ISSN : 2634-7806
Journal of Clinical and Molecular Pathology : Open Access
Necrotizing Myopathy in the Setting of Antisynthetase Syndrome
Richard A. Prayson1* and Elizabeth E. O’Toole2
1Cleveland Clinic Department of Anatomic Pathology, Cleveland Clinic, Ohio, USA
2MetroHealth Medical Center, Cleveland, Ohio, USA
- *Corresponding Author:
- Richard A. Prayson, MD, MEd
Department of Anatomic Pathology
L25 Cleveland Clinic 9500 Euclid Avenue Cleveland, Ohio, USA
Tel: 216-444-8805
E-mail: praysor@ccf.org
Received date: December 24, 2016; Accepted date: January 31, 2017; Published date: February 06, 2017
Citation: Prayson RA, O’Toole EE (2017) Necrotizing Myopathy in the Setting of Antisynthetase Syndrome. J Clin Mol Pathol 1: 04.
Copyright: © 2017 Prayson RA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Antisynthetase syndrome is a systemic autoimmune process characterized by a constellation of clinical manifestations (myositis, interstitial lung disease, polyarthritis, fevers, Raynaud’s phenomenon and cutaneous rashes). We report the muscle biopsy findings in a 64-year-old male with a past medical history of multiple sclerosis, hypertension, diabetes and depression who presented with an upper respiratory and muscle weakness and pain. He had an elevated white blood cell count, serum creatinine kinase, Westergren sedimentation rate and C reactive protein. Electromyography studies demonstrated evidence of a necrotizing myopathy. He was treated initially with antibiotics for pneumonia and subsequently received methylprednisolone and IVIG. A left deltoid muscle biopsy showed inflammatory myopathic changes. Extensive antibody testing was performed and revealed an OJantibody. The paucity of inflammation on his biopsy may be related to his prior treatment with immunosuppressive agents or may be related to tissue sampling.
Keywords
Antisynthetase syndrome; Inflammatory myopathy; Myositis; Anti-OJ antibody
Introduction
Antisynthetase syndrome (AS) is a systemic autoimmune syndrome marked by the presence of anti-synthetase antibodies and clinical manifestations of interstitial lung disease, non erosive symmetric polyarthritis of small joints, fever, Raynaud’s phenomenon, mechanic’s hand rash and myositis [1,2]. A variety of antibodies have been described in association with the syndrome with anti-Jo-1 (anti-histidyl-tRNA synthetase) being the most common; anti-OJ (anti-isoleucyl-tRNA synthetase) antibody is relatively infrequent and has been observed in only 1% of cases in patients with inflammatory myopathy [2]. Making the diagnosis is challenging because the clinical presentation is quite varied and is often nonspecific. Patients with milder disease symptoms may go undiagnosed. This paper reports on the muscle biopsy results in a patient with AS and OJ Antisynthetase antibody.
Case Presentation and Pathological Findings
The patient is a 64-year-old male with a past medical history of multiple sclerosis, hypertension, diabetes mellitus and depression who presented with an upper respiratory infection, cough and fevers for about 1 month duration. His symptoms progressed to include shoulder and hip pain, muscle weakness and increasing shortness of breath. A chest x-ray showed infrahilar opacities. He had an elevated white cell count of 24.44 (normal 3.70-11.00 k/uL). He was treated for pneumonia. A serum creatine kinase level of 1336 u/L (normal 30-220) was noted. Worsening muscle pain and weakness prompted an electromyography study which showed evidence of necrotizing myopathy affecting muscles of the right upper and right lower extremities. Elevated Westergren sedimentation rate (48 mm/hr; normal 1-14) and C reactive protein (6.15 mg/dL; normal 0.0-1.0) were noted. ANA was negative. The patient was treated with methylprednisolone and IVIG. He demonstrated no evidence of skin rashes or Raynaud’s phenomenon. An extensive antibody panel evaluation was notable for detection of OJ antibody, one of the antisynthetase autoantibodies; testing was negative for PL7, PL12, Mi2, Ku, EJ, SRP, Sm, RNP, SSA, SSB, Centromere, Scleroderma, Jo1, Ribosomal RNP and Chromatin antibodies.
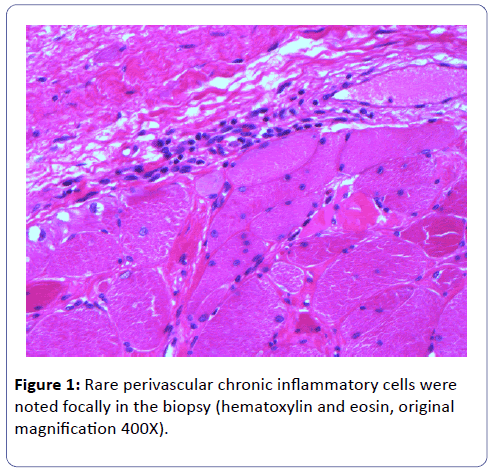
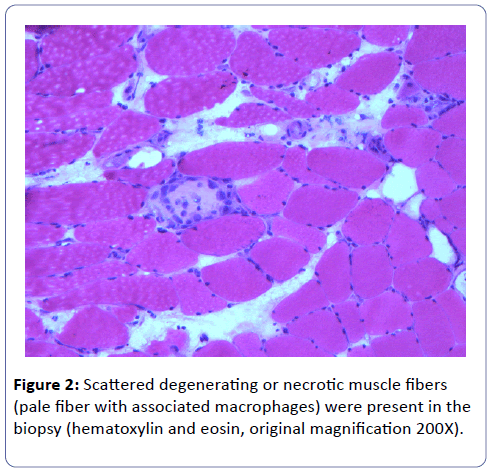
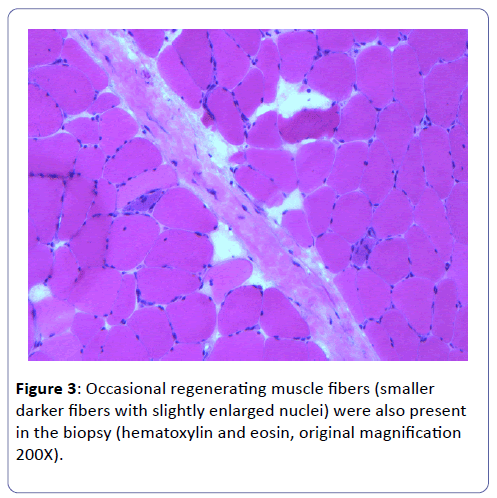
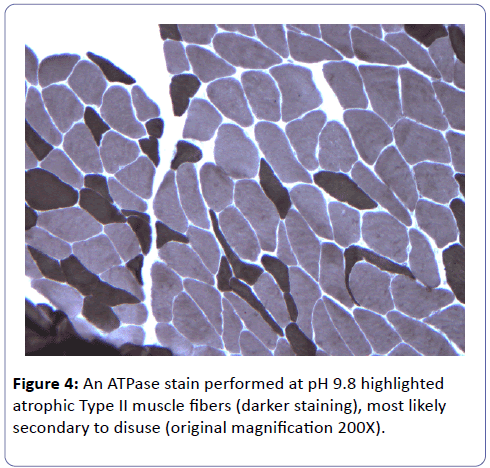
A biopsy of the left deltoid muscle was performed. Rare chronic inflammatory cells comprised of benign appearing lymphocytes and plasma cells, situated proximal to blood vessels, and was observed (Figure 1). There was no evidence of vasculitis or granulomas. Scattered degenerating muscle fibers were present (Figure 2) along with occasional regenerating muscle fibers (Figure 3). There was no evidence of perifascicular atrophy or rimmed vacuoles. Overall, there was a mild variation in muscle fiber size; ATPase stains performed at pH 4.6 and 9.8 highlighted Type II muscle fiber atrophy (Figure 4) and showed no evidence of fiber type grouping.
Figure 1: Rare perivascular chronic inflammatory cells were noted focally in the biopsy (hematoxylin and eosin, original magnification 400X).
Discussion
The spectrum of muscle disease in patients with AS can vary from subclinical with transient elevated creatinine kinase (CK) levels which respond to therapy to patients who experience significant proximal muscle weakness and pain [1-4]. Inflammatory myopathic changes, as encountered in this patient’s muscle biopsy, are found in over 90% of patients [5]. Typical symptoms associated with the usual abrupt onset of skeletal muscle involvement include muscle weakness and muscle tenderness/pain. Some patients have been reported to have weakness in muscles of the cricopharynx, hypopharynx and upper esophagus which can manifest as dysphagia and increased risk of aspiration.
Shortness of breath may result from muscle weakness of the intercostal and diaphragmatic muscles. Atrophy of the muscle with fibrosis may develop over time.
Electromyography can be useful in suggesting the presence of an inflammatory or necrotizing myopathy and findings are most likely to be noted in involved muscle groups. Magnetic resonance imaging studies may demonstrate increased signal intensity in involved muscles and the surrounding tissue [6] and these findings are nonspecific but may be utilized in guiding the muscle biopsy. Elevated serum levels of creatinine kinase, aldolase, alanine aminotransferase, lactate dehydrogenase and aspartate aminotransferase indicative of a necrotizing myopathy are often seen.
Changes observed in a skeletal muscle biopsy, as in the current case, can resemble those seen in other inflammatory myopathic conditions. Chronic inflammation can be observed in the endomysium and perivascular areas and consists of benign appearing lymphocytes, plasma cells and macrophages. Muscle fiber degeneration or necrosis and muscle fiber regeneration is observed in scattered myofibers. Perifascicular atrophy such as one sees in dermatomyositis is not seen. Rimmed vacuoles, such as one encounter in inclusion body myositis, are also not present. Anti –OJ associated disease more frequently manifests with cutaneous features similar to dermatomyositis, a feature which was not present in this patient; it is also almost invariably associated with interstitial lung disease, as in the current patient [7].
From a muscle biopsy perspective, the pathologic findings are not specific and similar features may be encountered in a variety of other inflammatory myopathic conditions including polymyositis, collagen vascular disease associated inflammatory myopathies, certain drug reactions, viral myositis, and certain muscular dystrophies. Correlation of the pathology with clinical manifestations and antibody testing is key to making the correct diagnosis. The amount of inflammation in the current case was minimal, which may be the result of immunosuppressive therapy or may represent a tissue sampling issue. It has been hypothesized that tissue-specific changes in the skeletal muscle may lead to the production of target autoantigens i.e. the aminoacyl-tRNA synthetases [2]. The synthetases function in protein synthesis by catalyzing the acetylation of tRNA. These enzymes may be involved with the recruitment of antigenpresenting inflammatory cells to the muscle. Myositis is most commonly associated with Jo-1 antibodies but may be encountered in association with a variety of other antisynthetase antibodies (OJ, PL-7, PL-12, EJ, Wa, YRS, and Zo) [2].
The mainstay of treatment is glucocorticoids, which may need to be given for a prolonged period of time. Muscle strength may take several weeks to improve with therapy. Creatine kinase levels may take longer to return to normal with treatment. An initial improvement in muscle strength on steroid therapy with subsequent decline in strength may indicate the development of a treatment related steroid myopathy. A subset of patients may require other immunosuppressive agents such as methotrexate or azathioprine to ameliorate symptoms
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- Katzap E, Barilla-LaBarca ML, Marder G (2011) Antisynthetase syndrome. Curr Rheumatol Rep 13: 175-181.
- Chatterjee S, Prayson R, Farver C (2013) Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 80: 655-666.
- Solomon J, Swigris JJ, Brown KK (2011) Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol 37: 100-109.
- Plastiras SC, Soliotis FC, Vlachoyiannopoulos P, Tzelepis GE (2007) Interstitial lung disease in a patient with antisynthetase syndrome and no myositis. Clin Rheumatol 26: 108-111.
- Tzioufas AG(2001) Antisynthetase syndrome. Orphanet encyclopedia.
- Reimers CD, Finkenstaedt M (1997) Muscle imaging in inflammatory myopathies. Curr Opin Rheumatol 9: 475-485.
- Sato S, Kuwana M, Hirakata M (2007) Clinical characteristics of Japanese patients with anti-OJ (anti-isoleucyl-tRNA-synthetase) autoantibodies. Rheumatology (Oxford) 46: 842-845.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences