions of chalcones with hydrazine derivatives under the ordinary conditions. The present derivatives contains indole moiety. Literature survey revealed that incorporation of indole moiety in pyrazoline ring enhanced anti-inflammatory activity. Indole alkaloids have been proved to be medicinally important natural compounds. Indole ring was an important template for drug design such as the classical NSAIDS indomethacin and indoxole. Further indole derivatives had been reported to possess number of potent biological activities including analgesics, antipyretics antifungal, anti-inflammatory, anthelmintic, cardiovascular, anticonvulsant, antimicrobial, and selective COX-2 inhibitory activities. Hence the efficient synthesis of novel substituted indolyl derivatives compounds still represents highly pursued target. The substitution of heterocyclic moiety at the 3-position of indole ring markedly influences the anti-inflammatory activity [1].
Promoted by the number of research studies, the present article aimed at gathering the two bioactive entities (i.e. indole and pyrazole) into one compact structure and evaluating its antiinflammatory activity.
Result and Discussion
Chemistry
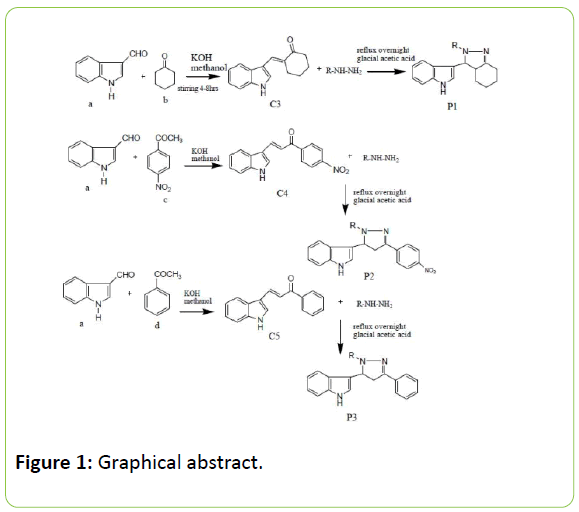
The 3-indolaldehyde is 3H-indene-1-carbaldehyde, prepared using Vielsmeier Haack method was utilized for synthesis of chalcones by reacting with selected aryl ketone. Chalcone [1] is a generic term given to compounds bearing the 1, 3-diphenyl-2- propen-1-one framework and belong to the flavonoid family [2-4]. Chemically they are open-chain flavonoids in which the two aromatic rings are joined by a three carbon α, β- unsaturated carbonyl system. We had three synthesized chalcones (E)-2-((1H-indol-3-yl) methylene) cyclohexanone (C3); (E)-3-(1H-indol-3-yl)-1-(4-nitrophenyl)prop-2-en-1-one (C 4) and (E)-3-(1H-indol-3-yl)-1-phenylprop-2-en-1-one (C5) which were synthesized [5] by using 3-indolaldehyde with cyclohexanone, pnitro phenylacetone and phenylacetone respectively. The reaction which produces chalcones is base catalysed claisen schimdt condensation reaction [6-10]. Formed chalcones than made to react with hydrazine hydrate, phenyl hydrazine and isonicotinyl hydrazine respectively to produce different derivatives with different N1 substitution. The scheme 1 represents the scheme of reaction for the synthesis of indolyl pyrazoline. The substituted hydrazine hydrate had synthesized the substituted pyrazoline derivatives (P1, P2, P3) (Table 1). The table enlisted these substituted derivatives as compounds 7a-7g. The completion of reaction was monitored by TLC using acetic acid: ethyl acetate as solvent system in 1:1 and the spots were visualized by exposure to iodine vapour or spraying dilute sulphuric acid. The structures of synthesized pyrazoline derivatives (7a-7i) were confirmed on the basis of IR, 1H NMR, Mass Spectroscopy and elemental analysis. The pharmacological activity taken over was anti-inflammatory by the Carrageenan induced paw oedema method (Figure 1).
 |
P1 | P2 | P3 |
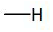 |
7a | 7d | 7g |
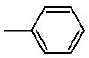 |
7b | 7e | 7h |
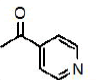 |
7c | 7f | 7i |
Table 1: Pyrazoline derivatives.
Spectral Studies
IR spectra of synthesized novel indolyl pyrazoline derivatives (7a-7i) showed v(C=N) stretching at 1550.77-1598.99 cm-1 due to closure of ring. The absorption band at 1340-1361.74 cm-1 were attributed to the v(C-N) stretching vibrations, these confirms the formation of the pyrazoline ring in all the compounds. The mono-substituted amines showed additional sharp bands in the region 3100-3273.20 cm-1 due to the v (NH) stretch. The indolyl at v (N-H) observed at range 3215.34-3547.09 cm-1 and other characteristic spectra’s band appeared. The compound 7a-7c exhibits v(CH-CH) stretch at the range of 1006.84-1008.77 cm-1. The v(C=O) stretching ranging 1650.18-16662.64 isonicotinyl attached with N1 in 7c, 7f and 7i of pyrazoline derivatives. 7d, 7e and 7f show v (Ar-NO2) between 1514.12-1517.9 cm-1 (Table 2).
| Compound | IR (KBr, cm-1) studies of synthesized compounds. |
|---|---|
| 7a | 1576.64(C=N ); 1335.49(C-N); 3167.58(N-H); 3448.71(N-H) indole 1006.38(CH-CH) |
| 7b | 1582(C=N ); 1327.03(C-N); 3273.20(N-H); 3416.29(N-H) indole 1008.77(CH-CH) |
| 7c | 1550.77(C=N ); 1336.19(C-N); 3165.19(N-H); 3390.86(N-H) indole; 1006.84(CH-CH);1650.18(C=O) |
| 7d | 1579.70(C=N); 1336.67(C-N); 3160(N-H); 3215.34(N-H) indole 1514.12(Ar-NO2) |
| 7e | 1598.99(C=N); 1361.74(C-N); 3115.34(NH); 3340(N-H) indole 1517.98(Ar-NO2) |
| 7f | 1598.99(C=N); 1361.74(C-N); 3150(NH); 3547.09(N-H) indole1517.98(Ar-NO2); 1658.78(C=O) |
| 7g | 1584.36(C=N); 1361.74(C-N); 3176.76(NH); 3429.95(N-H) indole |
| 7h | 1579.70(C=N); 1359.82(C-N); 3100(NH); 3410(N-H) indole |
| 7i | 1597.06(C=N); 1340(C-N); 3160(NH); 3410(N-H) indole; 1662.64(C=O). |
Table 2: IR spectral studies of synthesized compound.
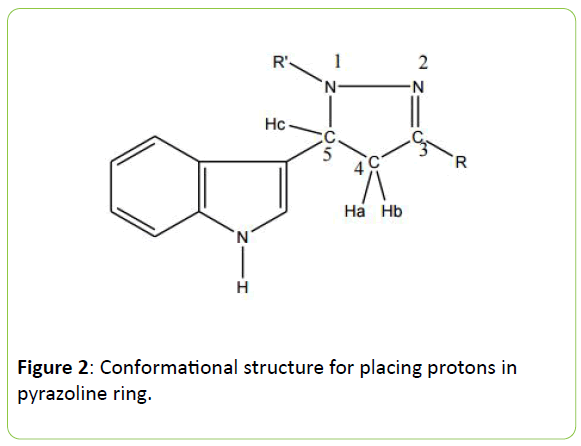
1H NMR spectra observed for the synthesized compounds in CDCl3 supported as evidence to the synthesized structures (Table 3). All the pyrazoline containing derivatives exhibit N-H of indole as doublet at δ range 11.398-11.500 ppm. The proton Hc at carbon C5 appears downfield in the region δ 4.630-4.875 ppm as doublet in all the compounds. The proton Ha and Hb at C4 carbon exhibits two signals, one as triplet in the δ range 3.05-4.624 ppm and the other as doublet of doublet ranging from δ 3.279-4.873 ppm which is due to vicinal coupling with two magnetically nonequivalent protons of the –CH2- group at position 5 and germinal proton at 4 of all synthesized compounds. But this type of characteristic range is total absent in three compounds 7a, 7b and 7c. It is due to the only reason because pyrazoline is found fused with cyclohexanone ring at C3 and C4 of pyrazoline. This shows signals range of δ for 3.00-4.01 ppm of CH-CH of cyclohexanone. The mass spectra (EI-MS) of compounds are also in agreement with their molecular formula. The elemental analysis results were within ± 0.4% of the theoretical values (Figure 2).
| Compound | Molecular Formula | Nitrogen content(estimated) | ESI-MS (m/z) | Molecular weight |
|---|---|---|---|---|
| 7a | C15H17N3 | 17.56 | 239.1 | 239.32 |
| 7b | C21H21N3 | 13.32 | 314.93 | 315.41 |
| 7c | C21H20N4O | 16.27 | 345.8 | 344.41 |
| 7d | C17H15N3 | 18.29 | 260.13 | 261.32 |
| 7e | C23H18N4O2 | 17.27 | 382.8 | 382.41 |
| 7f | C23H17N5O3 | 17.02 | 411.4 | 411.41 |
| 7g | C17H15N3 | 16.08 | 261.13 | 261.32 |
| 7h | C23H18N4O | 12.45 | 370.9 | 366.42 |
| 7i | C23H19N3 | 15.29 | 335.2 | 337.42 |
Table 3: Physical data, (Nitrogen content and Mass spectroscopy data) of synthesized compounds (7a-7i).
Pharmacological Activity
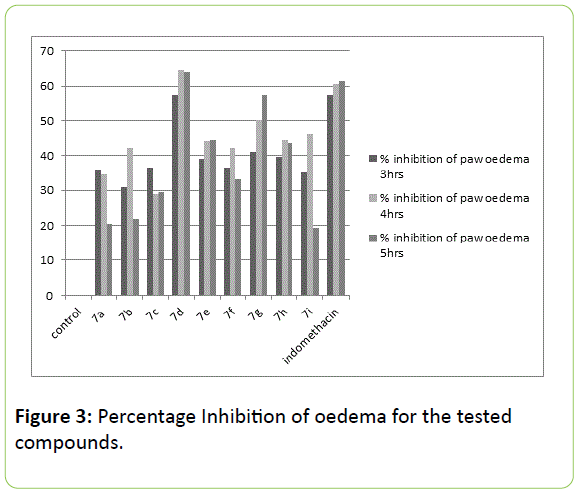
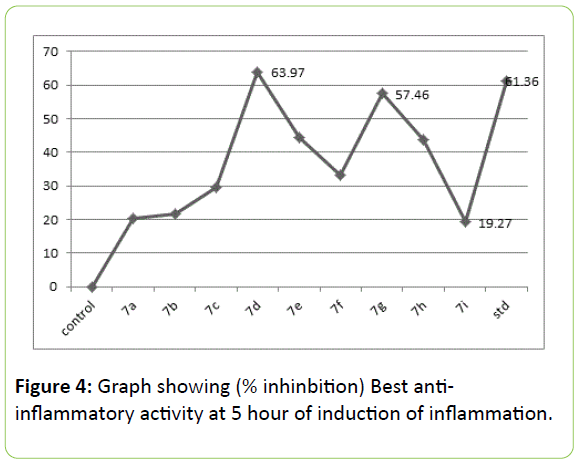
The activity revealed by novel synthesized 7 series in Table 4, that in vivo anti-inflammatory activity at the dose 100 mg/kg in rats by carrageenan induced paw oedema method. It is nonspecific inflammation resulting from a complex of diverse mediators; therefore oedema of this type is highly sensitive to NSAIDs. The entire investigated compounds exhibited moderate to good anti-inflammatory activity. In general the unsubstituted N1 of 2- pyrazoline shows better activity than substituted one. Although not significant inhibitory ratios for 7i, 7a, 7b and 7c were above 19% but below 30% for 5th hour of measurements. However the inhibitory effects of these compounds were above 30% at all the readings during 3rd and 4th hour of measurement, but reached significant values after 5 hour. It is found that 7d and 7g shows most significant inhibition which was very near to the standard indomethacin.
| Compounds | 1H-NMR spectra 400 MHz(CDCl3) |
|---|---|
| 7a | Indole proton11.50(1H,d,N-H); 7.17-7.89(4H,m,C-H Ar.); 7.15(1H,d,C-H) Cyclohexan proton1.27-1.31(9H,m,CH2); 3.27(1H,d,C-H) Pyrazoline proton7.91(1H,s,N-H); 4.63(1H,d,C-H) |
| 7b | Indole proton11.35(1H,d,N-H); 8.46-8.48(4H,m,C-H Ar.); 7.95(1H,d,C-H) Cyclohexan proton1.31-1.34(9H,m,CH2); 4.66(1H,d,C-H) Pyrazoline proton 4.66(1H,d,C-H); Proton of N-substituted Phenyl at pyrazoline 7.65-7.66(5H,m,C-H) |
| 7c | Indole proton11.40(1H,d,N-H); 7.44-7.49(4H,m,C-H Ar.); 7.90(1H,d,C-H) Cyclohexan proton1.29-1.33(9H,m,CH2); 3.27(1H,d,C-H) Pyrazoline proton 4.63(1H,d,C-H) Proton of N-substituted isonicotinyl at pyrazoline 8.47-8.50(4h,m,C-H) |
| 7d | Indole proton 11.39(1H,d,N-H); 7.41-7.47(4H,m,C-H Ar.); 7.90(1H,d,C-H) Proton at phenyl of pyrazoline 8.47-8.50 (5H,m,C-H) Pyrazoline proton7.91(1H,s,N-H); 4.63(1H,d,C-H) |
| 7e | Indole proton11.66(1H,d,N-H); 7.64-7.78(4H,m,C-H Ar.); 8.82(1H,d,C-H) Proton at phenyl of pyrazoline8.02-8.06(5H,m,C-H) Pyrazoline proton 4.66(1H,d,C-H) Proton of N-substituted Phenyl at pyrazoline 7.43-7.60(5H,m,C-H) |
| 7f | Indole proton11.49(1H,d,N-H); 8.00-8.02(4H,m,C-H Ar.); 8.77(1H,d,C-H) Proton at phenyl of pyrazoline 7.54-7.58(5H,m,C-H) Pyrazoline proton 1.19(2H,d,C-H); 3.25(1H,d,C-H) Proton of N-substituted isonicotinyl at pyrazoline 7.37-7.41(1H,d,N-C-H); 7.41-7.47(1H,d,C-CH) |
| 7g | Indole proton 11.51(1H,d,N-H); 7.78-7.83(4H,m,C-H Ar.); 8.05(1H,d,C-H) Proton at phenyl of pyrazoline 7.72-7.74(5H,m,C-H) Pyrazoline proton 1.37(2H,d,C-H); 4.03(1H,d,C-H);8.65(1H,d,N-H) |
| 7h | Indole proton 10.79(1H,d,N-H); 7.47-7.50(4H,m,C-H Ar.); 7.67(1H,d,C-H) Proton at phenyl of pyrazoline 7.37-7.41(5H,m,C-H) Pyrazoline proton 1.28(2H,d,C-H); 3.70(1H,d,C-H) Phenyl at N-substitution of pyrazoline 7.65-7.67(5H,m,C-H) |
| 7i | Indole proton11.50(1H,d,N-H); 7.72-7.75(4H,m,C-H Ar.); 7.89(1H,d,C-H) Proton at phenyl of pyrazoline 7.54-7.58(5H,m,C-H) Pyrazoline proton 1.28(2H,d,C-H); 3.98(1H,d,C-H) Proton of N-substituted isonicotinyl at pyrazoline 7.46-7.48(1H,d,N-C-H); 7.41-7.47(1H,d,C-CH) |
Table 4: 1H-NMR mass spectral data of synthesized compounds.
Relative to the structure the results clearly indicating that indolyl-pyrazoline with substituted phenyl at C4 and unsubstituted N1 orient the structure to the best activity. However unsubstituted N1, compounds like 7d and 7g given best inhibitory activity than all the compounds. Among all these p-nitro substituted phenyl at C3 position of pyrazolines, like 7d, 7e given promising inhibition, which was more than 44%, but the N1 substituted with isonicotinyl moiety shows poor inhibitions. The compounds which had fused cyclohexanone with pyrazoline moiety at C3 and C4 exhibits very poor results below 30%. Most prominent in the series were 7d, 7e, 7g, 7h and 7e; which had phenyl substitution at C3 of pyrazoline. The N1 having phenyl group (7e, 7f) had also given notable inhibitions.
Experimental Materials
All the reagent and solvent used in synthesis were of laboratory grade. All the solvents were dried and distilled before use. Melting point was determined in open capillaries and where uncorrected. Purity of the compounds was checked TLCusing Silica gel-G (Merck). IR (KBr, cm-1) of compounds are recorded on Shimadzu-2100s spectrometer and Perkin Elmer BX, UK spectrometer. The 1H-NMR was recorded in DMSO on Bruker 300 MHz spectrometer using TMS as internal standard. The mass spectrums were recorded on an API 3000 LC-MS. The completion of reaction was monitored by TLC using acetic acid: ethyl acetate as solvent system in 1:1 and the spots were visualized by exposure to iodine vapor or spraying dilute sulphuric acid.
Synthesis of (a) 3H-indene-1-carbaldehyde
The required indolylaldehyde were prepared by following Vielsmeier Haack complex method [11]. In a 100 ml round bottom flask, Vielsmeier Haack complex was prepared using phosphorus oxychloride (1 ml) and N, N-dimethylformamide (3.15 ml) and wasstirred using magnetic stirrer at 10-15°C. Indole (0.01 mol) was dissolved in minimum quantity of N, Ndimethylformamide and added with stirring at 10-15°C in Vielsmeier Haack complex. The reaction mixture was allowed to concentrate at 45°C and kept for 30 minutes and then poured into ice cold water (100 ml), a clear red colored solution was obtained, and 10% sodium hydroxide (20 ml) was added to get the precipitate [12]. This was boiled for 1 min and filtered, on cooling crystals were formed. Then these crystals were separated by filtration Color of was yellow. Recrystallization was done using aqueous DMF. Crystal was clear yellow in color. Melting point was found to be 192-194°C, Rf 0.52 (petroleum ether: ethyl acetate, 80:20), yield 76%. The formation of the aldehyde was confirmed by the Tollen's test and 2,4- dinitrophenylhydrazine test.
Synthesis of chalcones
Synthesis of (E)-2-((1H-indol-3-yl) methylene) cyclohexanone [C3]: Equimolar quantities (0.01 mol) of indole-3-aldehyde (a) and cyclohexanone were taken in 100 ml conical flask and dissolved in 20 ml of methanol to this (0.01 mol) of NaOH, which is dissolved in minimum quantity of water. The mixture was stirred on a magnetic stirrer. Reaction mixture was diluted with water and acidified with concentrated hydrochloric acid. The precipitated chalcones was filtered and recrystallized from absolute ethanol. The completion of reaction checked by TLC using solvent system of petroleum ether: ethyl acetate [70:30]. Color-bright yellow; % yield -81%; m.p.-189°C; Rf value-0.66.IR (KBr, v, cm-1): 1636.09(C=O); 1612.76(CH=CH); 1576.98(C=C).
Synthesis of (E)-3-(1H-indol-3-yl)-1-(4-nitrophenyl)prop-2-en-1- one [C4]
Equimolar quantities (0.01 mol) of indole-3-aldehyde (a) and para nitro phenylacetone were taken in 100 ml conical flask and Equimolar quantities (0.01 mol) of indole-3-aldehyde (a) and para nitro phenylacetone were taken in 100 ml conical flask and British Biomedical Bulletin Vol.5 No.1:298 2017 4 This article is available from: https://www.imedpub.com/british-biomedical-bulletin/ dissolved in 20 ml of methanol to this (0.01 mol) of NaOH, which is dissolved in minimum quantity of water. The mixture was stirred on a magnetic stirrer. Reaction mixture was diluted with water and acidified with concentrated hydrochloric acid. The precipitated chalcones was filtered and recrystallized from absolute ethanol. The completion of reaction checked by TLC using solvent system of petroleum ether: ethyl acetate [70:30]. Color-muddy yellow; % yield-87%; m.p.-198°C; Rf value-0.87.IR (KBr, v, cm-1): 1709.76(C=O); 1620(CH=CH); 1513.70(C=C).
Synthesis of (E)-3-(1H-indol-3-yl)-1-phenylprop-2-en-1-one [C5]
Equimolar quantities (0.01 mol) of indole-3-aldehyde (a) and phenylacetone were taken in 100 ml conical flask and dissolved in 20 ml of methanol to this (0.01 mol) of NaOH, which is dissolved in minimum quantity of water. The mixture was stirred on a magnetic stirrer. Reaction mixture was diluted with water and acidified with concentrated hydrochloric acid. The precipitated chalcones was filtered and recrystallized from absolute ethanol. The completion of reaction checked by TLC using solvent system of petroleum ether: ethyl acetate [70:30]. Color–brownish yellow; %yield- 78%; m.p.-190°C; Rf value-0.71. IR (KBr, v, cm-1):1658.78 1673.7(C=O); 1620(CH=CH); 1520(C=C).
Synthesis of pyrazoline derivatives
Synthesis of 3, 3a, 4, 5, 6, 7-hexahydro-3-(1H-indol-3-yl)-2- subtituted-indazole [P1]
(A) Synthesis of 3, 3a, 4, 5, 6, 7-hexahydro-3-(1H-indol-3- yl)-2H-indazole (7a): Synthesis of 7a carried out using solution of synthesized chalcone [C3] (0.01 mol) and hydrazine hydrate (0.01 mol) ,taken in mixture of ethanol (20 ml) and glacial acetic acid (60 ml),the mixture was refluxed for 4 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. Color-pale yellow; % yield -76%; m.p.-320°C.
(B) Synthesis of 3, 3a, 4, 5, 6, 7-hexahydro-3-(1H-indol-3- yl)-2-phenyl-2H-indazole (7b): Synthesis of 7b was done by using synthesized chalcone [C3] (0.01 mol) and phenylhydrazine (0.01 mol) ,taken in mixture of ethanol (20 ml) and glacial acetic acid (60 ml), the mixture was refluxed for 8 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. The completion of reaction and product purity was checked by TLC. Color-reddish brown; % yield-66%; m.p.-148°C.
(C) Synthesis of (3, 3a, 4, 5, 6, 7-hexahydro-3-(1H-indol-3- yl)indazol-2-yl)(pyridin-4-yl)methanone (7c): The 0.01 mol of synthesized chalcone C3 was dissolved in ethanol in a round bottom flask and 0.01 mol of isonicotinyl hydrazide was added on it. Glacial acetic acid (60 ml) was also added and refluxed for 10 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. C The completion of reaction and the purity of product was checked by TLC. The completion of reaction and product purity was checked by TLC. Color-muddy yellow; % yield-54%; m.p.-122°C.
Synthesis of 3-(4, 5-dihydro-3-(4-nitrophenyl)-substitutedpyrazol- 5-yl)-1H-indole [P2]
(D) Synthesis of 3-(4,5-dihydro-3-(4-nitrophenyl)-1Hpyrazol- 5-yl)-1H-indole (7d): The 0.01 mol of synthesized chalcone C4 was dissolved in ethanol in a round bottom flask and 0.01 mol of hydrazine hydrate was added on it. Glacial acetic acid (60 ml) was also added and refluxed for 6 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. C The completion of reaction and the purity of product was checked by TLC. The completion of reaction and product purity was checked by TLC. Color- brown; % yield-66%; m.p.-197°C.
(E) 3-(4,5-dihydro-3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-5- yl)-1H-indole (7e): The 0.01 mol of synthesized chalcone C4 was dissolved in ethanol in a round bottom flask and 0.01 mol of phenyl hydrazine was added on it. Glacial acetic acid (60 ml) was also added and refluxed for 8 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. C The completion of reaction and the purity of product was checked by TLC. The completion of reaction and product purity was checked by TLC. Color-orangish yellow; % yield -54%; m.p.-219°C.
(F) (4,5-dihydro-5-(1H-indol-3-yl)-3-(4-nitrophenyl)pyrazol-1- yl)(pyridin-4-yl)methanone(7f): The 0.01 mol of synthesized chalcone C4 was dissolved in ethanol in a round bottom flask and 0.01 mol of isonicotinyl hydrazide was added on it. Glacial acetic acid (60 ml) was also added and refluxed for 10 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. C The completion of reaction and the purity of product was checked by TLC. The completion of reaction and product purity was checked by TLC. Color-yellowish brown; %yield -76%; m.p.-320°C.
3-(4, 5-dihydro-3-phenyl-1substituted-pyrazol-5-yl)-1H-indole (P3)
(G) 3-(4,5-dihydro-3-phenyl-1H-pyrazol-5-yl)-1H-indole (7g): Synthesis of 7g carried out using solution of synthesized chalcone [C5] (0.01 mol) and hydrazine hydrate (0.01 mol), taken in mixture of ethanol (20 ml) and glacial acetic acid (60 ml), the mixture was refluxed for 6 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. Color-yellow; %yield-68%; m.p.-172°C.
(H) 3-(4,5-dihydro-1,3-diphenyl-1H-pyrazol-5-yl)-1H-indole (7h): Synthesis of 7h was done by using synthesized chalcone [C5] (0.01 mol) and phenylhydrazine (0.01 mol), taken in mixture of ethanol (20 ml) and glacial acetic acid (60 ml), the mixture was refluxed for 8 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. The completion of reaction and product purity was checked by TLC. Color-brownish yellow; % yield-72%; m.p.-254°C.
(I) (4,5-dihydro-5-(1H-indol-3-yl)-3-phenylpyrazol-1-yl) (pyridin-4-yl)methanone (7i): The 0.01 mol of synthesized chalcone C5 was dissolved in ethanol in a round bottom flask and 0.01 mol of isonicotinyl hydrazide was added on it. Glacial acetic acid (60 ml) was also added and refluxed for 9 hour and kept overnight. The synthesized product was poured into ice water and the crude product was filtered. The filtered derivative is crystallized with Acetone. C The completion of reaction and the purity of product was checked by TLC [13-15]. The completion of reaction and product purity was checked by TLC. Color-brown; % yield-64%; m.p.-210°C.
Anti-inflammatory activity screening
Anti-inflammatory activity screening for the prepared compound was determined in vivo by the acute Carrageenan induced paw oedema standard method in rats.
Procedure: Preparation of Sodium CMC suspension: 1 g of Sodium CMC was triturated in 100 ml of distilled water to give the required stock suspension of Sodium CMC. This stock suspension was used for suspending all the test compounds as well as the standard drug.
Preparation of Carrageenan suspension: 100 mg of Carrageenan powder was sprinkled in 10 ml of saline and set aside for 1 hour. Then it was mixed with the help of a magnetic stirrer to get a homogenous 1% suspension of carrageenan.
Experimental procedure: The synthesized compounds 7a-7i were evaluated for their anti-inflammatory activity using Carrageenan induced paw oedema method described by Winter et al. [16] Albino rats of either sex, weighing between 150-200 mg, divided into 11 groups of six animals each. All these groups were kept for fasting overnight and only allowed water ad libitum 0.05 ml of 1% carrageenan suspension was slowly injected subcutaneously into the subplantar region of the left hind paw to produce inflammation in all the groups. Groups III to XI were treated with samples with the dose 100 mg/kg. Group I used as carrageenan treated control was given only 1% sodium CMC gel (1 ml/kg) whereas group II received indomethacin (100 mg/kg). All these doses were administered orally and the induced paw oedema in each group was measured to assess the anti-inflammatory activity [17,18]. Paw volumes were measured volumetrically after every 1, 2, 3, 4 and 5 hours of induction with plethysmogometer apparatus and compared. The antiinflammatory activity was expressed as a percentage inhibition of oedema weight in treated animals in comparison with the control group [13-15] (Table 5).
| Group | Paw volume after induction(% inhibition) | |||||
|---|---|---|---|---|---|---|
| 3 hour | % In. | 4hour | % In. | 5hour | % In. | |
| control | 0.66 | 0 | 0.86 | 0 | 0.92 | 0 |
| 7a | 0.42 ± 0.12 | 36.01 | 0.56 ± 0.23 | 34.61 | 0.73 ± 0.20 | 20.48 |
| 7b | 0.46 ± 0.13 | 31 | 0.50 ± 0.21 | 42.3 | 0.72 ± 0.21 | 21.79 |
| 7c | 0.42 ± 0.14 | 36.55 | 0.61 ± 0.23 | 28.84 | 0.64 ± 0.21 | 29.62 |
| 7d | 0.28 ± 0.13 | 57.4 | 0.30 ± 0.20 | 64.62 | 0.33 ± 0.22 | 63.97 |
| 7e | 0.40 ± 0.14 | 39.1 | 0.48 ± 0.21 | 44.33 | 0.51 ± 0.23 | 44.45 |
| 7f | 0.42 ± 0.12 | 36.7 | 0.50 ± 0.20 | 42.3 | 0.61 ± 0.21 | 33.13 |
| 7g | 0.39 ± 0.14 | 41.2 | 0.43 ± 0.22 | 50.03 | 0.39 ± 0.22 | 57.46 |
| 7h | 0.40 ± 0.15 | 39.82 | 0.48 ± 0.23 | 44.61 | 0.51 ± 0.24 | 43.74 |
| 7i | 0.43 ± 0.14 | 35.2 | 0.46 ± 0.21 | 46.15 | 0.74 ± 0.21 | 19.27 |
| std | 0.28 ± 0.15 | 57.5 | 0.34 ± 0.19 | 60.51 | 0.35 ± 0.20 | 61.36 |
Table 5: Anti-inflammatory effect of the tested compounds using Paw oedema Carageenan induced method and its percentage inhibition at a dose 100 mg/kg body weight.
Percentage inhibition of oedema=V c-V t/V c*100 (Figures 3 and 4)
Where, Vc and Vt are the volume of oedema for the control and drug-treated animal groups, respectively.
Acknowledgement
The authors are thankful to Dr. Amit Roy, Principal, Dr. Ram Sahu, HOD, department of Pharmacology, Columbia college of Pharmaceutical Sciences, Raipur (Chhattisgarh) for providing laboratory facilities to conduct pharmacological activities.
References
- Caron S, Vazquez E, Stevans RW, Nakao K, Koike H, et al. (2003) Efficient synthesis of [6-Chloro-2-(4-chlorobenzoyl)-1H-indol-3-yl]-acetic acid, a novel COX-2 inhibitor. J Org Chem 68: 4104-4107.
- Maayan S, Ohad N, Solima K (2005) Chalcones as potent tyrosinase inhibitors: the importance of a 2, 4-substituted resorcinol moiety; Bioorg. Med Chem 13: 433.
- Nowakowska (2007) A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem 42: 125.
- Go ML, Wu X, Liu XL (2005) Chalcones: an update on cytotoxic and chemoprotective properties; current medicinal chemistry 12: 483.
- Ali MA, Shaharyar M (2007) Discovery of novel phenoxyacetic acid derivatives as antimycobacterial agents. Bioorg Med Chem 15: 1896–1902.
- Kohler HM, Chadwell H (1967) In Organic syntheses; Gillman, H., Blatt, A. H., Eds.; Wiley: New York (Coll. Vol. I) p: 78.
- Shaharyar M, Siddiqui AA, Ali MA (2006) Synthesis and evaluation of phenoxy acetic acid derivatives as a anti-mycobacterial agents. Bioorg Med Chem Lett 16: 3947–3949.
- Raja PP, Riyazulah MS, Kumar VS (2010) Synthesis and biological valuation of some chalcone derivatives. Int J Chem Tech Res 2: 208-215.
- Bekhit AA, Ashour HMA, Ghany YSA, El-DinA Bekhit A, Baraka A (2008) Synthesis and biological evaluation of some thiazolyl and thiadiazolyl derivatives of 1H-pyrazole as anti-inflammatory antimicrobial. Eur J Med Chem 43: 456-463.
- Bansal E, Srivastava VK, Kumar A (2001) Synthesis and anti-inflammatory activity of 1-acetyl-5-substitute daryl-3-(β-aminonaphthyl)-2-pyrazolines and β-(substitute daminoethyl) amidonaphthalenes. Eur J MedChem 36: 81.
- Manna F, Chimenti F, Bolasco A, Cenicola M L, D’Amico M, et al. (1992) Synthesis and in vitro selective anti-Helicobacter pylori activity of pyrazoline derivatives. Eur J Med Chem 27: 633.
- Mielens ZE, Drobeck HP, Rozitis J, Sansone V (1969) Effect of hepatic injury on adjuvant arthritis. J Toxicol Appl Pharmacol 14: 293.
- Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc soc Exp Biol Med 111: 544-547.
- Sahu SK, Banerjee M, Samantray A, Behera C, Azam MA (2008) Synthesis, analgesic, anti-inflammatory and antimicrobial activities of some novel pyrazoline derivatives. Tropical J Pharm Research 7: 961-968.
- Block John H (2003) “Wilson & Gisvold’s text book of organic medicinal and pharmaceutical chemistry”, 11th Edition 1-3.
- David A Williams, Thomas L Lemke (2008) Foye’s principles of medicinal chemistry. Sixth edition.
- Remington, the science and practice of pharmacy, 20th edition, 2008 2: 458.
- Singh H, Kapoor VK (2003) Medicinal and pharmaceutical chemistry II, 29.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences