New Findings for the Zero Waste Technologies; Demodex Folliculorum
Engineering Faculty, Energy Systems Engineering Department, Turkey
- *Corresponding Author:
- Cemil Koyunoğlu
Engineering Faculty, Energy Systems Engineering Department, Turkey
Tel: +90 226 815 5323
Email: ckoyunoglu@itu.edu.tr
Received Date: November 12, 2018; Accepted Date: December 17, 2018; Published Date: December 24, 2018
Citation: Koyunoğlu C (2018) New Findings for the Zero Waste Technologies; Demodex Folliculorum. J Waste Recycl Vol.3 No.2:12
Abstract
There are two types of worms that live on the face: Demodex folliculorum and Demodex brevis. The first one lives in our pores and hair follicles, and the second in fat glands in the lower layers of the skin. There are more pores and oil glands on my face than other parts of our body. Experts believe that there are hundreds of pieces of these grubs. But what they feed on is not yet known. Some claim that they feed on dead skin, while others feed on bacteria on the face. It is thought that they come back from the pores at night and come back again. Since Demodex does not have anus, it is believed that they kept the waste materials in their bodies until they died and they dissolved with their deaths. In this short review study, It will be discussed the determination of new waste evaluation technology by using these microorganisms.
Keywords
Demodex families; Zero waste; Waste recycling; Biotechnology for biomass
Introduction
It has been reported that mites are found in every habitat, from polar regions to deserts, in every period between free life and parasitic life. In addition to free living mites in nature, there are living things living in various plants and humans as parasites [1-5].
Demodex has defined over 65 species in the world. 10 of the Demodex species found in humans, horses, sheep, cattle, goats, pigs and cats have been reported as pathogenic parasites [1-5].
D. folliculorum is the first defined hair follicle mite. In recent years, the Demodexidae family of two species of the genus Demodex has been accepted to be located in the human body. These species are D. folliculorum and D. brevis. Human beings can be found in various places, especially in the face, nose, eyelash, ear and genital area of the face. They prefer hair follicles and skin glandular secretions [1-5].
The long D. folliculorum, short, is named D. brevis. D. folliculorum was discovered by Henle and Berger in 1841 and was first described by Simon in 1842. D. brevis first distinguished by Akbulutova as a subspecies of D. folliculorum in 1963. In 1972, Desch and Nutting D. brevis were considered as a separate species living on human skin by their laboratory studies. Lawrence first reported the association of Demodicosis with the facial diseases in 1916. In 1919, Hirst made the taxonomic classification of D. folliculorum, and in 1933 Fuss reported the moth's life cycle. In 1934, Stokes and Beerman published the pathogenesis of D. folliculorum and in particular the relationship between rosacea and mites [1,2]. The historical development of Demodex which is parasitic in humans is shown in Tables 1 and 2. There are various types of demodex; Demodex folliculorum, Demodex brevis, Demodex bovis, Demodex cavis, Demodex caprae, Demodex erminae, Demodex cati, Demodex equi, Demodex ovis, Demodex phylloides [1].
Table 1: The demodex chronology.
| Scientific contributions | Researcher | Year | References |
|---|---|---|---|
| Demodex in humans | Henle and Berger | 1841 | [1,2] |
| Type identification (Acarus folliculorum) | Simon | 1842 | [3,4] |
| Renaming the genus Demodex | Owen | 1843 | [1,3] |
| Lesion and flows | Raehlmann | 1899 | [2,5] |
| Carrying sickness | Borrel | 1908 | [2,3] |
| Mite biology and Demodicidosis | Gmeiner | 1908 | [3,5] |
| The relationship between bacteria and bacteria | Gmeiner | 1909 | [3,4] |
| Findings of the disease | Lawrance | 1916 | [1,4] |
| Demodex's drawing | Hirst | 1919 | [2,5] |
| Mites and cancer | Chamber and Somerset | 1925 | [3,4] |
| Incidence according to skin conditions | Fuss | 1933 | [2,3] |
| The relationship between mite and disease | Beerman and Stokes | 1934 | [3,5] |
| Behavior and biology | Spickett | 1961 | [3,4] |
| Host-parasite relationship | Nutting | 1965 | [1,5] |
| Two sub-types of Demodex | Akbulatova | 1966 | [2,3] |
| Characteristics of two species in humans | Desch and Nutting | 1972 | [2,5] |
| Pathogenesis | Grosshans et al. | 1974 | [3,4] |
| D. folliculorum anotomy and morphology (Simon) | Desch and Nutting | 1976 | [1,3] |
Table 2: Demodexes’s taxonomy.
| Kingdom | Animalia |
| Phylum | Arthropoda |
| Subphylum | Chelicerata |
| Class | Arachnida |
| Subclass | Acari |
| Order | Trombidiformes |
| Suborders | Prostigmata |
| Family | Demodicidae |
| Genus | Demodex |
Morphology
Body, gnathosoma, podosoma and opistosoma consists of three parts. The head and chest is also called as propodosoma. However, as in all mites, all of their bodies are single parts under the name of idiosome[1-3].
The posterior part of the body, ie the abdominal region (opistosoma), is long in the form of a cigar and is transverse. The mouth parts are surrounded by a cuticular sheath (epistoma). The shrink is short, thick and scissors. The pallets are large and two-part. The hypostoma is small and hole-shaped. No eyes. Legs are 4 pairs, short, thick and three knuckles. The last parts of the legs have a suction cup and two claws [1-4].
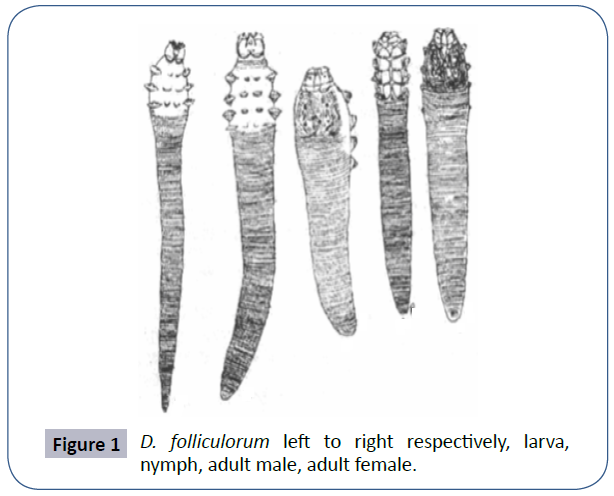
D. folliculorum, the largest member of the genus, male and female 280 ğum lengths are generally the same size, but the length of the 440-μm female mites are reported to be encountered. The body parts of male and female D. folliculorum and the dimensions of eggs, larvae and nymphs are detailed in Table 3 [1,3,5].
Table 3. The dimensions of the body parts of D. folliculorum.
| Name of body parts | Length/Width | Male (µm) | Female (µm) | Non-adults |
|---|---|---|---|---|
| Gnathosoma | Length | 19.5 ± 0.9 | 21.3 ± 0.7 | |
| Gnathosoma | Width | 24.0 ± 0.9 | 26.5 ± 1.0 | |
| Podosama | Length | 67.7 ± 2.8 | 75.0 ± 2.8 | |
| Podosama | Width | 45.0 ± 2.0 | 51.9 ± 3.0 | |
| Opisthosoma | Length | 191.0 ± 49.4 | 197.9 ± 55.7 | |
| Opisthosoma | Width | 32.7 ± 1.7 | 40.3 ± 3.3 | |
| Total length | - | 279.7 ± 52.0 | 294.0 ± 58.1 | |
| Penis | 24.2 ± 0.9 | - | ||
| Vulva | 8.5 ± 0.6 | |||
| Proctodeum | Length | 13.3 ± 1.5 | ||
| Opistosoma-proctodeal zone | 50.3 ± 13.3 | |||
| Egg | Length | 104.7 ± 6.3 | ||
| Egg | Width | 41.8 ± 1.8 | ||
| Egg | Depth | 37.6 ± 2.6 | ||
| Larva | Length | 282.7 ± 45.1 | ||
| Larva | Width | 33.5 ± 2.6 | ||
| Protonimf | Length | 364.9 ± 36.4 | ||
| Protonimf | Width | 36.3 ± 4.4 | ||
| Nimf | Length | 392.0 ± 46.8 | ||
| Nimf | Width | 41.7 ± 6.3 |
In the male gnathosoma narrows to the front and the horseshoeshaped pharyngeal bubble opens to the back. The subgnathosomal seta extends from the small farengeal bubble. The subrakoksal projections are seen as hooked with smaller lateral extensions and larger medial extensions [1,2,5].
There are four pairs of legs, each with a pair of tarsal paws, throughout the Podosoma. The claws are divided into two distal and large spurs extending to the posterior of the limbs 1 and 2 are noteworthy. Epimeral plaques are fused to the midline, the genital opening is dorsal and there is a narrow slit in the small triangular protuberance at the level of the second limbs. The dorsal podosomal seta was located closer to the anterior pair at the posterior double genital slit level. It is known that opistosome which has a posteriorly placed penis with a length of 24.2 μm does not have an anus with transverse striae and proctodeum [1,3,4].
Tooth; gnathosoma related structures, such as men, but the average height and width are reported to be 2 μm more. Leg and epimeral plaques such as men and dorsal podosomal cecans in the form of drops, the rear pair is more distinct from the anterior pair. The vulva has a simple longitudinal slit and a length of 8.5 μm. The opisthosomal transverse striae and the terminus are in the form of a male proctodeum and are 13.3 μm long1. The larval, nymph and adult periods of D. folliculorum are shown in Figure 1.
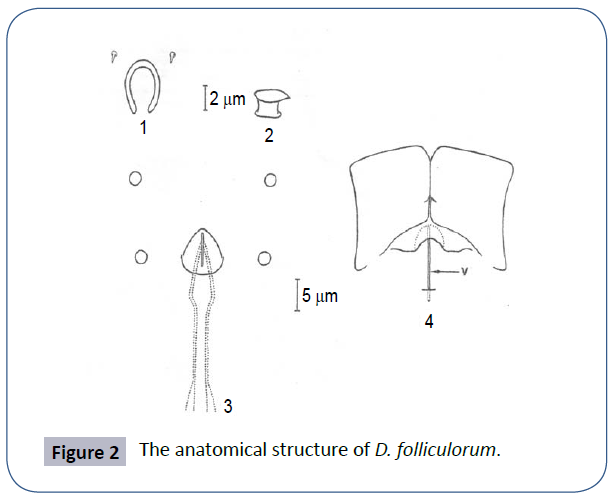
Figure 2 for a D. folliculorum’s; 1-Pharyngeal round and subgnathsomal hole 2-Left supracocytic bulge 3-Podosomal tubercles showing male genital organs-genital holes and penis 4-Epimeral plate showing the relationship with the female genital organs [2].
The eggs are 104.7 μm in the shape of an arrowhead and the width is reported to be 41.8 μm in the middle bulge and this bubble extends toward the ventral [1].
The larvae are 282.7 μm long and 33.5 μm between the 2nd and 3rd legs of the maximum width. It was observed that the supracoxal spines with no subgnathosomal cecans were laterally dislocated like simple hooks, the legs were two-segmented, and there was a single triple-forked paw and protruding dorso-lateral spurs on the anterior face of each tarsus [1].
The fine worm nymph has a length of 392 μm and the widest part of the 3rd leg is 41.7 μm. Nimfin gnathosoma and related structures are similar to larvae but have a longer and broader structure. It has been reported that there are mild transverse striae on the part of the body behind the fourth leg pair of 4 pairs of epimeral scales such as the larva [1].
Possible İnteractions for Eco- Biotechnological Processes
Demodex-mediated multitrophic level microbial cycle interactions, bioremediation, wastewater treatment, stimulation of plant growth, and directing of economic and agro-biotechnological processes such as the functioning of the ecosystem may be related to the fact that enzymes are too strong to release nutrients until death. These microbial interactions need to be investigated to what extent the context dependence is in performing biotechnological and ecosystem processes. It is important to investigate the above effects of these species under both theory-oriented and experimental application research. It may promote the understanding of eco-evolutionary processes that underlie microbial interactions, behavior, and even the functioning of microbial interactions for the successful development of biotechnologies advancing together with the discovery of enzymes used by Demodexes. This can also be a great way to test the validity or limits of ecology theory to manage various microbial sources in an age that changes the loss of microbial diversity caused by microbiological niches, multitrophic interactions and changes in climate and land use [6,7].
Methods to Use Demodex to the Waste Remediation
The microbial improvement of organic pollutants, which are produced directly or indirectly in nature and industry, generally are utilized as a natural processes by microorganisms. Contrary to manipulate these processes to the industrial pathways, issues such as the cost and unfavorable use should be addressed.
The following questions should be sought for the transfer of Demodex species to waste reduction technologies.
1. What types are there?
2. What type can demodex do?
3. What kind of environmental problem can be the solution?
The first two questions help in predicting the degradation potential and ability of the natural microbial community, while the third question provides valuable information on the preferred environmental conditions for better growth and activity of microbial functional species, which is important to increase the bioremediation efficiency. With the rapid development of molecular technologies, the identification and characterization of the microbial species and the community is easier, from the classical RNA sequence to highly efficient metagenomic sequencing. However, it is difficult to identify basic organisms and to understand their ecological interactions and reactions. Most information in the third question is derived from the cultivation of microbial isolates or enriched consortia in manipulated bioremediation microcosmoses. Therefore, it is necessary to determine for the biodiversity technology to isolate and identify microbial candidates for cultivation techniques demodex [8-10].
In the literature review there has been no varies data. In this case, the lacking of the information is addressed to give some examples for only referring to manipulate microorganisms to such a process. For example, Clark et al studied (2014) manipulating microbial process to tied for metal health of human [11]. Brune stated (2014) the manipulating of the termite digestive enzymes can be the best approach for evaluating a bioreactor process for the industry [12]. The reference study was expressed by Sernetz et al 1985 that highlighted the method how microorganisms can be manipulate which are presented below with,
Expressing;
- In terms of heterogeneous catalysis was expressed due to the kinetics of the process for the enzyme reactors
- According to fractal geometry and the structure of the organism
- Transport processes due to nonlinear relationships
- Structural property of the organisms turbulence as a kinetic approach.
- The law of reduction and limits of validity in the key metabolism [13].
Conclusion
Today, the interest in biofuels and chemicals, which have technological outputs, and their dependence on petroleumbased fuels and chemicals, which have technological outputs from the Demodex family, can be attributed to the behavior of these animals in 100% of their life without feeding them to the outside environment. Like waste treatment, it can be associated with a dramatic increase in demand over the past few years due to the potential for reduction. Since the whole process is naturally carbon-neutral and can be used for environmental cleaning applications, the demodex family can be considered the most suitable and sustainable model source for producing green energy, such as other living species (eg, algae, bacteria, and various microorganisms). In general, a physiological study of the folliculorum member of the demodex family has been carried out. In the theoretical, scientific and experimental studies necessary to present these data as a zero waste generation model, an energy production or chemical production of demodex folliculorum as an efficient and economically suitable zero waste production model focus on how it can be used in the facility. For this reason, an efficient production model using the Demodex family can only be created if it is integrated in an industry. For this reason, in order to understand the concept of demodex family zero waste generation model, there is a need to establish a correct connection between the various input and output flows of a chemical raw material product, for example, and the services to be provided by the participating industries. Furthermore, in the zero waste production model of the demodex family, which is highlighted in this article, it is possible to adapt the mechanism of the digestive system to the whole energy and non-energy production of the industrial process. It can be obtained by understanding not only the digestion mechanism but also the use of enzymes as a raw material sources from the participant industry (such as energy, chemical intermediate or end product step) evaluating for the main aim of this study with purpose of contributing to science.
References
- Anofel, Botterel F, Darde ML, Debourgogne A, Delhaes L, et al. (2017) In Parasitologie et Mycologie Médicales - Guide des Analyses et des Pratiques Diagnostiques Paris pp: 383-385.
- Oconnor BM, Resh VH, Carde RT (2009) In Encyclopedia of Insects (Second Edition) pp : 643-649.
- Norn MS (1970) Demodex Folliculorum: Incidence and Possible Pathogenic Role in the Human Eyelid.
- Zouboulis CC, Katsambas AD, Kligman AM (2014) Pathogenesis and Treatment of Acne and Rosacea
- Plewig G, Kligman AM, Jansen T (2000) Pathogenesis and Treatment Acne and Rosacea.
- Schommer NN, Gallo RL (2013) Structure and function of the human skin microbiome. Trends in Microbiology 21: 660-668.
- Barash Y, Perrin Y (2009) Bioremediation: Wastewater treatment in diesel power stations. Filtration & Separation 46: 28-31.
- Wang Y, Tam NFY (2019) Microbial Remediation of Organic Pollutants. In World Seas: an Environmental Evaluation (Second Edition) pp: 283-303.
- . Meng F, Yang A, Zhang G, Wang H (2018) Effects of dissolved oxygen on key enzyme activities during photosynthetic bacteria wastewater treatment.
- Aksu Z (2005) Application of biosorption for the removal of organic pollutants 40: 997-1026.
- 11. Anisman H, Hayley S, Kusnecov A (2018) In The Immune System and Mental Health pp :77-102.
- Brune A (2014) Symbiotic digestion of lignocellulose in termite guts 12:168
- . Sernetz M, Gelléri B, Hofmann J (1985) The organism as bioreactor. Interpretation of the reduction law of metabolism in terms of heterogeneous catalysis and fractal structure 117: 209-230.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences