Nanogels as Next Generation Drug Delivery Systems
K Sri Sruthi*
Department of Pharmaceutics, Vignan Institute of Pharmaceutical Technology, Duvvada, Visakhapatnam, India
- *Corresponding Author:
- Sruthi KS
Department of Pharmaceutics
Vignan Institute of Pharmaceutical Technology
Duvvada
Visakhapatnam
India
E-mail: sruthikommoju@gmail.com
Received Date: October 20, 2020; Accepted Date: November 03, 2020; Published Date: November 10, 2020
Citation: Sruthi KS (2020) Nanogels as Next Generation Drug Delivery Systems. J Pharm Prac Edu Vol.3 No.4:36
Abstract
Nanogels are that innovative drug delivery system which plays an integral part in pointing out many issues related to an old and modern course of treatment such as nonspecific effects and poor stability. Nanogels can may be defined as highly cross-linked nano-sized hydrogels ranges from 20-200 nm. These can also be administered through various routes, including oral, pulmonary, nasal, parenteral, intra-ocular etc. These have high degree of drug loading capacity and it also shows better permeation capabilities due to a smaller size. These also release the drug by pH responsive, thermosensitive, volume transition, photochemical internalization and Photoisomerization mechanism. Actually, the term “nanogels” defined as nanosized particles formed by physically or chemically cross-linked polymer networks that is swell in a good solvent. With emerging field of polymer sciences, it has now become an inevitable to prepare smart nano-systems which can prove effective for treatment as well as clinical trials progress. The brief review aims at the providing comprehensive illustrations on novel applications, drug loading technique, mechanism of the drug release from nanogels. Further, current status, clinical trial status, and future perspective of the nanogels have been summarized.
Keywords
Nanogel; Nanosized particles; Clinical trial status
Introduction
The term ‘nanogels’ defined as nanosized particles formed by the physically or chemically crosslinked polymer networks that is a swell in a good solvent. The term “nanogel” (NanoGel™) was first introduced to define cross-linked bifunctional networks of the polyion and nonionic polymer for the delivery of polynucleotides (cross-linked polyethyleneimine (PEI) and poly (ethylene glycol) (PEG) or PEG-cl-PEI). A Sudden outbreak in the field of nanotechnology have been introduced the need for developing nanogel systems which proven their potential that deliver drugs in controlled, sustained and targetable manner. With the emerging field of polymer sciences which has been now become an inevitable to prepare smart nano-systems which can also prove an effective for treatment as well as the clinical trials progress.
Nanogel
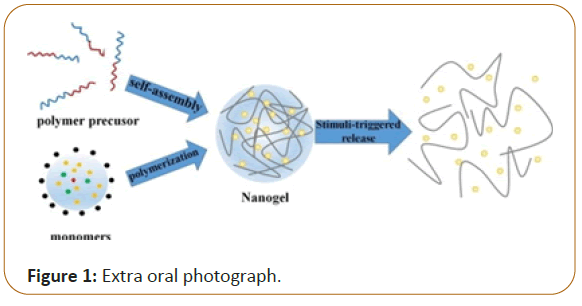
Nanogels defined as highly cross-linked nano-sized hydrogel systems which are either co-polymerized or monomers which are ionic or non-ionic [1,2]. The size of nanogels ranges from 20-200 nm [3]. These escape renal clearance and the prolonged serum half-life period due to their size. Nanogels are the threedimensional hydrophilic networks that have tendency to imbibe water or physiological fluid in large amount, without changing in internal network structure. Chemical modifications can be made to help incorporating plenty of ligands which can be used for the targeted drug delivery, stimulus responsive drug release or preparation of composite materials [4]. Nanogels are known to be exhibit great qualities which contribute to the drive towards it as a delivery system. These include remarkable thermodynamic stability, elevated capacity of the solubilization, relatively low viscosity, and the capability of undergoing vigorous sterilization techniques [5]. Nanogels may entrap drugs and the biological molecules. Therefore, they are vastly employed in protein and gene delivery. Some nanogels possess a hydrophilic nature which limits good encapsulation property of hydrophobic drugs. This issue was faced with encapsulation of anticancer drugs which are hydrophobic in nature. For this purpose, suitable structure engineering of the polymer was adopted to permit high encapsulation of them. Thereby, Nanogels provided a new mean of drug delivery for poorly soluble drugs which doesn’t only improve their solubility and stability but increasing the opportunity of their cellular uptake than the free drug [4] (Figure 1).
Advantagses
High biodegradability, which is a crucial to avoid the accumulation of nanogel material in the bodily organs, thereby leading to toxicity and adverse effects [6].
High biocompatibility, which makes nanogels are very promising approach to drug delivery systems [7].
Extremely small size, which can induce a number of effects such as:
• Enhanced permeation capability [7].
• Avoidance of the rapid renal exclusion. Escaping renal clearance leads to the prolonged serum half-life [7].
• Avoidance of clearance by the phagocytic cells and uptake by the reticuloendothelial system, which permits both passive and active drug targeting [7].
• Capability to cross the Blood Brain Barrier [7].
• Enhanced penetration of endothelium in pathological sites like solid tumors, inflammation tissue and infracted areas. Since Tumor tissues have a high capillary permeability, more nanoparticles permeate into the tumor tissue and accumulate there, which increases the amount of drug delivered and the selectivity of the drug delivery [8].
• Safe delivery of drug carrying nanogel particles into the cytoplasm of target cells, therefore making them ideal for intracellular drug delivery.
• Rapid responsiveness to environmental changes such as pH and temperature [4].
Nanogels are inert in the blood stream and the internal aqueous environment, meaning that they do not induce any immunological responses in the body [8].
Nanogels are suitable to administer both hydrophilic and hydrophobic drugs, as well as charged solutes and other diagnostic agents. This property is highly influenced by the type of functional groups present in the network of polymer chains, the crosslinking density and the type of crosslinking agent incorporated in the polymeric network [7].
Drug loading in nanogels is relatively high when compared to other nanocarriers and drug delivery systems. This is due to the effect of the functional groups present in the polymeric network. By forming hydrogen bonds or other weak linkages within the polymeric network and interacting with drug or protein molecules at the interface, functional groups on the polymeric network tremendously increase the drug loading capacity of nanogels [4].
Nanogels are prepared to be capable of releasing drug in a controlled and sustained pattern at the target site, thereby enhancing the therapeutic efficacy of the drug and avoiding its adverse reactions [7].
Nanogels can be formulated in the form of polymeric micellar nanogel systems that exhibit slower rates of dissociation, better stability over the surfactant micelles, lower critical micelle concentrations, and, most importantly, longer retention of loaded drugs [7].
Bio-macromolecules as well as delicate compounds with low or high molecular weights can be successfully and efficiently encapsulated in the nanogels for the purpose of prolonging the activity of these molecules in the biological environment [4].
Properties of Nanogels
Biocompatability and degradability
Nanogel is made up of either natural or synthetic polymers. They are the highly biocompatible and biodegradable thereby avoiding its accumulation in organs. Chitosan, ethyl cellulose, methyl cellulose and various polysaccharide-based polymers like the dextran, pullulan and the dextrin can be used to prepare the nanogel. Polysaccharides are the mostly carbohydratebased polymers, formed of the repeating monosaccharide units linked by glycosidic bonds. These polymers are stable, non-toxic, hydrophilic and biodegradable in nature [9].
Permeability and particle size
What distinguishes nano delivery systems is that tiny manipulation in the particle size, surface charge and hydrophobicity can be remarkably improve permeability. In spite of the fact these nanoparticles are capable of the permeation by diffusion through tissues or compromised areas of the endothelium and in other some cases through a particular transport system, they created a challenge crossing Blood Brain Barrier (BBB) [9]. So, in order to overcome such dilemma, nanogels were formulated in a way where these possess a diameter of 20-200 nm. It’s small enough to cross (BBB) and in same time avoid rapid clearance mechanisms [7].
Higher drug loading capacity
WJust like any of the other nanodelivery system, nanogels are expected to have the greater loading capacity compared to the conventional dosage forms. This is mainly due to swelling property which allows the formulation to absorb a large quantity of water. Thus, upon the incorporation and loading of the water will provide the cargo space sufficient to contain salts and biomaterials [2]. Loading takes place through three methods:
Physical entrapment: It can be referred to the linkage between hydrophilic chains and the hydrophobic regions of polymer or to dissolving hydrophobic molecules in the hydrophilic vehicle.
Covalent attachment of bioactive molecules which can leads to formation of dense drug-loaded core.
Controlled self-assembly: It is generally for the polyelectrolyte based nanogel. The high loading efficiency is attributed to interaction between oppositely charged electrolytes [2].
Other factors also contribute to the high loading capacity, such as the composition, molecular weight, the possible of interactions between the drug and the employed polymer and different functional groups in each of polymeric unit [6,7].
Colloidal stability
When handling nanoparticle, there is always a propensity of aggregation that compromises the colloidal stability. Formulators are tending to alter surface charge to avoid formation of aggregates in bloodstream and further complications. It can be achieved by increasing zeta potential (minimum of ± 30 mV) that results in the larger repulsive forces between particles that electrostatically stabilize them. Other techniques involve the incorporation of surface modifier like PEG that can produce steric effects and hydration forces to give a stable nanosuspension [9]. If we compare polymeric micellar nanogel systems and surfactant micelles on basis of the stability we will find that the former exhibits better stability lower critical micelle concentrations, decrease in the dissociation rates, and longer retention of loaded drugs. They can also have a high water content that assure good dispersion stability [7,10].
Swelling property in acqueous media
Due to the fact of Nanogels are very small, soft materials, they have ability to swelling presence of an aqueous medium. They are considered to be the fundamental property influencing the mechanism of action followed by this drug delivery system. It depends on:
1. The structure of nanogels includes the Polymer chain’s chemical nature as well as the cross-linking degree and in case of the polyelectrolyte gels; the charge density.
2. Environmental parameters which are related to the variables of the aqueous medium. For instance, in polyelectrolyte gels pH as well as ionic strength and ions’ chemical nature are influential factors. Likewise, temperature is a trigger of swelling in cases of thermoresponsive gels [2].
Providing appropriate circumstances allows rapid swelling/ deswelling. Regardless of the trigger, swelling takes place only when the osmotic pressure exerted by medium ions and the polymer’s network swelling pressure are imbalanced [11].
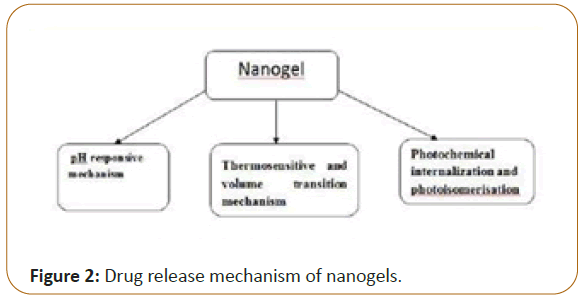
Drug release mechanism of nanogels
There are multiple mechanisms to which release of the drug or the biomolecule is attributed to including: simple diffusion, degradation of the nanogel structure, pH and temperature changes, counterion displacement or induced due to external energy source [2] (Figure 2).
pH responsive mechanism
As of the name indicates, drug release responds to pH changes in surrounding environment. In other words, the release of drug can be take place in different physiological environments which acquire different pH values. The most release will take place in appropriate pH which means that the release is mainly by achieved in a targeted area of the body that possesses that pH. This mechanism that is based on the fact that polymers employed in the synthesis of a nanogel contain pH sensitive functional groups that deionize in the polymeric network. The deprotonation results in increase in osmotic pressure, swelling and porosity of the polymer which triggers the release of the electrostatically bound molecules [2,5].
Photochemical internalization and photoisomerization
Photoisomerization refers to a process in which is a bond of restricted rotation undergoes some conformational changes due to the exposure of light. Double bond containing molecules which are good example; these isomerize usually from a trans orientation to cis orientation upon light irradiation [12]. When photosensitizers loaded nanogel are excited, they produce two species of the oxygen (singlet and reactive) which can be a result in oxidation in the cellular compartment walls that highly influence the release of therapeutic agents into the cytoplasm [1]. Azodextran snanogel loaded with aspirin was a subject of release studies. The observations showed that Cis-trans isomerization of azobenzene by photoregulation causes the formation of E-configuration of azo group. This results in better release profile of aspirin compared to the previous Z-configuration [1,12,13].
Thermosensitive and volume transition mechanism
Some nanogels are reactive to a specific temperature known as volume phase transition temperature (VPTT) which means they display a change in the volume according to temperature. If surrounding medium is below VPTT, the polymer becomes quenched and hydrated which makes it swell and release the drug loaded. Above VPTT that opposite occurs and the nanogel shrinks too abruptly and the content flows out [14]. Previously, the thermoresponsive nanogels used to rupture cellular network when they expand and increase in the volume. So, some of the alterations were applied on thermosensitive drug-containing nanogels like changing of polymers ratio to the achieve lower critical solution temperature. One of good example is the biocompatible magnetic field targetibility of poly stimulus are termed as multi-responsive nanogels [7].
Based on the type of linkages present in the network chains of polymeric gel structure
Chemically cross linked nanogels: Where the physically crosslinked nanogels which are linked by weak forces, chemically crosslinked nanogels are formed by networks of strong covalent bonds and other permanent chemical linkages. The strength of the linkage is highly dependent on the type of functional groups present in the molecules of the nanogel network.
(N-isopropylacrylamide) and chitosan nanogel which is quiet employed in hyperthermic cancer treatment.
Limitations
It is expensive to remove the surfactant and the solvent at the end of preparation process although the manufacturing process itself is not very pricey.
Classification of Nanogels
Based on their behavior towards a specific stimuli
Non responsive nanogels: When non-responsive nanogels come in contact with the water, they can absorb it, resulting in the swelling of nanogel.
Stimuli responsive nanogels: Environmental conditions, such as temperature, pH, magnetic field, and ionic strength, control
whether swelling will occur or not and the extent of swelling or deswelling of the nanogels. Any of the changes in any of these environmental factors, which act as stimuli, will lead to alteration in the behavior of nanogels as of response, hence the term is stimuli-responsive nanogels (Table 1).
| Type of nanogel | Drug | Disease | Activity |
|---|---|---|---|
| 1.PAMA-DMMA nanogels | Doxorubicin | Cancer | Increase in the release rate as the pH value decreased. Higher cytotoxicity at pH 6.8 in cell- viability studies |
| 2.PCEC nanoparticles in Pluronic hydrogels | Lidocaine | Local anesthesia | Produced long-lasting infiltration anaesthesia of about 360 min |
| 3.Cholesterol bearing pullulan nanogels | Recombinant murine interleukine-12 | Tumor immunotherapy | Sustained release nanogel |
| 4.Poly(N-isopropylacrlamide) and chitosan | Hyperthermia cancer treatment and targeted drug delivery | Thermosensitive magnetically modalized | |
| 5.Cross-linked branched network of polyethyleneimine and PEG Polylexnanogel | Fludarabine | Cancer | Elevated activity and reduced cytotoxicity |
| 6.Biocompatible nanogel of cholesterol-bearing pullulan | As artificial chaperone | Treatment of Alzheimer’s disease | Inhibited aggregation of amyloid β-protein |
| 7.DNA nanogel with photocross- linking | Genetic material | Gene therapy | Controlled delivery of plasmid DNA |
Table 1: Types of nanogels and its activity.
Nanogels which are responsive to more than one of environmental In order to synthesize this type of nanogels, polymeric chains are the cross-linked at a specific point, called cross-linking points, which are determined by multifunctional of cross-linking agent present. Using different polymers and different chemical linking strategies leads to the production of nanogels with a variety of properties for a number of applications. In addition, the physiochemical properties of the nanogel can be modified depending on the type of cross-linking agent used to produce the polymer and the position of cross-linking points. Hydrophilic polymers or amphiphilic copolymers, produced by polymerization of vinyl monomers, are usually used to produce chemically crosslinked nanogels.
For example, a nanogel ranging in the size from 20 to 200 nm, in which polymeric chains containing pendant thiol groups were crosslinked by an environment friendly chemical method, was produced [7].
Physically cross linked nanogels: Physically cross-linked nanogels, which are also called pseudo gels, depend greatly on the characteristics of the polymer used in their production including polymer composition, temperature, concentration of the polymer, type of cross-linking agent, and the ionic strength of the medium. Weak linkages like [1] van der Waals forces, [2] hydrogen bonding or [3] hydrophobic, electrostatic interactions are the forces that form this type of the nanogels. Physical crosslinked nanogels can be produced within a short time via a number of simple methods. These methods involve a variety of processes such as association of amphiphilic blocks, self-assembly, aggregation of polymeric chains as well as the complexation of the oppositely charged polymeric chains.
Liposome modified nanogels: Liposome modified nanogels are physically cross-linked, stimuli-responsive nanogels, which are being studied as transdermal drug delivery devices, due to their unique properties. These are the nanogels which involve the incorporation of poly [N-isopropyl-acrylamide] copolymeric groups into the liposomes, resulting in a high degree of responsiveness to both pH and temperature. In addition, Succinylated poly[glycidol]s are infused to the liposomes, under pH of less than 5.5, in order to create nanogels that effectively and efficiently deliver Calcein to the cytoplasm of target cells [15].
Micellar nanogels: Micellar nanogels are produced by supramolecular self-assembly of the both hydrophilic and hydrophobic blocks or by the graft copolymers in an aqueous solution. Micellar nanogels consist of a hydrophilic shell (corona), made of polymer blocks, surrounding a hydrophobic core, and stabilizing the whole micelle. The purpose of this conformation is to provide sufficient space to contain drugs or biological macromolecules just by physically entrapping these particles inside the borders of a shell, thereby acting as a drug delivery system. As the micelle enters the body, the hydrophilic shell interacts with an aqueous media by forming hydrogen bonds in order to protect the hydrophobic core which is carrying the drug to its target cells. This process protects the drug molecules from being the hydrolyzed or degraded by the enzymes.
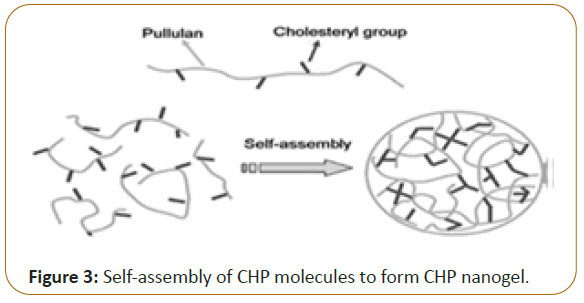
Hybrid nanogels: When the particles of a nanogel are dispersed in an organic or inorganic medium, it is known as the Hybrid nanogel. Self-assembly and the aggregation of an amphiphilic polymers, such as pullulan-PNIPAM, hydrophobized polysaccharides, and hydrophobized Pullulan, were the processes used for the formation of nanogels in aqueous medium. Specifically, Cholesterol-Bearing Pullulan (CHP) nanogels were investigated. These are stable monodispersed nanogels formed by selfaggregation of the CHP molecules (formed of pullulan backbone and cholesterol branches) with hydrophobic groups providing physical crosslinking points. CHP nanogels were found to have an unique abilities to not only the complex with molecules like DNA, proteins and the various drugs but also to coat solid surfaces like liposomes, particles and an even cells. Hybrid nanogels have significance, particularly, as drug delivery systems for insulin and anticancer drugs [9] (Figure 3).
Preparation techniques of nanogels
Modified pullulan technique: The example that can be given for this category is selfassembled hydrophobized pullulan nanogel. The pullulans are modified in to stages; initially methacrylates are used, then with hydrophobic 1-hexadecanethiol. The end product is an amphiphilic material that upon addition of water starts to assemble itself by hydrophobic interaction among alkyl chains [16].
Another example is Cholesterol based pullulan nanogel. Here, pullulan was substituted with 1.4 cholesterol and the nanogel is fabricated by simply reacting cholesterol isocynate in dimethyl sulfoxide and pyridine. This mixture was freeze dried and in aqueous phase it formed nanogel which further formed a complex with W-9 peptide, a TNF-alpha and RANKL antagonist for delivery of osteological disorder [18].
Reverse microemulsion polymerization technique: Lithium loaded Polyacrylic acid (PAA) nanogels were formulated by reverse microemulsion polymerization technique. 3.43 g span 80 and 2.62 g span 80 were added to 100 ml hexane that is oil phase and kept for stirring using magnetic stirrer. Aqueous phase was prepared by adding 1.5 ml of 10% (w/w) LiOH in water to 500 μl acrylic acid. Add 214 μl of 5% (w/v) N, N’-Methylenebisacrylamide (MBA) suspension, 500 μl of 2% (w/v) potassium persulfate and 40 μl of 20% (w/v) N, N, N’, N’Tetramethylene-diamine (TEMED) to an aqueous phase [19].
Microemulsion was formed by addition of an aqueous phase drop wise into an oil phase. Emulsion was transferred to 60°C water bath and the stirred at an 400 rpm using the magnetic stirrer, kept overnight at a room temperature. Supernatant were decanted and the pellets were collected. Microemulsion is thermodynamically stable [19].
Free radical crosslinking polymersization technique: Photocrosslinked Biodegradable Photoluminescent Polymers (PBPLPs) nanogel was prepared by the free radical crosslinking of a vinyl-containing florescent prepolymer for the drug delivery and cell imaging. Development of a PBPLPs nanogel which shows a new era to develop nanobiomaterials in theranostic nanomedicine for drug delivery and cell imaging [20].
Photolithographic techniques: Photolithographic techniques, photochemical reaction for activation and subsequent reaction which have explored in strive of producing 3D hydrogel particles and nanogels for drug delivery. In this method, stamps or replica molds are treated to give the surface specific properties that also allow the molded gels to release the incorporated agents [21]. Microfabrication of such gels follow a general strategy where poly (dimethylsiloxane) (PDMS) stamps are utilized to mold, release, and which stack gels into 3 dimensional structures. Surface modification enhances the release or adhesion of the molded gels to a substrate. The most known as techniques to modify PDMS stamps are usually achieved by hexa (ethylene glycol)-terminated self-assembled monolayers (SAMs), or by the adsorbed monolayers of bovine serum albumin (BSA) [22].
Emulsion polymerization technique: l-proline functionalized PMMA [poly (methyl methacrylate)] nanogel with a range of catalyst functionalization (0.5-15 wt%) and cross-linking densities (0-50 wt%) were prepared by the emulsion polymerization technique [23]. In emulsion polymerization technique monomer droplets are formed by mechanical stirring [24].
Inverse miniemulsion polymerisation technique: Fluorescent dye Rhodamine B or the Fluorescein labeled nanogels were prepared by the activators generated electron transfer atom transfer radical polymerization (AGET ATRP) of oligo (ethylene oxide) monomethyl ether methacrylate (OEO300MA) by an inverse mini-emulsion polymerization of the water/cyclohexane at an ambient temperature. Hydroxyl containing ATRP initiator was used to control the polymerization to produce functional HO-POEO300MA nanogels. Cell adhesive nanogels were synthesized using ACRLPEO-GRGDS as a co-monomer during the polymerization [3]. In O/W mini-emulsion technique monomer droplets are formed by the applying high shear stress by ultrasonication or high-pressure homogenizer. Miniemulsion is the kinetically stable [24].
Applications
Vaccine delivery
Vaccination is based on the induction of an immune response that is antigen-specific. In order to the enhance potency and performance of the vaccines, polymeric nanogels are being utilized as the novel, alternative means of a vaccine delivery. The advantage of nanogels over a conventional vaccine lies in the ability of nanogel network to protect vaccine antigens from enzymatic degradation. Target specificity of the vaccine delivery can be significantly enhanced by using surface modified nanogels with attached antibodies and other ligands [25].
Bone regeneration
For a successful regeneration of the bones, where biodegradable cell scaffolds should release lithium as well as the other medicament slowly and locally. Bone growth can be increased by the lithium, hence, lithium nanogels, synthesized by a micro-emulsion polymerization of the polyacrylic acid and is incorporated into the biodegradable polyhydroxybutyrate matrix, are formulated for the controlled release of lithium into bone tissue [19].
Anti-inflammatory
Nanogels have found an application to dermatology and a cosmetology as topical delivery systems of non-steroidal antiinflammatory drugs (NSAIDs) and for the treatment of allergic contact dermatitis and psoriatic plaque. Nanogels are being ideal for this application since they overcome major limitation of the topical delivery systems, which is relatively the short contact time between the active drugs and the application site. This is done by the retaining water into gel matrix and forming a uniform dispersion of the nanogel. The simultaneous topical delivery of two anti-inflammatory drugs, Spantid II and ketoprofen was successfully achieved through a nanogel of the poly-(lactideco- glycolic acid) and chitosan. Oleic acid was used for surface modification. A variety of inflammatory disorders can be treated using this nanogel system as it can effectively permeate to deep layers of the skin [26].
Local aneastics (la)
Local anesthetics are one of the classes which drugs that induce analgesia and eliminate pain. The analgesic effect of the local anesthetics is due to the blockage of the nerve impulses in the nerve cell membrane by shutting the voltage gated Na+ channels. The manner and intensity of the nerve stimulation as well as its resting membrane potential will determine the degree of the numbness induced by a specific concentration of a local anesthetic. Local anesthetics are clinically classified into two classes, depending on their chemistry: amino esters and amino amide. An over dosage of local anesthetics leads to their high toxicity, which has been sparked the interest in formulating controlled release the drug delivery systems of them. Incorporating local anesthetics into the drug delivery systems like a nanogels can improve their regional administration. A delivery system of a procaine hydrochloride, which is an amino ester local anesthetic, loaded into methacrylic acid ethyl acrylate nanogel via hydrophobic and hydrogen bonds exhibited a high release rate at a high pH. The mechanism of release is based on deprotonation of the acid on the nanogel which leads to an increase in the osmotic pressure and the swelling of the whole system, which increases the porosity, thus promoting the release of the procaine hydrochloride [5].
Cancer treatment
Biodegradable nanogel prepared by a cross linking of the polyethyleneimine and PEG/pluronic used for 5’triphosphorylated ribavirin reduced toxicity [27]. Doxorubicine loaded selforganizing nanogel formulated by acetylated chondroitin sulphate used for the cancer treatment [26]. pHresponsive doxorubicine uptake accelerated nanogel containing glycol chitosan, which was grafted with the 3-diethylaminopropyl groups [28]. Selfquenching polysaccharide-based pullulan/folate-pheophorbide used in minimal toxicity of pheophorbide [29]. Cross linked branched network of a polyethyleneimine and PEG [Polyplex nanogel] used for elevated activity and reduced by the toxicity of fludarabine [30]. Self-assembled nanogel composed of heparin pluronic used to deliver the RNaseA enzyme to internalize in cell [31]. Cholesterol bearing pullulan sustained release nanogels used in recombinant murine onterlikine-12 sustained tumor immunotherapy [32]. Reducible heparin with the disulfide linkage nanogel used in an internalization of heparin for apoptotic death of melanoma cells [33]. Specific targeting nanogel of a doxorubicin loaded acetylated hyaluronic acid used in cancer treatment [34]. pH and temperature responsive cadmium (II) ions quantum dots, made of Hydroxypropylcellulose – poly (acrylic acid) used in the cell imaging [35]. In-situ Poly (Nisopropylacrylamide-coacrylamide) gelatinized thermosensitive nanogel used to deliver the 5-fluorouracil [36]. Cholesterol bearing pullulan with modified amino group, quantum dot hybrid nanogel used for bioimaging [37]. Generally, the nanoparticles possess an average diameter of nearly 100 nm, neutrality and a surface hydrophilicity which results in a prolonged blood circulation and an increased level of tumor delivery [38].
Diabetics
As diabetes becomes more and more prevalent in the world’s population, revolutionized approaches are being considered for its treatment. An injectable nanogel network that is sensitive to changes of glucose levels in the blood and releases specific amounts of insulin accordingly has been formulated, containing a network of oppositely charged nanoparticles. These nanoparticles attract each other, forming a gel matrix that remains intact and responds to changes in pH. By utilizing dextran, the nanogel network will carry insulin and other enzymes necessary for the conversion of glucose into gluconic acid. Under conditions of hyperglycemia, glucose molecules, being easily diffusible through the nanogel, pass the gel network and trigger the conversion process of glucose into gluconic acid, thereby decreasing the pH of the medium. This will, in turn, stimulate the release of insulin. Even though this approach is very promising for the treatment of diabetes, it is still new and needs some work to be done before this nanogel is suitable for human trials [39].
Antibacterial and anti-microbioly activity
Infections have been becoming increasingly difficult to cure due to the resistance to conventional delivery systems of antibiotics. In order to treat a microbial infection, where a quick and localized action is required, which is possible in a nanogel delivery systems. Dextran crosslinked polyacrylamide nanogels (polysaccharide based nanogels) loaded with zinc nitrate (zinc ions) as antibacterial agent were prepared by mini-emulsion method. The crosslinking agent used was methacrylated hyaluronic acid. The purpose of this nanogel was to target the methicillin-resistant strains of staphylococcus aureus [40].
Ophthalmology
Dexamethasone containing eye drop was prepared by a solvent evaporation or emulsification method using 2-hydroxypropyl-γ- clclodextrin (HP γ CD) medium containing γCD nanogel for the sustain release. pH-sensitive polyvinylpyrrolidone-poly [acrylic acid] (PVP/PAAc) nanogels, formulated by the γ radiation-induced polymerization of an acrylic acid (AAc) in an aqueous solution of polyvinylpyrrolidone (PVP) acting as a template, were used to encapsulate pilocarpine, thus the enhancing of bioavailability as well as the stability of pilocarpine and maintaining an adequate concentration of the drug at a site of action for a prolonged period of time [41,42].
Auto-immune disease
DThe treatment of an autoimmune disorders is based on ability of the drug delivery system to a selectively disable the immune cells that mediate an autoimmunity response. The incorporation of the immunosuppressant drugs into the nanogel delivery systems have been extensively studied for this purpose which since nanogels can improve that the immunosuppression effect by targeting the antigen presenting cells that contribute to disease and enabling systemic accumulations of the loaded drug. A nanogel system of the mycophenolic acid compexed with non-methylated βcyclodextrin was formulated by loading of liposomes with a diacrylate terminated copolymer of poly (lactic acid-co-ethyleneglycol) and are tested for the treatment of systemic lupus erythematosus, an autoimmune disease. Where the cross linking between acrylated monomers and the gelation of the particles into a stable mix was achieved by exposing the nanogel system to ultraviolet radiation.
Transdermal drug delivery
Transdermal route of an administration has many advantages of over other routes in that it bypasses first pass effect, improves the efficiency of drugs, provides steady state drug concentration in the plasma and also increases patient compliance. There are a variety of approaches which were considered to enhance the penetration of drug into site of action. A promising approach is the use of nanogels for topical delivery of active pharmaceutical ingredients to the stratum corneum. As an oral administration of aceclofenac causes a number of side effects like ulcers and gastric bleeding, transdermal delivery of the drug, was studied as an alternative, and showed better stability and permeability. Through the emulsion solvent diffusion method, a dispersion of aceclofenac was formed and incorporated into a gel matrix to formulate a nanogel for the transdermal delivery of the drug [43- 45].
Conclusion
As it is a new and improved approach to diagnosis and the treatment of a wide range of diseases, nanogels have been proved to bring about a huge advancement in this field. Nanogels have been versatile properties that make them which are capable of efficient delivery of biologically active molecules, particularly biopharmaceuticals. This has given rise to a number of therapeutic applications; nanogels are used in a controlled delivery of an active drug compounds. They can also be used as a carrier, or chaperone, to treat diabetes, cancer, neurodegenerative disease, etc. Unique properties of nanogels, like of their tailoring characteristics and easy encapsulation of therapeutics, have promoted these applications of nanogels. They can also been used to minimize the side effects of drugs and lower their therapeutic dose, resulting in improved efficacy of therapeutic agents and increased benefit to the patient.
References
- Dorwal D (2012) Nanogels as novel and versatile pharmaceuticals. Int J Pharm Pharm 4: 67-74.
- Kabanov AV, Vinogradov SV (2009) Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl 48: 5418-5429.
- Bencherif SA, Siegwart DJ, Srinivasan A, Horkay F, Hollinger JO, et al. (2009) Nanostructured hybrid hydrogels prepared by a combination of atom transfer radical polymerization and free radical polymerization. Biomaterials 30: 5270-5278.
- Soni G, Yadav KS (2016) Nanogels as potential nanomedicine carrier for treatment of cancer: A mini review of the state of the art. Saudi Pharm J 24: 133-139.
- Tan JP, Tan MB, Tam MK (2010) Application of nanogel systems in the administration of local anesthetics. Local Reg Anesth 3: 93-100.
- Gonçalves C, Pereira P, Gama M (2010) Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 3: 1420- 1460.
- Sultana F, Manirujjaman, Md Imran-Ul-Haque, Arafat M, Sharmin S (2013) An overview of nanogel drug delivery system. J Appl Pharm Sci 3: 95-105.
- Rigogliusoa S, Sabatinob MA, Adamoa G, Grimaldib N, Dispenzab C, et al. (2012) Nanogels: Nanocarriers for drug delivery application. Chemical Engineering Transactions 27: 247-252.
- Vinogradov SV, Batrakova EV, Kabanov AV (2004) Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem 15: 50-60.
- Vinogradov SV (2010) Nanogels in the race for drug delivery. Nanomedicine 5: 165–168.
- Kazakov S, Levon K (2006) Liposome-nanogel structures for future pharmaceutical applications. Curr Pharm Des 12: 4713-4728.
- Fomina N, Sankaranarayanan J, Almutairi A (2012) Photochemical mechanisms of light-triggered release from nanocarriers. Adv Drug Deliv Rev 64: 1005-1020.
- Patnaik S, Sharma AK, Garg BS, Gandhi RP, Gupta KC (2007) Photoregulation of drug release in azo-dextran nanogels. Int J pharm 342: 184-193.
- Lu X, Sun M, Barron AE (2011) Non-ionic, thermo-responsive DEA/DMA nanogels: Synthesis, characterization, and use for DNA separations by microchip electrophoresis. J Colloid Interface Sci 357: 345-353.
- Labhasetwar V, Leslie-Pelecky DL (2007) Biomedical applications of nanotechnology.
- Ferreira SA, Coutinho PJG, Gama FM (2011) Synthesis and characterization of self-assembled nanogels made of pullulan. Materials 4: 601-620.
- Alles N, Soysa NS, Hussain MA, Tomomatsu N, Saito H, et al. (2009) Polysaccharide nanogel delivery of a TNF-α and RANKL antagonist peptide allows systemic prevention of bone loss. Euro J Pharm Sci 37: 83-88.
- Akiyoshi K (2007) nanogel-based materials for drug delivery system. European Cells and Materials 14: 36.
- Larsson M, Bergstrand, A, Mesiah, L, Vooren CV, Larsson SA (2014) Nanocomposites of polyacrylic acid nanogels and biodegradable polyhydroxybutyrate for bone regeneration and drug delivery. J Nanomaterials 2014: 1-9.
- D Manry, D Gyawali, J Yang (2011) Size optimization of biodegradable fluorescent nanogels for cell imaging. High School Res 2: 1.
- Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K (2008) The development of microgels/nanogels for drug delivery applications. Prog Polym Sci 33: 448–477.
- Tang MD, Golden AP, Tien J (2003) Molding of three-dimensional microstructures of gels. J Am Chem Soc 125: 12988-12989.
- Lu A, Moatsou D, Longbottom DA, O’Reilly RK (2013) Tuning the catalytic activity of L-proline functionalized hydrophobic nanogel particles in water. Chem Sci 4: 965-969.
- Sanson N, Rieger J (2010) Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym Chem 1: 965-977.
- Ferreira SA, Gama FM, Vilanova M (2013) Polymeric nanogels as vaccine delivery systems. Nanomedicine 9: 159-173.
- Shah PP, Desai PR, Patel AR, Singh M (2012) Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials 33: 1607-1617.
- Kohli E, Han HY, Zeman AD, Vinogradov SV (2007) Formulation of biodegradable nanogel carriers with 5’-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Controlled Release 121: 19-27.
- Singka GSL, Samah NA, Zulfakar MH, Yurdasipe A, Heard CM (2010) Enhanced topical delivery and anti-inflammatory activity of methotrexate from an activated nanogel. Euro J Pharm Biopharm 40: 234-253.
- Bae B, Na K (2010) Self-quenching polysaccharide based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials 31: 6325-6335.
- Vinogradov SV, Zeman AD, Batrakova EV, Kabanov AV (2005) Polyplex nanogel formulation for drug delivery of cytotoxic nucleoside analogs. J Controlled Release 107: 143-157.
- Choi JH, Jang JY, Joung YK, Kwon MH, Park KD (2010) Intracellular delivery and anti-cancer effect of assembled heparin-pluronic nanogel with RNase. J Control Release 2: 32-45.
- Shimizu T, Kishida T, Hasegawa U, Ueda Y, Imanishi J, et al. (2008) Nanogel DDS enables sustained release of IL-12 for tumor immunotherapy. Biochem Biophys Res Commun 367: 330-335.
- Bae KH, Mok H, Park TG (2008) Synthesis, characterization and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 29: 3376-3383.
- Park W, Kim KS, Bae B, Kim Y, Na K (2010) Cancer cell specific targeting of nanogels from acetylated hyaluronic acid with low molecular weight. Euro J Pharm Sci 40: 367-375.
- Wu W, Aiello M, Zhou T, Bernila A, Banerjee P, et al. (2010) In situ immobilization of quantum dots in polysaccharide based nanogel for integreation of optical pH sensing, tumor cell sensing and drug delivery. Biomaterials 31: 3023-3031.
- Wang Q, Xu H, Yang X, Yang Y (2008) Drug release behavior from in situ gelatinized thermosensitive nanogel aqueous dispersions. Int J Pharm 361: 189-193.
- Hasegawa U, Nomura ICM, Kaul SC, Hirano T, Akiyoshi K (2005] Nanogel quantum dots shybrid nanoparticles for live cell imaging. Biochem Biophys Res Commun 331: 917-921.
- Look M1, Stern E, Wang QA, DiPlacido LD, Kashgarian M, et al. (2013)] Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J Clin Invest 123: 1741-1749.
- Paddock C (2013) Nanogel to Manage Type 1 Diabetes. Medical News Today.
- Malzahn K, Jamieson WD, Droge M, Mailander V , Jenkins ATA, et al. (2014) Advanced dextran based nanogels for fighting Staphylococcus aureus infections by sustained zinc release. J Mater Chem B 2: 2175-2183.
- Moya-Ortega MD, Alves TF, Alvarez-Lorenzo C, Concheiro A, Stefánsson E, et al. (2013) Dexamethasone eye drops containing γ Cyclodextrin based nanogel. International journal of Pharmaceutics 441: 507-515.
- Abd El-Rehim HA1, Swilem AE, Klingner A, Hegazy el-SA, Hamed AA (2013) Developing the potential ophthalmic applications of pilocarpine entrapped into polyvinylpyrrolidone-poly nanogel dispersions prepared by γ radiation. Biomacromolecules 14: 688- 698.
- Phatak AA, Praveen DC (2012) Development and evaluation of nanogel as a carrier for transdermal delivery of aceclofenac. Asian J Pharm Tech 2: 125-132.
- Singh N, Nisha, Gill V, Gill P (2013) Nanogel based artificial chaperone technology: an Overview.American. American J Adv Drug Del1: 271- 276.
- Park W, Park SJ, Na K (2010) Potential of self-organizing nanogel with acetylated chondriotin sulfate as an-anti-cancer drug carrier. Colloids Surf B 79: 501-508.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences