ISSN : 2348-9502
American Journal of Ethnomedicine
Neurocognitive Values of Evolvulus alsinoides and Centella asiatica on Scopolamine Induced Amnesia in Mice
Mukesh Kumar Yadav1*, Santosh Kumar Singh2, Manish Singh3, Shashank Shekhar Mishra4, Anurag Singh2, Jyoti Shankar Tripathi1 and Yamini Bhusan Tripathi5
1Department of Kayachikitsa, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
2Centre of Experimental Medicine and Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
3Department of Pharmacology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
4Department of Vikriti Vigyan, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
5Department of Medicinal Chemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India
- *Corresponding Author:
- Mukesh Kumar Yadav
Department of Kayachikitsa
Institute of Medical Sciences, Banaras Hindu University, Varanasi
Uttar Pradesh-221005, India
Tel: 919792353549
E-mail: mukesh.yadav@bhu.ac.in
Received date: December 26, 2018; Accepted date: January 14, 2019; Published date: January 22, 2019
Citation: Yadav MK, Singh SK, Singh M, Mishra SS, Singh A, et al. (2018) Neurocognitive Values of Evolvulus alsinoides and Centella asiatica on Scopolamine Induced Amnesia in Mice. Am J Ethnomed Vol.6 No.1:15
Abstract
Aim of the study: To study the comparative neuroprotective activity of ethanolic extracts of E. alsinoides and C. asiatica.
Method: The ethanolic extracts of E. alsinoides and C. asiatica were administered orally daily along with scopolamine for a period of 14 days following which the behavioral tests i.e. elevated plus maze and Morris water maze tests were performed to assess learning and memory. Animal groups were divided in nine different groups. In-vivo antioxidant enzymes activity, inflammatory markers inhibition activity and acetylcholinesterase (AChE) inhibition activity in the brain of mice were also measured at the end of the study.
Results: The study demonstrate that scopolamine induction resulted in learning and memory deficits which were partially and significantly ameliorated by the ethanolic extracts of E. alsinoides and C. asiatica. The extracts also counteracted scopolamine-induced decreases in acetylcholine levels, increases in AChE activity, and decreases in activities of the antioxidant enzymes.
The study demonstrates the ability of the ethanolic extracts of E. alsinoides and C. asiatica to reverse scopolamine-induced learning and memory deficits in mice which may at least partially be explained by the reversal of scopolamine-induced reductions in brain antioxidant enzymes activity, inflammatory markers inhibition activity and acetylcholinesterase (AChE) inhibition activity by the both extracts.
Keywords
Ethanolic extracts; Scopolamine; In-vivo antioxidant; Inflammatory markers; Acetylcholine esterase.
Introduction
Alzheimer’s disease is a progressive neurodegenerative disease which is characterized by the loss of learning and memory abilities with aging. This impairment of memory is correlated with the loss of cholinergic neurons. Scopolamine-induced amnesic animal models are used to screen for drugs that potentially have anti-dementia activities by stimulating the cholinergic system, make them a candidate for the treatment of Alzheimer’s disease. Ayurveda, the Indian system of medicine describe a group of medicinal plants under the category of ‘Medhya Rasayana’, which possess the memory-enhancing effect and facilitate learning acquisition. Medicinal plants are rich sources of important metabolites, which are potential sources of antioxidant, antimicrobial, anti-inflammatory, and anticancer activities [1-3]. The utilization of herbal medicine in treating infectious diseases have been practiced for 1000s of years and will continue to provide mankind with new remedies [4].
Evolvulus alsinoides L., an important medicinal plant is employed for different ailments in India traditionally and grows in the open and grassy places almost throughout India and mostly in the region from India to west Cameroon and widely dispersed elsewhere in tropical Africa and worldwide. Some vernacular names of the plant in India are vishnukranta, vishnugandhiy (sanskrit), shankapushpi (hindi). Evolvulus alsinoides L. is used in Ayurveda as a brain tonic in the treatment of neurodegenerative diseases, asthma and amnesia [5].
Centella asiatica is a very important medicinal herb also known as Mandukparni, which has been used as a medicine in the Ayurvedic tradition of India for thousands of years and mentioned in many classical texts of Ayurveda. Centella asiatica (CA) is a rejuvenative nervine recommended for nervous disorders, epilepsy, senility and premature aging and a number of other medical conditions. The extract of Centella asiatica especially from roots and leaves contain a high anti-oxidative activity, which was as good as tocopherol, a natural anti-oxidant, have been reported to play a significant role in wound healing [6]. Centella asiatica extract contains four principle bioactive compounds: asiatic acids, madecassic acid, asiaticoside and madecassoside [7], in which asiaticoside was identified as the main active constituent responsible for wound healing.
We are working on the development of anti-dementia natural drug using an in vivo screening of herbal materials due to their safety and cost effectiveness [8].
Material and Methods
Plant material
Whole plant material of Centella asiatica and Evolvulus alsinoides were collected from village Ramnapur, Varanasi, Uttar Pradesh, India in October 2015 and authentication was done by Department of Botany, Banaras Hindu University, India and also herbarium of Centella asiatica (voucher specimen no. Apia/02/2015) and Evolvulus alsinoides (voucher specimen no. Convolvul./03/2015) of plant was deposited in the Department of Botany, Banaras Hindu University, India.
Preparation of extracts
The extraction of both plants was done with Soxhlet method in ethanolic solvents at 72-82°C for 72 hours. The Soxhlet extraction has widely been used for extracting valuable bioactive compounds from various natural sources. It is used as a model for the comparison of new extraction alternatives.
Preliminary phytochemical analysis
For preliminary phytochemical analysis, the freshly prepared crude ethanolic extracts of the whole plant were tested for the presence or absence of phyto-constituents such as alkaloids, tannins, flavonoids, saponins by using standard phytochemical procedures [9].
Animals
The experimental adult Swiss albino mice (female) 7-8 weeks old of 25-30 gm weight were issued by Animal house of Institute of Medical Sciences, Banaras Hindu University Varanasi, Uttar Pradesh. Animals were divided into experimental groups, housed in plastic cages and maintained on a 12-hour light and 12- hour dark cycle. They were given standard food and water ad libitum. The Central Animal Ethical Committee of Banaras Hindu University approved all experimental procedures (CAEC/196).
Experimental Design and Drug Administration
All the solutions were freshly prepared prior to use. Scopolamine was purchased from Sigma-Aldrich Chemical Co. India. A solution of scopolamine (1.0 mg/kg; dissolved in distilled water) was administered to the experimental animals through the intraperitoneal (IP) route. The ethanolic extracts of Evolvulus alsinoides (EEA) and Centella asiatica (ECA) in the following doses: EEA 250 mg/kg/day and 500 mg/kg/day, ECA 250 mg/kg/day and 500 mg/kg/day and combination of EEA+ECA 250 mg/kg/day and 500 mg/kg/day, dissolved in 0.3% CMC and was administered orally (PO). Nine groups, each consisting of 6 animals, were included in the study. Group I (normal) was treated with vehicle daily (0.3% CMC; PO). Group II (Sco control) was treated with scopolamine (1.0 mg/kg/day; IP). Group III (Sco+Doz) was treated with a donepezil (1.5 mg/kg/day; IP) and scopolamine (1.0 mg/ kg/day; IP). Group IV (Sco+EEA) was treated with low dose EEA (250 mg/kg/day; PO) and scopolamine (1.0 mg/kg/day; IP). Group V (Sco+EEA) was treated with high dose of EEA (500 mg/kg/day; PO) and scopolamine (1.0 mg/kg/day; IP). Group VI (Sco+ECA) was treated with low dose ECA (250 mg/kg/day; PO) and scopolamine (1.0 mg/kg/day; IP). Group VII (Sco+ECA) was treated with high dose of ECA (500 mg/kg/day; PO) and scopolamine (1.0 mg/kg/ day; IP). Group VIII (Sco+EEA+ECA) was treated with low dose of EEA (250 mg/kg/day; PO) and ECA (250 mg/kg/day; PO) and scopolamine (1.0 mg/kg/day; IP). Group IX (Sco+EEA+ECA) was treated with high dose of EEA (500 mg/kg/day; PO) and ECA (500 mg/kg/day; PO) and scopolamine (1.0 mg/kg/day; IP).
Behavioral study
Elevated plus maze: The elevated plus maze (EPM) is designed to study the behavioral pattern of experimental animals such as sensitivity to external stimuli (exteroceptive behavior), anxiety, exploration as well as learning and memory [10,11]. The EPM consists of four arms (two open and two closed), each 49 cm x 10 cm, with 40 cm high walls in closed arms and the open roof. The whole structure is elevated 50 cm above the ground. On the 10th day, 60 min after the drug treatment, each mice was placed at the end of an open arm, facing away from the central platform. The time is taken by the mice to enter any of the closed arms was recorded and considered as the transfer latency (TL) and served as a parameter for acquisition/learning. If the mice did not enter into any one of the closed arms within 180 sec, it was gently pushed into one of the two closed arms and the TL was assigned as 180 sec. For the next 15 sec, the mice were allowed to explore the maze before returning it to its home cage. On the 14th day, TL was recorded again, which served as a parameter for retention of memory. Between each session, the maze was carefully cleaned with 30% ethanol tissue to remove any olfactory cues.
Morris water maze: The Morris water maze (MWM) [12,13] test was performed to assess the learning and spatial memory of the experimental animals. The advantage of MWM over other models of learning and memory lies in the fact that no motivational stimuli such as food and water deprivation, electrical stimulation and buzzer sounds are applied. The apparatus consists of a circular pool (45 cm in height x 100 cm in diameter) with a featureless inner surface. The pool was filled with opaque water, maintained at the temperature of 22 ± 2 °C, to a height of 30 cm, and was divided into four quadrants of equal area, marked by different visual cues. A platform (29 cm X 6 cm) was placed one centimeter below the level of water at the center of one of the four quadrants, which was considered as the target quadrant. The position of the platform was kept unaltered throughout the duration of the experiment. The MWM test was performed on the 10th day after drug administration was started. On the first experimental day, the mice were allowed to acclimatize in the pool and swim for 120 sec without the platform. During the next four consecutive days, each animal received four learning trials of 120 sec with an inter-trial interval of 60 sec.
For each learning trial, the mice were placed in the water facing the pool wall diagonally opposite to the quadrant in which the platform was kept. The time taken by the animal to locate the submerged platform was recorded as Escape latency time (ELT) for each trial. If the animal were unable to locate the platform within 120 sec, it was directed to the platform and allowed to rest there for 60 sec, and in this case, the ELT was recorded as 120 sec. These sessions were recorded as hidden platform trials for acquisition test. On the 14th day after the learning trial session, the platform was removed from the pool and the mice were subjected to a Probe trial session to assess memory retention. Each mouse was placed into the water diagonally opposite to the target quadrant, and for 60 sec was allowed to swim and find the quadrant in which the platform was previously placed. The swimming time of the animal to reach the target quadrant was recorded as probe trial memory retention test.
Preparation of brain homogenate
On the terminal experimental day, animals were euthanized and out of 6 animals per group, the whole brains of 3 mice were isolated after performing cardiac perfusion with normal saline for biochemical estimations. Then further rinsed in ice-cold isotonic saline and were homogenized with ice-cold 0.1 M phosphate buffer saline (pH 7.4) to form 10% w/v homogenates. These homogenates were then further centrifuged at -4°C (10,000 rpm; C-24 cooling centrifuge instrument, Model no. C-24 Remi, India) for 15 min and the supernatant was used for estimation of biochemical parameters [14].
In-vivo antioxidants assessment
Assessment of superoxide dismutase activity: Every 3 ml of reaction mixture contained 2.8 ml of potassium phosphate buffer (0.1 M, pH 7.4), 0.1 ml of brain homogenate and 0.1 ml of pyrogallol solution (2.6 mM in 10 mM HCl). The change in absorbance was recorded at 325 nm for a period of 5 min with 30 sec. interval. One unit of SOD is equivalent to the amount of enzyme required to cause 50% inhibition of pyrogallol autoxidation per 3 ml of the assay mixture (Li X, 2012).
Assessment of catalase activity: The reaction mixture contained 2.0 ml of diluted homogenate in 0.1 M phosphate buffer (enzyme extract). The reaction was started by adding 1.0 ml of 200 mM H2O2. The decrease in OD per min was recorded against the blank (all the reagents except enzyme extract) for 3 min at 240 nm at intervals of 15 sec. CAT activity was expressed as U/mg protein [15].
Assessment of lipid peroxidation activity: To 100 μl of tissue homogenate, 1.5 ml of 10% TCA solution was added. After 10 min, centrifuge at 5000 rpm for 10 min. The supernatant was separated and mixed with 1.5 ml of TBA. The tubes were kept in boiling water bath for 30 min to complete reaction and were cooled under tap water. The absorbance of the sample at 535 nm against distilled water [16].
Assessment of reduced glutathione (GSH) activity: The GSH assays were performed as described by Smith I K et al. [17]. In GSH assay, 3 ml of reaction mixture consisted of 2.9 ml of 5, 5-dithiobis (2-nitrobenzoate) (DTNB) prepared in potassium phosphate buffer (0.1 M, pH 7.4) and 0.1 ml of tissue homogenate. The reaction mixture was incubated at 37°C for 15 min and the absorbance was recorded at 412 nm and the results were expressed as GSH/mg protein.
Analysis of IL-1β, IL-6 and TNF-α in the brain: The levels of IL- 1β, IL-6, and TNF-α in the brain homogenates were determined using commercial (Elabscience) ELISA kits according to the manufacturer’s instructions. The levels of these cytokines in the brain tissues were normalized to the protein content.
AChE estimation: AChE activity was estimated in the whole brain homogenates. Briefly, the brain homogenate was incubated for 5 min with 2.7 ml of phosphate buffer and 0.1 ml of 5, 5-dithiobis (2-nitrobenzoate) (DTNB). Further, 0.1 ml of freshly prepared acetylcholine iodide (pH 8) was added and the change in absorbance was recorded at 412 nm [18].
Statistical Analysis
The relative hematological and biochemical data were expressed as the mean ± standard error of the mean (SEM). Data were submitted to analysis of variance (one-way ANOVA) followed by Dunnett’s multiple comparison tests. The results were expressed as mean ± SEM. The software GraphPad Prism 6.0 (GraphPad Software, USA) was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Behavioral study
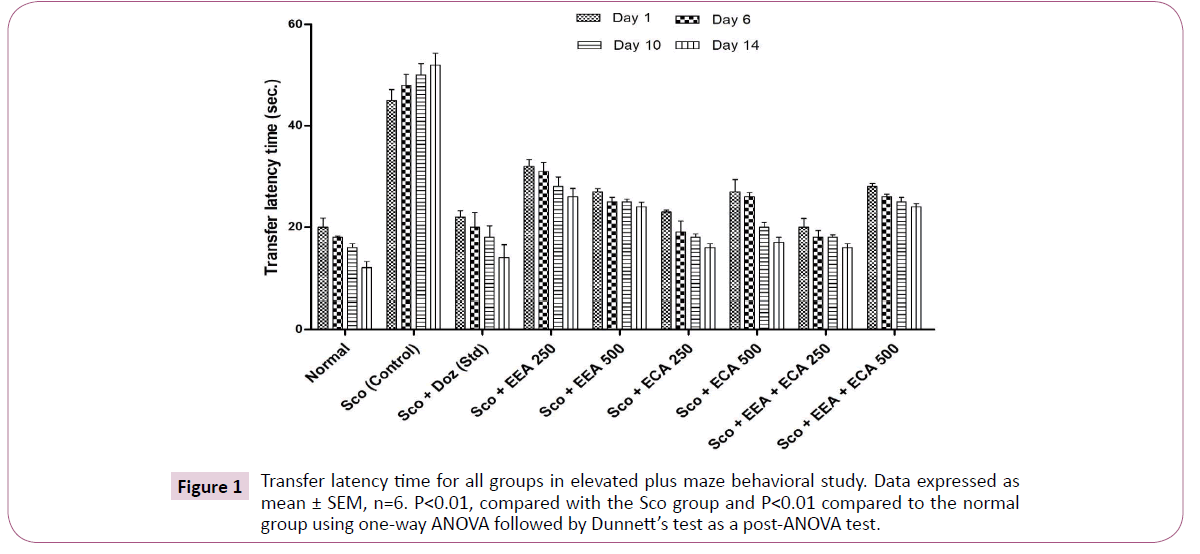
Elevated plus maze: The elevated plus maze (EPM) was used to study the behavioral pattern of mice such as anxiety, exploration as well as learning and memory and results are shown in Figure 1. At the days 1st, 6th, 10th and 14th with transfer latency time (sec) for Sco+ECA 250=23 ± 0.7, 19 ± 2.27, 18 ± 0.67, 16 ± 0.73; Sco+ECA 500=27 ± 2.39, 26 ± 0.79, 20 ± 0.97, 17 ± 0.97; Sco+EEA+ECA 250=20 ± 1.73, 18 ± 1.32, 18 ± 0.51, 16 ± 0.72 groups have shown good activity in respect to Sco (control)=45 ± 2.1, 48 ± 2.1, 50 ± 2.3, 52 ± 2.3 group and Sco+Doz (standard)=22 ± 1.28, 20 ± 2.86, 18 ± 2.3, 14 ± 2.52 group.
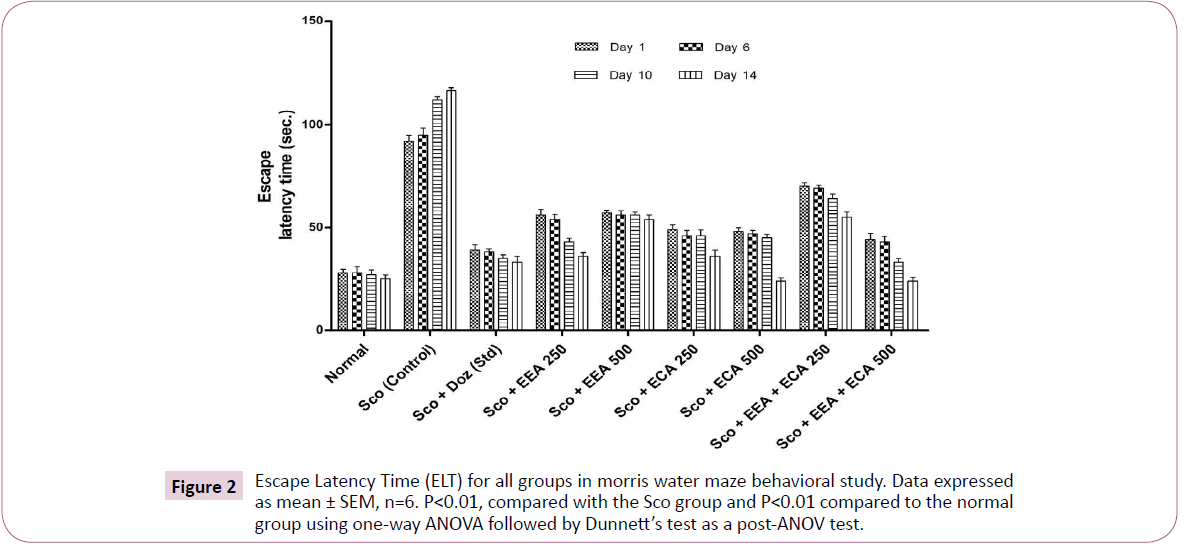
Morris water maze: The escape latency time (ELT) was measured to assess spatial memory in mice and the results are shown in Figure 2. On 1st and 6th day and we found that there was no significant difference in ELT in all mice groups. On the 10th and 14th day all plant extract treated groups were found to improve the spatial memory in a dose-dependent manner with a significant (P<0.01). On 14th day plant extracts treatment groups significantly reduced the ELT at Sco+EEA 250=56 ± 2.88, 54 ± 2.3, 43 ± 1.85, 36 ± 1.72; Sco+ECA 250=49 ± 2.33, 46 ± 2.5, 46 ± 2.88, 36 ± 2.98; Sco+ECA 500=48 ± 1.95, 47 ± 1.45, 45 ± 1.73, 24 ± 1.5; Sco+EEA+ECA 500=44 ± 3.2, 43 ± 2.66, 33 ± 1.85, 24 ± 1.76 and Sco+Doz (standard)=39 ± 2.66, 38 ± 1.62, 35 ± 1.73, 33 ± 2.88 and showed significantly improvement in spatial memory on both the days during treatment regimen (P<0.01) in comparison with Scopolamine treated mice group Sco (control)=92 ± 2.8, 95 ± 3.4, 112 ± 1.76, 116 ± 1.45.
In-vivo antioxidant activity
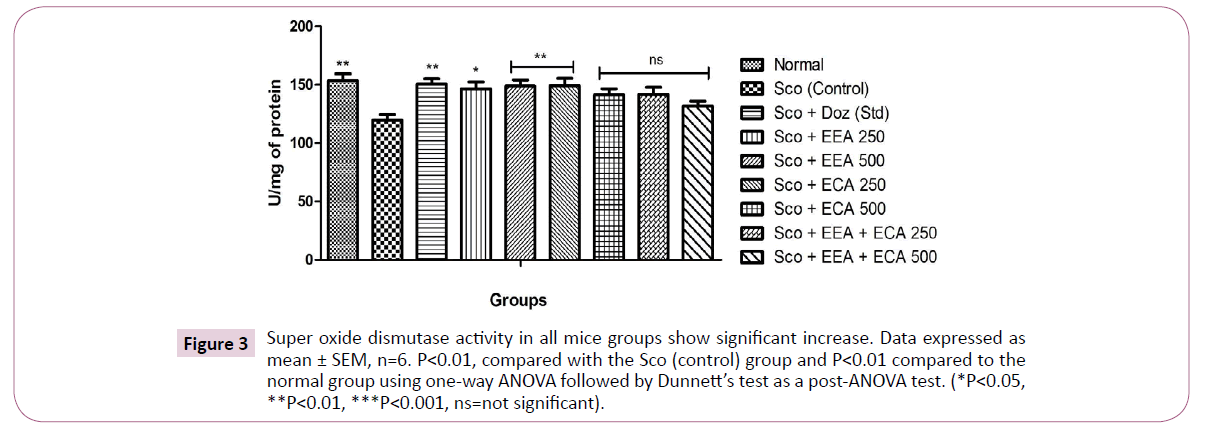
Super oxide dismutase (SOD): SOD is the major antioxidant enzyme scavenging the free radicals generated during oxidative stress were estimated and results are showed in Figure 3. The Sco+EEA 250=146 ± 6.01, Sco+EEA 500=148.66 ± 5.23 and Sco+ECA 250=148.89 ± 6.63 treatment groups significantly increased the SOD activity at all doses levels (P<0.01) except Sco+ECA 500=141.13 ± 5.18, Sco+EEA+ECA 250=141.63 ± 6.11 and Sco+EEA+ECA 500=131.55 ± 4.25 found not significant in comparison with Sco (Control)=119.78 ± 4.57 treated mice group. Sco+Doz (standard)=150.52 ± 4.61 administration also showed protection from oxidative stress by elevating the SOD activity as compared to Sco (control) mice (P<0.01).
Figure 3: Super oxide dismutase activity in all mice groups show significant increase. Data expressed as mean ± SEM, n=6. P<0.01, compared with the Sco (control) group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
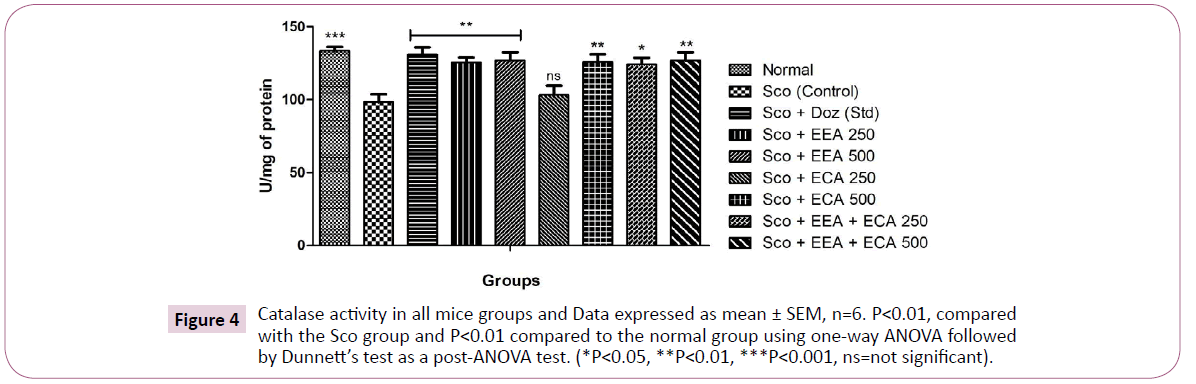
Catalase: In the all treatment groups; Sco+EEA 500=126.8 ± 5.7, Sco+ECA 500=126.0 ± 5.2 and Sco+EEA+ECA 500=126.7 ± 5.8 and Sco+Doz (standard)=131.1 ± 4.7 significantly increased the catalase activity (P<0.01) except Sco+ECA 250=103.1 ± 6.6 which found not significant in comparison with Sco (control)=98.32 ± 5.3 treated mice group (Figures 4-7).
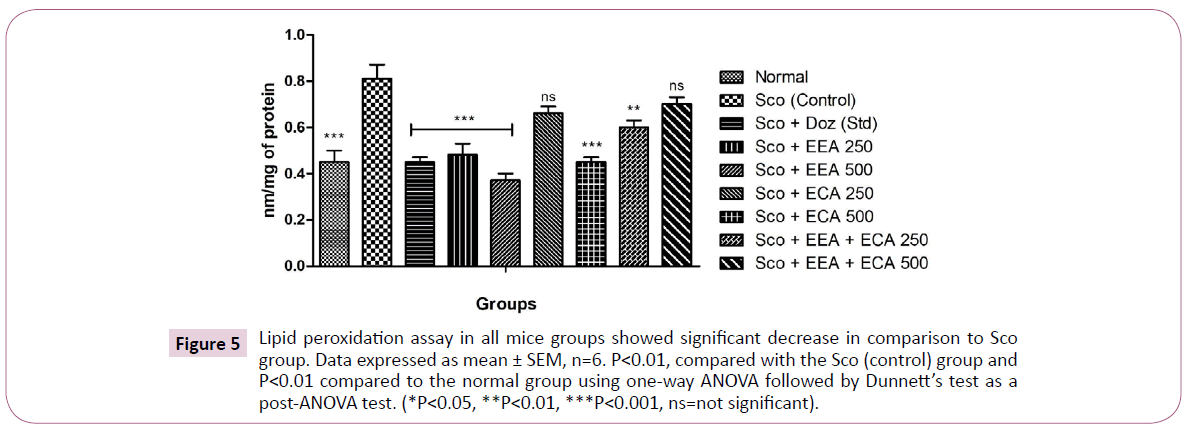
Figure 5: Lipid peroxidation assay in all mice groups showed significant decrease in comparison to Sco group. Data expressed as mean ± SEM, n=6. P<0.01, compared with the Sco (control) group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
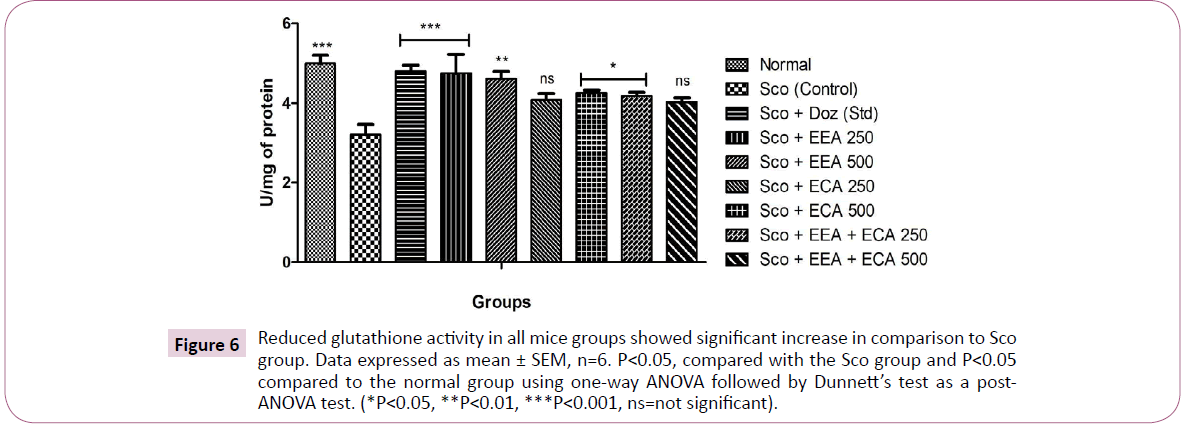
Figure 6: Reduced glutathione activity in all mice groups showed significant increase in comparison to Sco group. Data expressed as mean ± SEM, n=6. P<0.05, compared with the Sco group and P<0.05 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post- ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
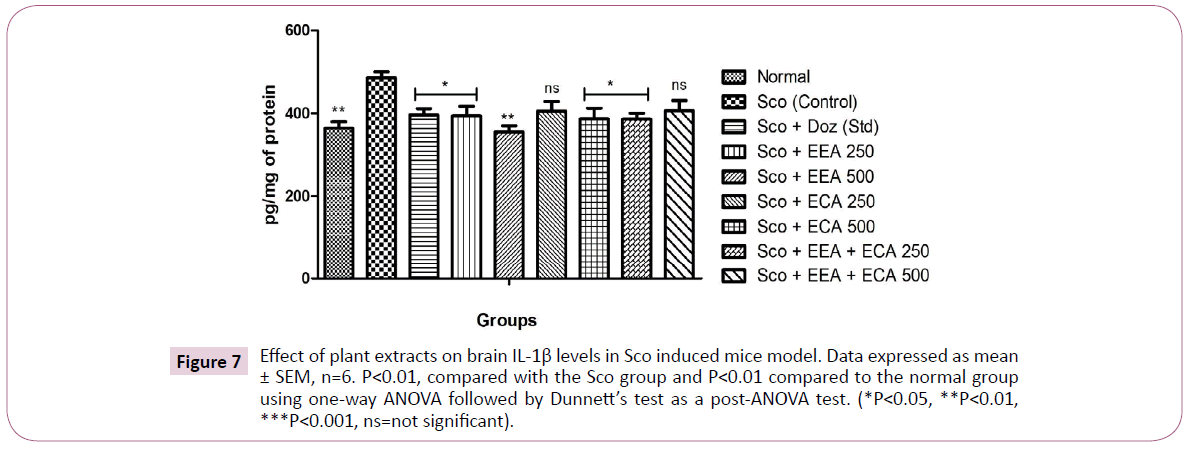
Figure 7: Effect of plant extracts on brain IL-1β levels in Sco induced mice model. Data expressed as mean ± SEM, n=6. P<0.01, compared with the Sco group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
Lipid peroxidation: In the all treatment groups; Sco+EEA 250=0.48 ± 0.05, Sco+EEA 500=0.37 ± 0.03 and Sco+ECA 500=0.45 ± 0.02 and Sco+EEA+ECA 250=0.6 ± 0.03 significantly increased the LPO activity (P<0.05) except Sco+ECA 250=0.66 ± 0.03 and Sco+EEA+ECA 500=0.7 ± 0.03 in comparison with Sco (control)=0.81 ± 0.06 treated mice group as well as Sco+Doz (standard)=0.45 ± 0.02 also decreased the elevated MDA levels.
Reduced glutathione (GSH): Reduced glutathione (GSH) level were increased after treatment with all the experimental animal groups; Sco+EEA 250=4.75 ± 0.47, Sco+EEA 500=4.61 ± 0.18, Sco+ECA 500=4.26 ± 0.06 and Sco+EEA+ ECA 250=4.18 ± 0.09 which found to be significant (P<0.05) except Sco+ECA 250=4.08 ± 0.16 and Sco+EEA+ECA 500=4.02 ± 0.11 which found not significant in comparison to Sco (control)=3.2 ± 0.26 treated group and Sco+Doz (standard)=4.8 ± 0.15 significantly elevated GSH levels (P<0.05).
Inflammatory markers
IL-1β: The effect of plant extracts treated at different concentrations Sco+EEA 250=394.1 ± 23.03, Sco+EEA 500=355.5 ± 14.64, Sco+ECA 500=387.26 ± 25.3 and Sco+EEA+ECA 250=386.21 ± 14.38 and Sco+Doz (standard)=396.38 ± 15.28 on whole brain markedly decrease the levels of IL-1β when compared with Sco (control)=485.85 ± 14.76 group (P<0.01) although Sco+ECA 250=405.5 ± 23.16 and Sco+EEA+ECA 500=406.38 ± 24.65 groups founds to be not significant.
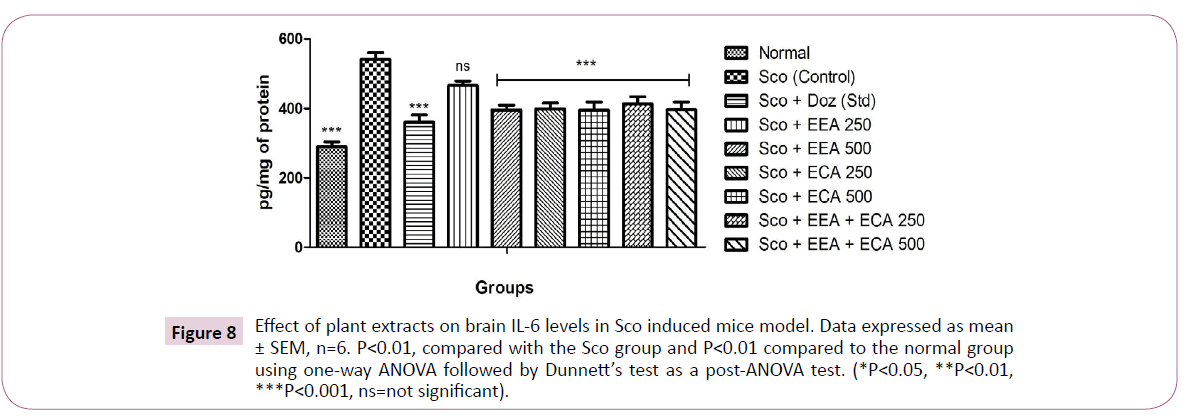
IL-6: Figure 8 shows the effect of plant extracts on the IL-6 levels in the whole brain and we found that Sco+EEA 500=395.54 ± 14.17, Sco+ECA 250=398.87 ± 16.25 Sco+ECA 500=395.12 ± 23.68 and Sco+EEA+ECA 250=413.66 ± 20.26 and Sco+Doz (standard)=360.54 ± 20.86 except Sco+EEA 250=465.75 ± 13.62 all treatment groups markedly decrease the levels of IL-6 when compared with Sco (control)=541.58 ± 19.82 group.
Figure 8: Effect of plant extracts on brain IL-6 levels in Sco induced mice model. Data expressed as mean ± SEM, n=6. P<0.01, compared with the Sco group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
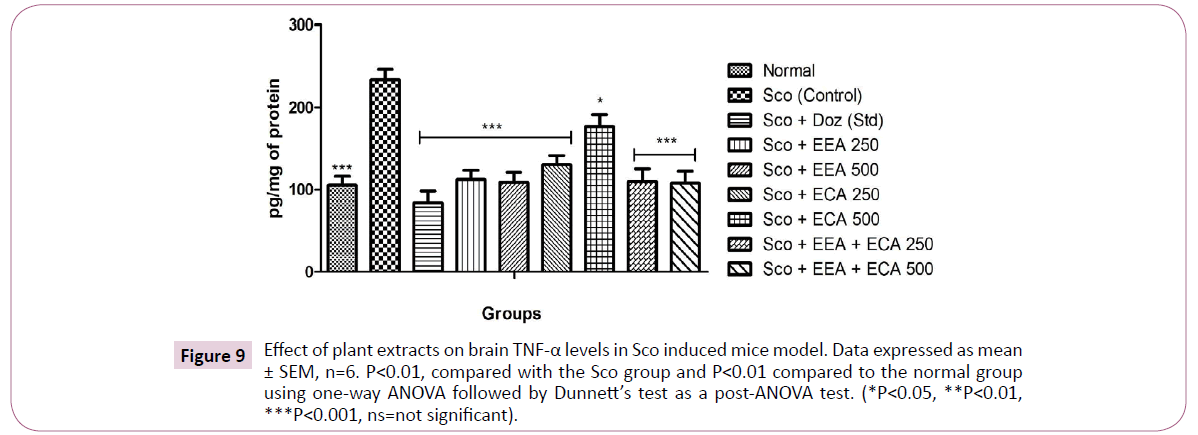
TNF-α: Figure 9 shows the effect of plant extracts on the TNF-α level in the whole brain on treatment with Sco+EEA 250=112.17 ± 11.52; Sco+EEA 500=108.97 ± 12.32; Sco+ECA 250=130.12 ± 11.50; Sco+ECA 500=176.92 ± 14.72; Sco+EEA+ECA 250=109.61 ± 15.86; Sco+EEA+ECA 500=107.69 ± 14.63 and Sco+Doz (standard)=83.97 ± 14.42 subsequently decrease the levels of TNF-α activity when compared with Sco (control)=233.97 ± 12.4 group.
Figure 9: Effect of plant extracts on brain TNF-α levels in Sco induced mice model. Data expressed as mean ± SEM, n=6. P<0.01, compared with the Sco group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
Acetylcholine esterase (AChE) activity
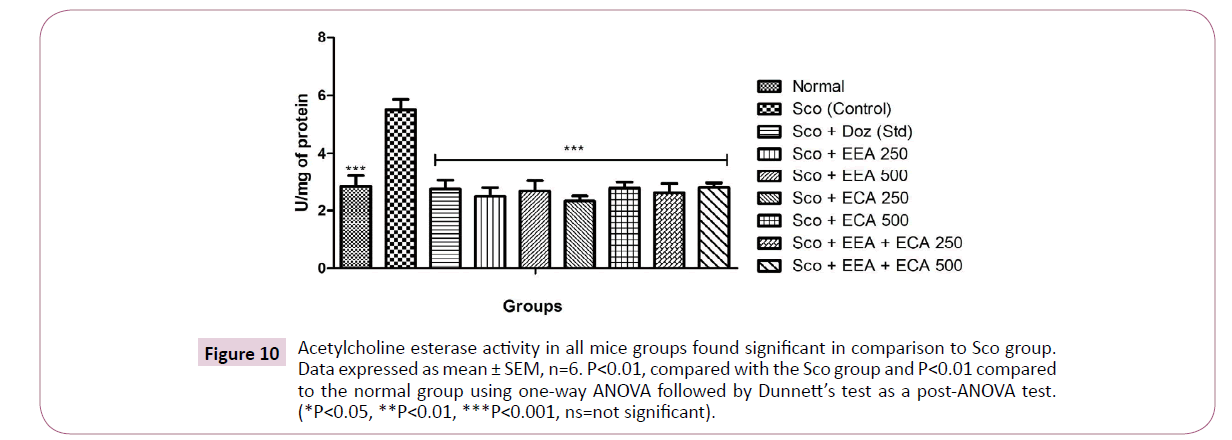
Sco administration resulted in significant increase in the brain AChE activity. All the treatment groups Sco+EEA 250=2.49 ± 0.29; Sco+EEA 500=2.67 ± 0.36; Sco+ECA 250=2.33 ± 0.17; Sco+ECA 500=2.77 ± 0.21; Sco+EEA+ECA 250=2.61 ± 0.32; Sco+EEA+ECA 500=2.79 ± 0.16 and Sco+Doz (standard)=2.74 ± 0.3 significantly inhibit the AChE activity in comparison with Sco (control)=5.51 ± 0.35 treated mice group (Figure 10).
Figure 10: Acetylcholine esterase activity in all mice groups found significant in comparison to Sco group. Data expressed as mean ± SEM, n=6. P<0.01, compared with the Sco group and P<0.01 compared to the normal group using one-way ANOVA followed by Dunnett’s test as a post-ANOVA test. (*P<0.05, **P<0.01, ***P<0.001, ns=not significant).
Discussion
In neurodegeneration, oxidative stress plays important role in ageing process and the brain is highly susceptible to the oxidative imbalance due to its high energy demand, high oxygen consumption [19]. In this study, Scopolamine treatment significantly decreased the reactive oxygen species scavenging enzymes activities like superoxide dismutase, catalase and also reduced glutathione levels in brain tissue. Scopolamine treatment also significantly increased lipid peroxidation as compared to the normal group. Both plants extracts treatments were found to significantly elevate the SOD, catalase activity, lipid peroxidation activity and reduced glutathione levels in comparison with Scopolamine group. The expression of cytokine receptors is temporally and spatially regulated in the central nervous system, and they are closely involved in cell proliferation, gliogenesis, neurogenesis, cell migration, apoptosis, and synaptic release of neurotransmitters [20,21]. The level of cytokines IL-1β, IL-6 and TNF-α are significantly decreased on treatment with plant extracts which increased by Scopolamine administration. The elevated activity of AChE leads to increased degradation of acetylcholine (Ach) neurotransmitter and which in turn declines the ACh pool in the brain which is essential in learning and memory [22]. Scopolamine administration amplifies the AChE activity which is one of the major causes of the cholinergic deficit occurrence after its administration. In this study we found that treatment with both plants ethanolic extracts significantly reduced the AChE activity as compared to the Scopolamine treated mice. It reveals that inhibition of AChE activity by plant extracts have a protective role in acetylcholine degradation and improved the cholinergic neurotransmission. Thus, plant extracts reduced the cholinergic deficits produced by Scopolamine administration resulting into enhanced neuroprotective effect [23,24].
Conclusion
Since the E. alsinoides and C. asiatica are already used in Indian traditional medicine as the neuroprotective agent and also found promising effects over inflammatory diseases, wound healing, and immunomodulatory activity. The neuroprotective effect of both plants extracts attributed to inhibition of AChE activity and make improvement in the spatial memory formation. The neuroprotective activity could be ascribed to extracts strong antioxidant potential, inhibitory role on AChE activity. The aforementioned research findings of this study prospect ethanolic extracts of both plants i.e. EEA, ECA, and EEA+ECA extracts as the promising therapeutic candidate for neurodegenerative diseases.
Conflict of Interest
The authors declare that there is no conflict of interest.
Acknowledgement
This research work was done in collaboration with Department of Kayachikitsa and Centre of Experimental Medicine and Surgery (CEMS), Institute of medical sciences, Banaras Hindu University, Varanasi. All authors contributed in this study equally.
References
- Hernandez NE, Tereschuk ML, Abdala LR (2000) Antimicrobial activity of flavonoids in medicinal plants from Tafí del Valle (Tucumain, Argentina). J Ethnopharmacol 73: 317-322.
- Polya GM (2003) Biochemical targets of plant bioactive compound. A pharmacological references guide to sites of action and biological effects. Boca Raton, FL: CRC Press.
- Maruthanila VL, Poornima J, Mirunalani S (2014) Attenuation of carcinogenesis and the mechanism underlying by the influence of indole-3-carbinol and its metabolite 3,3’-diindolylmethane: a therapeutic marvel. Adv Pharmacol Sci 1-7.
- Cragg GM, Newman D (2007) Drug from nature: past achievement, future prospect. Adv Phytomed 1: 23-37.
- Goyal PR, Singh KP (2005) Shankhpuspi (Evolvulus alsinoides Linn.): a medicinal herb. Int J Mendel 2:124.
- Zainol MK, Abd-Hamid A, Yusof S, Muse R (2003) Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chemistry 81: 575-581.
- Zheng CJ, Qin LP (2007) Chemical components of Centella asiatica and their bioactivities. Journal of Chinese Integrative Medicine 5: 348-351.
- Myers SP, Cheras P (2004) The other side of the coin: safety of complementary and alternative medicine. Med J Aust 181: 222-225.
- Solomon CU, Arukwe UI, Onuoha I (2013) Preliminary phytochemical screening of different solvent extracts of stem bark and roots of Dennetia tripetala G. Baker. Asian Journal of Plant Science and Research 3:10-13.
- Alicia AW, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322-328.
- Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA 101: 14515-14520.
- Gacar N, Mutlu O, Utkan T, KomsuogluCelikyurt I, Gocmez SS, et al. (2011) Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and morris water maze tests in rats. Pharmacol Biochem Behav 99: 316-323.
- Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM (2009) Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behav Brain Res 205: 550-558.
- Goverdhan P, Sravanthi A, Mamatha T (2012) Neuroprotective effects of meloxicam and selegiline in scopolamine induced cognitive impairment and oxidative stress. Int J Alzheimer’s Dis 974013.
- Aebi H (1974) Catalase. In: Bergmeyer HU (ed.) Methods of Enzymatic Analysis, Verlag Chemie/Academic Press Inc., Weinheim, USA, pp: 673-680.
- Ohkawa H, Nobuko O, Yagi K (1979) Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Smith IK, Vierheller TL, Thorne CA (1988) Assay of glutathione reductase in crude tissue homogenates using 5,5-dithiobis (2-nitrobenzoic acid). Anal Biochem 175: 408-413.
- Das A, Dixit M, Nath C (2005) Role of molecular isoforms of acetyl cholinesterase in learning and memory functions. Pharmacol Biochem Behav 81: 89-99.
- Nunomura A, Perry G, Hirai K, Takeda A, Balraj EK, et al. (2001) Oxidative damage is the earliest event in Alzheimer;s disease. J Neuropathol Exp Neurol 60: 759-767.
- Borsini A, Zunszain PA, Thuret S, Pariante CM (2015) The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38:145-57.
- Boulanger LM (2009) Immune proteins in brain development and synaptic plasticity. Neuron 64:93-109.
- Singh M, Kaur M, Kukreja H, Chugh R, Silakari O (2013) Acetylcholinesterase inhibitors as Alzheimer’s therapy: from never toxins to neuroprotection. Eur J Med Chem 70: 165-188.
- Li, X (2012) Improved pyrogallol autoxidation method: a reliable and cheap superoxide scavenging assay suitable for all antioxidants. J Agric Food Chem 60: 6418-6424.
- Organization for economic co-operation and development guideline 407 & 423.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences