ISSN : 2348-9502
American Journal of Ethnomedicine
Neuroprotective Activity of Crataeva nurvala Buch-Ham Stem Bark against Scopolamine- Induced Cognitive Impairment via Antioxidative Activities in Rats
1Department of Pharmacognosy, NGSM Institute of Pharmaceutical Sciences, Deralakatte, Mangalore – 574 018, Karnataka, India
2Department of Pharmacology, NGSM Institute of Pharmaceutical Sciences, Deralakatte, Mangalore – 574 018, Karnataka, India
Abstract
Background: Crataeva nurvala Buch-Ham is a well explored traditional Indian medicinal plant, routinely used as folkloric medicine to treat various ailments in particular urolithiasis and neurodegenerative disorders associated with cognitive dysfunction.
Objectives: The present study was undertaken with the objective to investigate the antioxidant and anti-dementia activities of Crataeva nurvala stem bark extract.
Methods: The antioxidant and anti-dementia activities of Crataeva nurvala extract was carried out using superoxide dismutase (SOD), catalase (CAT) specific activities, total content of reduced glutathione (GSH), malondialdehyde (MDA) level (lipid peroxidation) and Hebb’s-William maze assays respectively in Wistar rats subjected to scopolamine (1 mg/kg i.p.) induced dementia.
Results: Sub-acute exposures (daily, for 7 successive days) of Crataeva nurvala extract (100, 200 and 400 mg/kg) significantly increased antioxidant enzyme activities (SOD and CAT), total content of reduced GSH and reduced lipid peroxidation (MDA level) in rat brain homogenates in dose dependent manner, suggesting the antioxidant potential. Moreover, in Hebb’s-William maze test, Crataeva nurvala extract showed significant improvement in transfer latency, suggesting anti-dementia activity.
Conclusion: Taken together, our results suggest combination of antioxidant and anti-dementia activities may be associated with the neuroprotective effect of Crataeva nurvala extract against scopolamine-induced oxidative stress in rat brain.
Keywords
Crataeva nurvala, Neuroprotection, Antioxidant, Anti-dementia.
INTRODUCTION
Alzheimer disease (AD) is an irreversible age-related form of dementia that slowly erodes the brain. It slowly robs the person memory and cognitive skills and causes changes in personality and behavior [1]. The progression of the erosion is accompanied with neurodegeneration and characterized by abnormal amyloid β protein plaques, neurofibrillary tangles of tau protein and loss of cholinergic cells, and thereby decline in cholinergic neurotransmission [2,3].
Scopolamine, a muscarinic antagonist, had been widely used to study cognitive deficits in experimental animals [4]. Recently, it was reported that memory impairment induced by scopolamine in rats was associated with increased oxidative stress in brain [5]. The damaging effect of the oxidative stress was most vulnerable in AD [6]. Moreover, increased MDA level, one of the most harmful effects of oxidative stress had been considered as important biomarker for in-vivo lipid peroxidation [7,8]., previous studies indicated oxidative damage as an important early-stage factor in neurodegenerative disorders associated with cognitive dysfunctions [9]. Hence, it may be possible to delay the progression of neurodegeneration in AD with antioxidants.
Several synthetic drugs are used clinically to improve cognitive function and bolstering body’s defense against free radicals and thereby provide protection against AD. But the resulting side-effects associated with these synthetic drugs have made their utility limited [10]. Therefore, it is worthwhile to explore the efficacy of traditional medicines to treat various neuropharmacological disorders as they are considered to be safe and economical [11].
Crataeva nurvala (C. nurvala) Buch- Ham (Family: Capparidaceae) commonly known as Varuna, a well explored traditional Indian medicinal plant of Western ghats routinely used as folkloric medicine against urolithiasis and neurological disorders [12,13]. It is a medium sized branched deciduous plant distributed throughout the river banks of southern India and other tropical, sub-tropical countries of the world, wild or cultivated [14]. Hence, the present study was designed to evaluate the neuroprotective potentiality of C. nurvala through in-vivo neuropharmacological assay and biochemical estimations of several antioxidant parameters in Wistar rat model.
MATERIALS AND METHODS
Extraction and phytochemical analysis
The stem bark of C. nurvala was collected from the stream sides of Western ghat, India (voucher specimen no. NGSMIPS/Hb-04/2011), extracted by cold maceration with ethanol and concentrated through rotary flash evaporatr [15]. The yield was found to be 17 % w/w. A portion of concentrated ethanolic extract was subjected to different qualitative phytochemical screening to identify the presence of various phytoconstituents as described by Harborne [16].
Drugs and chemicals
Scopolamine hydrobromide, piracetam, sodium carboxy methyl cellulose (Sodium-CMC), Acetylthiocholine iodide, 5, 5′-Dithiobis (2-nitrobenzoic acid) (DTNB) were collected from Sigma-Aldrich, Bangalore, India. All the toxic, standard and test drugs (suspended in 0.6% w/v of sodium-CMC solution) were administered in the morning session i.e. 9 AM- 10 AM on each day.
Acute toxicity study
The acute toxicity of ethanolic extract of C. nurvala stem bark was evaluated in female Wistar rats as per OECD guidelines 425 (Up and Down Procedure). A fixed dosage study was adopted for acute toxicity study where the limit dose is 2000 mg/kg body weight of test animal. Clinical signs of toxicity, body weight changes, cage side parameters and mortality rate were observed every hour for the first 6 hours and every day for 7 days [17].
Experimental animals
Healthy young Wistar rats of either sex aged between 8-12 week old and weighing between 150-200 g obtained from the institutional animal house were used for in-vivo study. Prior to experiment animals were housed in a room under standard environmental condition for 7 days (25°C; 50-70% relative humidity; 12/12 h light/dark cycle) and fed with standard rat pellet and water ad libitum [18]. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) under the IAEC no.: KSHEMA/AEC/34/2011.
Experimental design
Sixty healthy Wistar rats of either sex were selected randomly (n=60) and divided into ten groups of six animals each to evaluate their responses on exteroceptive behavior models. The groupings of animals were summarized below.
Group I (Normal control): Vehicle (0.6% w/v sodium CMC) p.o.
Group II (Negative control): Scopolamine (1 mg/kg i.p.)
Group III (Positive control): Piracetam (50 mg/kg) p.o.
Group IV: C. nurvala ethanolic extract (100 mg/kg) p.o.
Group V: C. nurvala ethanolic extract (200 mg/kg) p.o.
Group VI: C. nurvala ethanolic extract (400 mg/kg) p.o.
Group VII: Piracetam (50 mg/kg) p.o. + Scopolamine (1 mg/kg i.p.)
Group VIII: C. nurvala ethanolic extract (100 mg/kg) p.o. + Scopolamine (1 mg/kg i.p.)
Group IX: C. nurvala ethanolic extract (200 mg/kg) p.o. + Scopolamine (1 mg/kg i.p.)
Group X: C. nurvala ethanolic extract (400 mg/kg) p.o. + Scopolamine (1 mg/kg i.p.)
The dosing for all groups were done for a period of 7 days; after 45 minutes of administration of the last dose on 7th day, amnesia was induced by administration of scopolamine (1 mg/kg i.p.) to Group II, VII, VIII, IX and X respectively. The negative control group (Group II) received just one dose of scopolamine (1 mg/kg i.p.) on 7th day and 45 minutes after the administration of scopolamine, trials were taken on Hebb’s- William maze and the retention was observed after 24 hours of the first exposure i.e. on day 8 [19].
Exteroceptive behavioral model
Hebb’s-William maze
The Hebb’s-William Maze is an incentive based exteroceptive behavioral model useful for measuring spatial and working memory of rats [20]. A typical Hebb’s-William maze (75x75x 25 cm) is a rectangular wooden box divided into three components viz. animal chamber (start box), which is attached to the middle chamber (exploratory area) and a reward chamber at the other end of the maze in which the reward (food) is kept.
The experiment was conducted under zero watt red coloured bulb to minimize the nocturnal cycle disturbances. During study, the animals were exposed to food and water ad libitum only for 1 h after the maze exposure to ensure motivation towards reward chamber.
Each rat was placed in animal chamber and the door of start box was closed immediately after the animal moved into the exploratory area to prevent its back entry. Time taken (in seconds) by the animals to reach reward chamber from start box (initial transfer latency, ITL) was noted for each animal. Each animal was allowed to explore the maze for additional 20 seconds. After experiment, the animals were returned to their home cages and transfer latency was recorded again after 24 hours of the first exposure (retention transfer latency, RTL). A fall in transfer latency on subsequent maze exposures was taken as an index of successful retention [21].
Estimation of antioxidant status
Collection of brain sample
Immediately after the experiment animals were sacrificed by cervical decapitation under light anesthesia and whole brain was carefully removed from the skull. The fresh whole brain was weighed, kept on ice bath, rinsed with ice-cold isotonic saline and homogenized (approximately 20 mg of tissue/ml of phosphate buffer (pH 8.0; 0.1M) in a Potter- Elvehjem homogenizer. The homogenate was centrifuged at 3000 rpm for 10 min and the resultant cloudy supernatant liquid was used for the estimation of antioxidant status [22].
Estimation of lipid peroxidation
Lipid peroxidation is an autocatalytic process, which is common consequence of cell death. Malondialdehyde (MDA), one of the end products of lipid peroxidation is formed during oxidative degeneration. Hence, to measure MDA, thiobarbituric acid (TBA) test was performed because of its higher sensitivity and simplicity in operation. During the process, MDA react with TBA and form a red colour complex which was extracted with n-butanol-pyridine mixture and estimated by UV-Visible spectrophotometer at absorption maxima 532 nm. Lipid peroxide levels were expressed in terms of μMol MDA/mg protein [24].
Estimation of super oxide dismutase (SOD)
Superoxide dismutase (SOD) catalyzes the dismutation of the superoxide radical (O2-) into hydrogen peroxide (H2O2) and elemental oxygen (O2) and as such provides an important defense against the toxicity of the superoxide radical [25]. In the assay, conversion of xanthine to uric acid and hydrogen peroxide is mediated via superoxide ions (O2-), generated by xanthine oxidase (XOD). Superoxide ions (O2-) convert NBT to NBT-diformazan, a blue colour complex with absorption maxima at 560 nm. SOD reduces the superoxide ion (O2-) concentration and thereby lowers the rate of NBT-diformazan formation. The extent of reduction in NBT-diformazan formation is a measure of SOD activity present in the sample. The absorbance was recorded for 3 minutes at 1 minute interval and % inhibition of NBT-diformazan formation by SOD was converted to the relative concentration. SOD levels were expressed in terms of unit/mg wet tissue [26].
Estimation of catalase (CAT)
Catalase (CAT) is an antioxidant enzyme ubiquitously present in mammalian and non-mammalian aerobic cells containing a cytochrome system. In UV range H2O2 shows continuous increase in absorption with decreasing wavelength. Catalase catalyses the rapid decomposition of H2O2 to water and oxygen. The decomposition of H2O2 can be followed directly by the decrease in absorbance at 240 nm. The difference in absorbance per unit is a measure of catalase activity [27].
Estimation of reduced glutathione (GSH)
Reduced glutathione (GSH) is a tripeptide (γ-glutamylcysteinylglycine) that contains a free thiol group. GSH is a major tissue antioxidant that provides reducing equivalents for the glutathione peroxidase (GPx) catalyzed reduction of lipid hydroperoxides to their corresponding alcohols and hydrogen peroxide to water [28].
DTNB (5, 5’-Dithiobis (2- nitrobenzoic acid)), known as Ellman’s Reagent, was developed for the detection of thiol compounds. DTNB reacts with free thiol group of glutathione (GSH) and generates 2-nitro-5-thiobenzoic acid and glutathione disulfide (GSSG). Since 2-nitro- 5-thiobenzoic acid is a yellow colored compound, GSH concentration in the sample can be determined by UV spectrophotometer at 412 nm. GSH is generated from GSSG by glutathione reductase, and reacts with DTNB again to produce 2-nitro-5- thiobenzoic acid. Therefore, this recycling reaction improves the sensitivity of total glutathione detection [29].
Statistical significance
The results were expressed as mean ± SEM (standard error of mean). The difference between the control and treated means were analyzed using one way analysis of variance (ANOVA) followed by Tukey test. P-values < 0.05 were taken to be statistically significant.
RESULTS
Phytochemical analysis
Preliminary phytochemical analysis showed presence of alkaloids, phenolic compounds and tannins, phytosterols and triterpene, flavonoids, saponins, and coumarins in ethanolic extract of C. nurvala stem bark.
Acute toxicity study
No mortality was observed following oral administration of C. nurvala ethanolic extract even with the highest dose (2000 mg/kg). Moreover, no significant changes in body weight and behavior were observed. Hence, C. nurvala could be safe up to the dose of 2000 mg/kg body weight of the animal. Hence doses of 100, 200 and 400 mg/kg were selected for in-vivo behavioral assay.
Hebb’s-William maze test
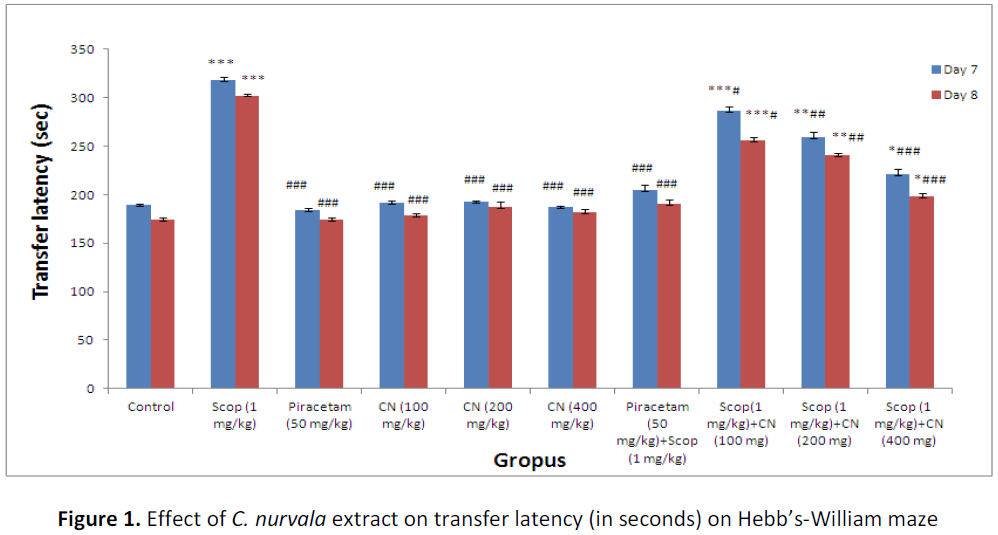
Results suggest pre-treatment with ethanolic extract of C. nurvala (100, 200 and 400 mg/kg body weight) for seven successive days did not exhibited much difference in ITL compared to normal control group but in presence of amnesia, higher dose of C. nurvala ethanolic extract (400 mg/kg body weight) afforded a significant (###p<0.001) decrease in TL (score: 220.66 ± 6.05) compared to other lower doses groups (score: 286.33 ± 5.10 and 259.33 ± 5.87 respectively) and closely approximated to standard drug Piracetam (score: 204.66 ± 5.48). However, all the three doses of extract showed improvement spatial learning and memory activity in dose dependant manner. Moreover, in Group VIII, IX and X, significant decrease in RTL on Day 8 compared to Day 7 were observed which elaborate the drugs responses to overcome the learning and memory deficits produced by scopolamine (Table 1 and Figure 1).
Table 1: Effect of C. nurvala extract on transfer latency (in seconds) on Hebb’s-William maze
| Groups | Treatment | ITL on Day 7 (sec) | RTL on Day 8 (sec) |
|---|---|---|---|
| Group I: (Normal control) | Vehicle (0.6% w/v sodium CMC) | 189.50 ± 1.40 | 174.56 ± 2.39 |
| Group II: (Negative control) | Scopolamine (1mg/kg i.p) | 317.83 ± 3.42 *** | 302.32 ± 1.45 *** |
| Group III: (Positive control) | Piracetam (50 mg/kg) orally | 184.28 ± 2.29 ### | 174.28 ± 2.29 ### |
| Group IV: | C. nurvala ethanolic extract (100 mg/kg) orally | 191.36 ± 2.40 ### | 178.33 ± 2.35 ### |
| Group V: | C. nurvala ethanolic extract (200 mg/kg) orally | 192.41 ± 1.16 ### | 187.54 ± 5.12 ### |
| Group VI: | C. nurvala ethanolic extract (400 mg/kg) orally | 187.2 ± 1.35 ### | 182.19 ± 3.29 ### |
| Group VII | Piracetam (50 mg/kg) orally + Scopolamine (1mg/kg i.p.) | 204.66 ± 5.48 ### | 190.42 ± 4.65 ### |
| Group VIII | C. nurvala ethanolic extract (100 mg/kg) orally + Scopolamine (1mg/kg i.p.) | 286.33 ± 5.10 ***# | 256.14 ± 3.23 ***# |
| Group IX | C. nurvala ethanolic extract (200 mg/kg) orally + Scopolamine (1mg/kg i.p.) | 259.33 ± 5.87 **## | 240.62 ± 2.56 **## |
| Group X | C. nurvala ethanolic extract (400 mg/kg) orally + Scopolamine (1mg/kg i.p.) | 220.66 ± 6.05 *### | 198.32 ± 2.89 *### |
Data were analyzed through one way ANOVA followed by Tukey’s test. Values were expressed as (mean ± S.E.M.), n = 6 in each group. *** p<0.001, ** p<0.01 and * p< 0.05 when compared to normal control; ### p< 0.00, ## p< 0.01 and # p< 0.05 when compared to negative control.
Measurement of lipid peroxidation
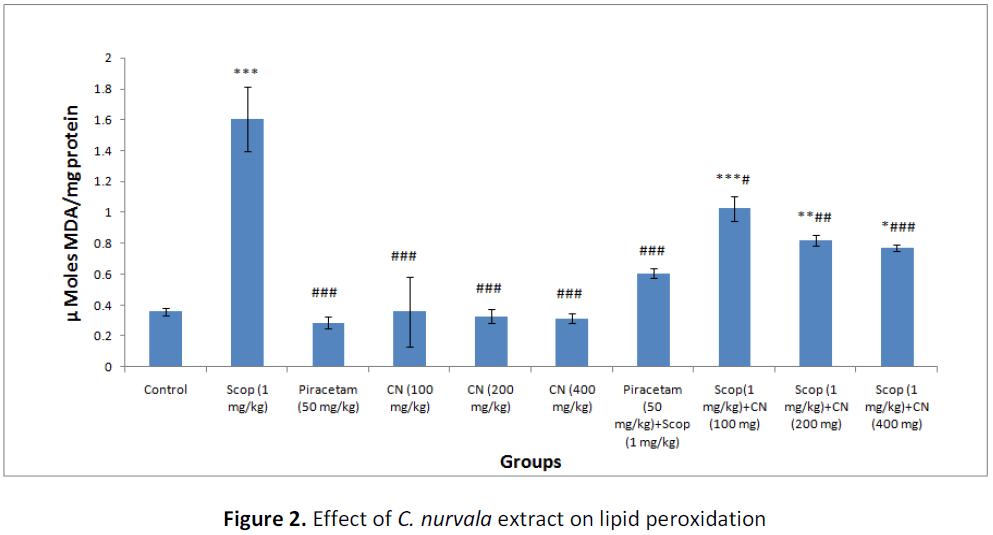
Results suggest pre-treatment with ethanolic extract of C. nurvala (100, 200 and 400 mg/kg body weight) for seven successive days did not exhibited much difference in MDA level compared to normal control group but in presence of amnesia, higher dose of C. nurvala ethanolic extract (400 mg/kg body weight) afforded a significant (###p<0.001) decrease in MDA level (score: 0.77 ± 0.022) compared to other lower doses groups (score: 1.025 ± 0.080 and 0.82 ± 0.033 respectively) and closely approximated to standard drug Piracetam (score: 0.606 ± 0.031). However, all the three doses of extract showed decline in MDA level in dose dependant manner (Table 2 and Figure 2).
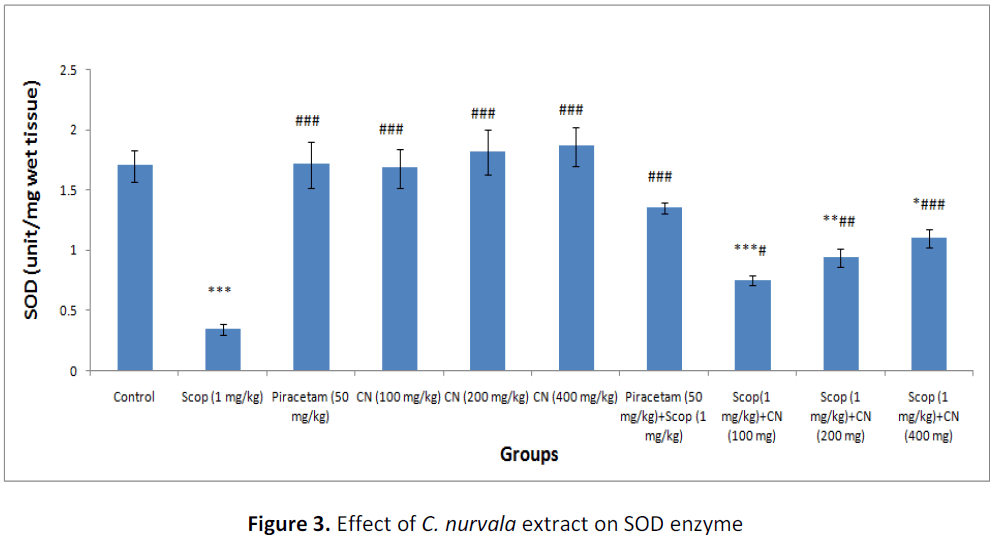
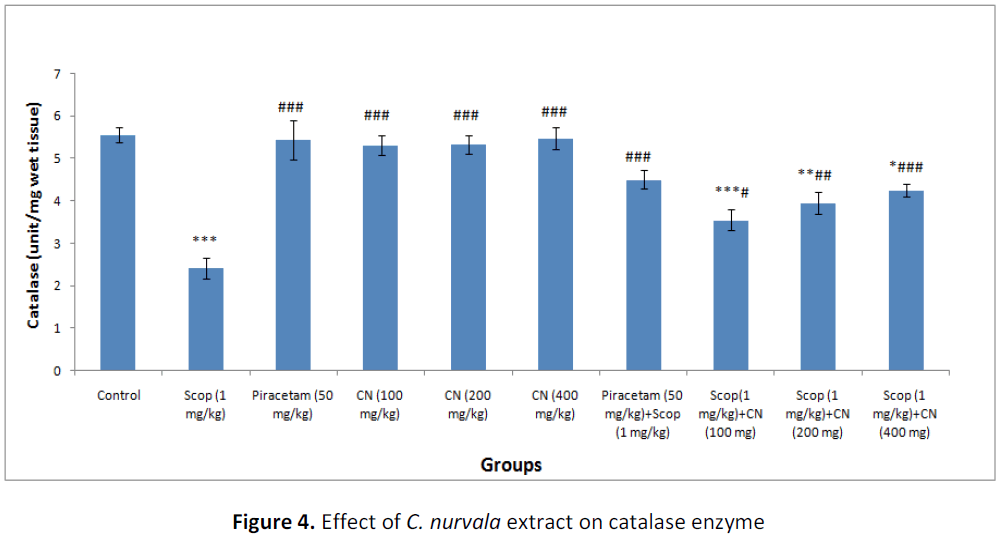
Table 2: Effect of C. nurvala extract on lipid peroxidation and antioxidant enzymes
| Groups | Treatment | MDA level µ Mol MDA/mg protein | SOD (unit/mg wet tissue) | Catalase (unit/mg wet tissue) | mMol GSH/mg wet tissue |
|---|---|---|---|---|---|
| Group I (Normal control) | Vehicle (0.6% w/v sodium CMC) | 0.358 ± 0.026 | 1.70 ± 0.131 | 5.563 ± 0.172 | 0.655 ± 0.004 |
| Group II (Negative control) | Scopolamine (1mg/kg i.p) | 1.605 ± 0.206 *** | 0.34 ± 0.046 *** | 2.416 ± 0.252 *** | 0.373 ± 0.031 *** |
| Group III (Positive control) | Piracetam (50 mg/kg) orally | 0.287 ± 0.038 ### | 1.71 ± 0.193 ### | 5.438 ± 0.452 ### | 0.623 ± 0.009 ### |
| Group IV | C. nurvala ethanolic extract (100 mg/kg) orally | 0.358 ± 0.228 ### | 1.68 ± 0.160 ### | 5.316 ± 0.239 ### | 0.631 ± 0.022 ### |
| Group V | C. nurvala ethanolic extract (200 mg/kg) orally | 0.328 ± 0.044 ### | 1.81 ± 0.186 ### | 5.333 ± 0.215 ### | 0.608 ± 0.017 ### |
| Group VI | C. nurvala ethanolic extract (400 mg/kg) orally | 0.315 ± 0.031 ### | 1.86 ± 0.160 ### | 5.480 ± 0.251 ### | 0.618 ± 0.024 ### |
| Group VII | Piracetam (50 mg/kg) orally + Scopolamine (1mg/kg i.p) C. nurvala ethanolic | 0.606 ± 0.031 ### | 1.35 ± 0.045 ### | 4.50 ± 0.220 ### | 0.580 ± 0.028 ### |
| Group VIII | extract (100 mg/kg) orally + Scopolamine (1mg/kg i.p) C. nurvala ethanolic | 1.025 ± 0.080 ***# | 0.75 ± 0.042 ***# | 3.55 ± 0.246 ***# | 0.443 ± 0.024 ***# |
| Group IX | extract (200 mg/kg) orally + Scopolamine (1mg/kg i.p) C. nurvala ethanolic | 0.82 ± 0.033 **## | 0.94 ± 0.077 **## | 3.951 ± 0.248 **## | 0.520 ± 0.021 **### |
| Group X | extract (400 mg/kg) orally + Scopolamine (1mg/kg i.p) | 0.77 ± 0.022 *### | 1.10 ± 0.073 *### | 4.25 ± 0.16 *### | 0.541 ± 0.013 *### |
Data was analyzed by one-way ANOVA followed by Tukey’s post-hoc test. Values were expressed as mean ± S.E.M., n = 6 in each group. *** p < 0.001, ** p < 0.01and * p < 0.05 when compared to control; ### p < 0.001, ## p < 0.01 and # p < 0.05 when compared to negative control.
Estimation of superoxide dismutase (SOD)
In presence of amnesia, higher dose of C. nurvala ethanolic extract (400 mg/kg body weight) afforded a significant (###p<0.001) increase in SOD level (score: 1.10 ± 0.073) compared to other lower doses groups (score: 0.75 ± 0.042 and 0.94 ± 0.077 respectively) and closely approximated to standard drug Piracetam (score: 1.35 ± 0.045). However, all the three doses of extract showed increase in SOD level in dose dependant manner (Table 2 and Figure 3).
Estimation of catalase (CAT)
In presence of amnesia, higher dose of C. nurvala ethanolic extract (400 mg/kg body weight) afforded a significant (###p<0.001) increase in catalase level (score: 4.25 ± 0.16) compared to other lower doses groups (score: 3.55 ± 0.246 and 3.951 ± 0.248 respectively) and closely approximated to standard drug Piracetam (score: 4.50 ± 0.220). However, all the three doses of extract showed increase in catalase level in dose dependant manner (Table 2 and Figure 4).
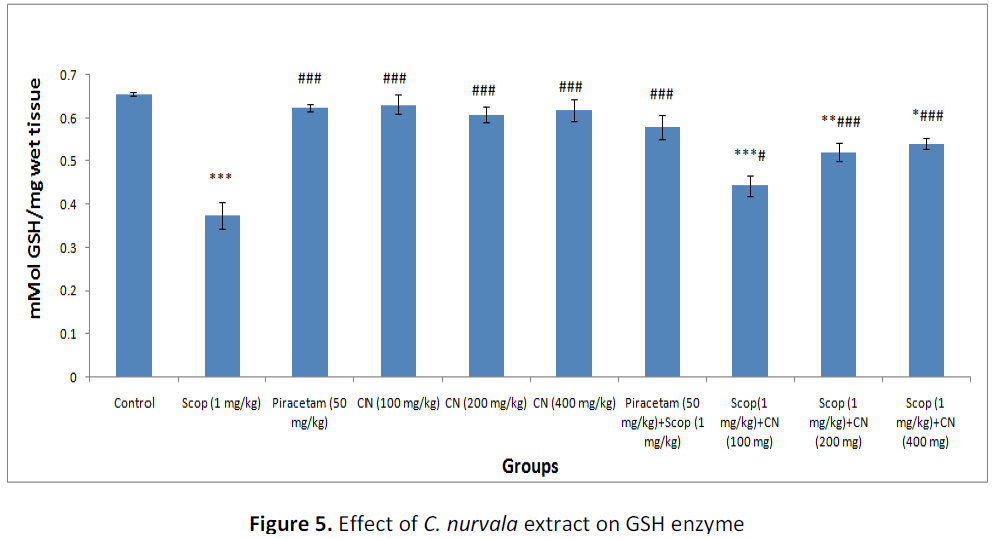
Estimation of reduced glutathione (GSH)
In presence of amnesia, higher dose of C. nurvala ethanolic extract (400 mg/kg body weight) afforded a significant (###p<0.001) increase in GSH level (score: 0.541 ± 0.013) compared to other lower doses groups (score: 0.443 ± 0.024 and 0.520 ± 0.021 respectively) and closely approximated to standard drug Piracetam (score: 0.580 ± 0.028). However, all the three doses of extract showed increase in GSH level in dose dependant manner (Table 2 and Figure 5).
DISCUSSION
Many clinical studies have reported oxidative stress as one of the early causative factors involved in the pathogenesis of AD [30]. Recent studies postulated that memory impairment in scopolamineinduced amnesic model is associated with increased oxidative stress within the brain [31,32]. Oxidative stress is the disturbance of balance between production of reactive oxygen species (ROS) and antioxidant defense systems. Therefore, it may be possible to delay the progression of AD with antioxidants [33]. The current hypothesis about the mechanisms by which neurons come into necrotic or apoptotic processes has led to believe that the therapeutic use of antioxidants may be beneficial in aging and neurodegenerative disorders like AD [34].
Increased MDA level, one of the most harmful effects of ROS has been considered as important biomarker for in-vivo lipid peroxidation [35]. The results of antioxidant study showed higher MDA level and lower levels of SOD, CAT, and GSH in scopolamine induced group compared to normal control group. Pretreatment with C. nurvala extract, significantly improve the levels of SOD, CAT and GSH whereas MDA level was decreased significantly. This demonstrated the antioxidant potentiality of C. nurvala extract. The findings from Hebb’s - William maze test suggest C. nurvala ethanolic extract showed protective effect in transfer latency against scopolamine induced amnesia in dose dependent manner. Moreover, the decrease in transfer latency during retention period suggested the drug response to overcome learning and memory deficit produced by scopolamine which provides an adequate scientific promise to validate the antidementia potency of C. nurvala extract.
CONCLUSION
Therefore, in the present study we observed that C. nurvala extract (i) showed improvement in antioxidant enzyme status (ii) inhibited lipid peroxidation in brain homogenate and (iii) showed improvement in memory of Wistar rats when tested on Hebb’s- William maze test. Thus, a combination of antioxidant and anti dementia effects exhibited by C. nurvala extract may be responsible for its neuroprotective potentiality.
Conflict of interest
We declare that we don’t have any conflict of interest.
ACKNOWLEDGEMENT
The authors acknowledge the financial support of Nitte University, Mangalore, India for the research work (Grant no. NU/PhD/Pharm/Res-10/2011).
REFERENCES
- Anil K, Samrita D, Atish P. Neuroprotective effects of Centella asiatica against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress. Int J Alzheimer’s Disease 2009; 9: 1-8.
- Puchchakayala G, Akina S, Thati M. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimer’s Disease 2012; 12: 1-8.
- Mahesh G, Tasneem S. Biomarker controversies and diagnostic difficulties in Alzheimer’s disease. AJPCT 2014; 2(4): 463-68.
- Mrugaya PK, Archana RJ. Effect of Alstonia scholaris (Linn.) R.Br. on stress and cognition in mice. Ind J Exp Bio 2008; 47: 47-52.
- Hala FZ, Abd-El-Fattah MA, Amina SA. Naringenin protects against scopolamine- induced dementia in rats. Bulletin of Faculty of Pharmacy, Cairo University 2014; 52: 15–25.
- Eun JJ, Ki YL, Seung HK, Sang HS, Young CK. Cognitive-enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine- treated mice. European J Pharmacol 2008; 588: 78–84.
- Joshi H, Parle M. Nootropic activity of calyces of Hibiscus sabdariffa Linn. IJPT 2006; 5(1): 15-20.
- Neeraj KS, Alok N, Dixit VK, Mishra SH. Cognition boosting effect of Canscora decussate (a south Indian Shankhapushpi). European J Integrative Med 2012; 4: e113- e121.
- Hanumanthachar J, Krupa M. Antiamnesic evaluation of C. phlomidis Linn. bark extract in mice. Brazilian J Pharm Sci 2008; 44(4): 717-25.
- Hanumanthachar J, Milind P. Pharmacological Evidences for the Antiamnesic Effects of Desmodium gangeticum in mice. Iranian J Pharm Res 2007; 6(3): 199-207.
- Muraleedharannair JM, Johnson MA, Mony M, Zachariah MP, Solomon J. Phytochemical studies on Azolla pinnata R. Br., Marsilea minuta L. and Salvinia molesta Mitch. Asian Pac J Trop Biomed 2011; 2: S26-29.
- Shiddamallayya N, Azara Y, Gopakumar K. Hundred common forest medicinal plants of Karnataka in primary healthcare. Indian J Trad Knowl 2010; 9(1): 90-95.
- Amod PK, Laurie AK, Girish JK. Herbal complement inhibitors in the treatment of neuroinflammation. Ann N Y Acad Sci 2005; 1056: 413–429.
- Atanu B, Shastry CS, Santanu S. Isolation, purification and structural elucidation of bioactive polyphenolic compound (catechin) from Crataeva nurvala Buch-Ham stem bark chloroform fraction. AJPCT 2014; 2(3): 301-09.
- Parvin S, Md. Abdul K, Md. Abdul M, Ekramul H, Md. Ashik M, Mir Imam IW. Triterpenoids and phytosteroids from stem bark of Crataeva nurvala buch ham. J Appl Pharm Sci 2011; 1(9): 47-50.
- Harbone JB. Phytochemical methods: a guide to modern techniques of plant analysis. 3rd ed. London: Thompson Publishing IT, 1998; 5-21.
- Atanu B, Shastry CS, Santanu S. Evaluation of acute and sub-chronic oral toxicity study of ethanolic extract of Crataeva nurvala Buch-ham stem bark on experimental Wistar rats. Res J Pharmacog & Phytochem 2013; 5(6): 293-96.
- Shirish SP, Shrikant SS. Acute toxicity study of Phyllanthus amarus. Int J Pharm Sci Rev Res 2011; 9(1): 81-4.
- Saxena V, Ahmad H, Gupta R. Memory enhancing effects of Ficus carica leaves in hexane extract on interoceptive behavioral models. Asian J Pharm Clin Res 2013; 6(3): 109-13.
- Hebb DO, Williams KA. A method of rating animal intelligence. J Gen Psychol 1946; 34: 59-65.
- Satoru K, Yasushi O, Susumu A. Effects of enriched environments with different durations and starting times on learning capacity during aging in rats assessed by a refined procedure of the Hebb-Williams maze task. J Neuro Res 2002; 70: 340–46.
- Vinutha B, Prashanth D, Salma K, Sreeja SL, Pratiti D, Padmaja R. Screening of selected Indian medicinal plants for acetylcholinesterase inhibitory activity. J Ethnopharmacol 2007; 109; 359–63.
- Barry H, Susanna C. Lipid peroxidation its mechanism, measurement, and significance. Am J Clin Nutr 1993; 57: 715S-25S.
- Ohkawa H, Ohishi N, Yogi K. Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-8.
- Yi S, Larry WO, Ylng L. A simple method for clinical assay of superoxide dismutase. Clin Chem 1988; 34(3): 497-500.
- Kussmaul L, Hirst J. The mechanism of superoxide production by NADH: ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. PNAS 2006; 103: 7607-12.
- Beers RF, Sizer IW. A spectrophotometric method for measuring breakdown of hydrogen peroxide by catalase. J Biol Chem 1952; 195: 133-40.
- Md. Nur A, Nusrat JB, Md. Rafiquzzaman. Review on in-vivo and in-vitro methods evaluation of antioxidant activity. Saudi Pharma J 2013; 21: 143–52.
- Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys 1959; 82: 70-77.
- Fereshteh J, Zohre G, Mehdi D, Jasem E, Abbas H. Evaluation of antidiarrhoeal and neuropharmacological activity of Cynanchum acutum in rat. Asian J Med Sci 2012; 4(4): 149-51.
- Hanumanthachar J, Krupa M. Antiamnesic evaluation of C. phlomidis Linn. bark extract in mice. Brazilian J Pharm Sci 2008; 44(4): 717-25.
- [ 32 ] Sampath M, Rachana C, Gayathri MR, Hema U. Neuroprotective effects of Mucuna pruriens against stress-induced oxidative damage. J Physiol Biomed Sci 2011; 24(2): 28-33.
- El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary ED. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain antioxidative status induced by amnstic dose of scopolamine. Pharmacol Biochem Behavior 2003; 76: 525-33.
- Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci 2008; 1147: 93–104.
- Denise G, Lucas SM, Juliana V, Clóvis P, Gabriela S. Importance of the lipid peroxidation biomarkers and methodological aspects FOR malondialdehyde quantification. Quim Nova 2009; 32(1): 169- 74.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences