Morphine Promotes Breast Cancer Cell Proliferation via μ-Opioid Receptor Independent-MRG Dependent Signaling: An in vitro Study
Tony L Yaksh1*, Tsutomu Mieda1, Roshni Ramachandran1, Yuelian Zhu1, Yajun He1, Patrick W Mantyh2 and Linda M Page3
1Department of Anesthesiology, University of California, San Diego, USA
2Department of Pharmacology, University of Arizona, Tucson, USA
3Implantables Research and Technology, Medtronic, Inc, Restorative Therapies Group, Minneapolis, Minnesota
- *Corresponding Author:
- Tony L Yaksh
Department of Anesthesiology, University of California, San Diego, La Jolla CA, USA
Tel: 16195433597
E-mail: tyaksh@health.ucsd.edu
Received date: March 26, 2020, Manuscript No. IPCBTO-20-3660; Editor assigned date: April 01, 2020, PreQC No. IPCBTO-20-3660(PQ); Reviewed date: April 15, 2020, QC No. IPCBTO-20-3660; Revised date: June 28, 2022, QI No. Q-IPCBTO-20-3660; Manuscript No. IPCBTO-20-3660(R); Published date: July 26, 2022
Citation: Mieda T, Ramachandran R, Zhu Y, He Y, Yaksh TL, et al. (2022) Morphine Promotes Breast Cancer Cell Proliferation via μ-Opioid Receptor Independent-MRG Dependent Signaling: An in vitro Study. Cancer Biol Ther Vol.6 No.4:001
Abstract
Systemic morphine results in significant pain relief in patients who are in advanced stage of cancer. However, the possibility that morphine may facilitate tumor development is a recent concern. Pre-clinical studies have shown that morphine may enhance tumor angiogenesis and promote cancer growth, reducing long term survival with few contradictory studies. The present study addresses the effects of morphine on cell proliferation using a rat mammary adenocarcinoma cell line (MTLn3). MTLn3 cells were cultured in MEM-alpha growth media supplemented with 5% heat-inactivated fetal bovine serum and 0.5% penstrep. Cells were incubated at 37Ë? C in a humidified 5% CO2 environment and grown to 70%-90% confluence. To assess proliferation, MTLn3 cells were seeded (8 x 104 cells/well) in a 12-well plate and incubated for 24 hours, followed by 48 hours incubation with four mu opioid agonists (morphine: 3 nM-30 M; fentanyl: 0.03 pM-0.3 TMD; M-DALDA: 3 nM-30 Pro M ZM 21: 3 nM-30 M). To examine antagonism Morphine+naloxone (3 nM-30 M), cell proliferation assays were performed after 48 hours by counting total number of cells. Morphine enhanced proliferation of MTLn3 cells following 48 hours incubation with a peak effect observed with 0.03 μM morphine. This effect was not reversed by naloxone or naltrindole. Of note, fentanyl PZM-21 nor DMTDALDA had no effect upon proliferation. The common feature is that morphine is a mu agonist that activates in a naloxone independent fashion Mas Related G-coupled (MRG) orphan receptors. The three agents not affecting cancer cell growth are potent mu agonists, but they are not considered to activate MRGs. Of note MTLn3 cells express MRGs. These results show that morphine enhanced proliferation of breast cancer cells and that the tumor enhancing effects, independent of u-opioid receptor, may reflect an effect of MRG signaling in this syngenic cell line.
Keywords
Cancer; MTLn3; Morphine; Opioids; Fentanyl
Introduction
Recent advances in the cancer therapies have been successful in extending the life of patients suffering from advanced form of cancer. However, pain secondary to cancer occurs with a high prevalence and negatively impacts the quality of life, resulting in adverse physiological events that arise from the stress of an unmanaged pain state [1]. The liberal use of analgesics is considered standard of care, not only for humane reasons, but based on the long-held appreciation that pain can lead to depression of immune function and perhaps enhanced tumor growth and viability [2]. Oral morphine is an effective agent for managing severe cancer pain states [3]. An appropriate concern is the potential effect of supportive therapeutics on tumor development and long-term patient survival. Several retrospective clinical studies examining the effects of anesthetics on the interval between tumor relapse suggested that cancer patients managed with perioperative morphine displayed worse outcomes compared with those receiving nonopioid regional anesthetic management [4,5]. Such commentary has led to the speculation that opioids promote tumor progression and relapse [6]. Several mechanisms proposed for this facilitatory effect have included modulation of the immune response or changes in cellular pathways regulating survival and metastatic behavior of the cancer cells [7]. Preclinical work has demonstrated that:
• Morphine may result in decreased mitogenic activity of lymphocytes and other inflammatory cells [8-10].
• Morphine may enhance tumor angiogenesis [11-14].
• Morphine may initiate survival-promoting signaling enhancing cancer growth and metastases [15-17]. Although the preponderance of studies suggests a facilitatory effect of morphine on tumor growth and vascularity, several papers report minimal opiate effect on either tumor progression [18] or angiogenesis [19].
The present study arose from work to develop a syngenic tumor model to study opiate effects on tumor growth in vivo using nonimmune suppressed rats. In this, we screened several syngenic cell lines and focused on the MTLn3 mammary tumor cell line. We took the opportunity to investigate in cell culture the effects of morphine on this cell line of several opiates, including morphine and several biased mu opioid ligands to determine their effect upon MTLn3 cell line proliferation.
In the course of these studies, we found a facilitating effect of morphine, but not the biased ligands, consistent with the discrepant agonist’s pharmacology and observed that the effects of naloxone were not reversed by naloxone. Additionally, the mu agonists that were biased ligands were found to lack the activation of MRGs. Morphine, in contrast, is a potent MRG agonist [20]. These results suggest that MRG activation by morphine may play a role in the proliferative action of the mu opioid.
Materials and Methods
MTLn3 cell culture
MTLn3 rat mammary adenocarcinoma cell line was obtained from John Condeelis/Jeffrey E. Segall/Robert Eddy at the Albert Einstein College of Medicine. The MTLn3 cell population was cultured according to the instructions provided by the provider. Briefly, MTLn3 cells were cultured in growth medium consisting of MEM-alpha (Life Technologies Corp; cat # 12561-056, stored at 4oC) culture medium containing 5% sterile FBS and 2.5 mL of penicillin and streptomycin (Sigma 10,000 units of penicillin and 10 mg of streptomycin/mL) for 500 mL volume.
Culture flasks of 150 cm2 were used to grow the cells, and once>90% confluence was reached; The cells were detached by the addition of 3.5 mL of 0.05% trypsin-EDTA (Sigma). The cells were spun down in a centrifuge (5 minutes at 1500 RPM) then resuspended in freeze media and were sent for MAP impact testing. Following the impact testing, cells at passages 2-13 were used for the proliferation assay.
Proliferation assay
To assess proliferation of MTLn3, cells were detached from the flask and centrifuged and rehydrated in the growing media. The cells were then seeded at a density of 8 x 104 cells/ well in a 12-well plate and incubated for 24 hours, following which MTLn3-GFP cells were incubated with Morphine sulfate (3 nM-30 M), fentanyl (0.03 pM- 0.3 M), Morphine+naloxone (3 nM-30 M), DMT-DALDA (30 nM-30 M) and PZM21 (3 nM-30 M) for 48 hours at 37°C. The control group were incubated with the medium alone. The effect of the drug on cell proliferation was determined after 48 hours by counting the number of cells in each well using hemocytometer by the observer blinded to the treatment.
Quantitative real-time Polymerase Chain Reaction (PCR) for the expression of μ-opioid receptor, Tolllike receptor 4, MrgprD and MrgprX2
The presence of mRNA for μ-opioid receptor, Toll-like receptor 4, MrgprD and MrgprX2 were determined via qPCR in MTLn3-GFP breast adenocarcinoma cells. For this the total RNA was isolated from the cells (n=6) using Qiagen mini RNA kit (Qiagen, USA) and 1 g of total RNA was used for cDNA synthesis by using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, USA) according to the manufacturer’s instructions. cDNA was amplified using Real-Time PCR in an ABI 7300 Real-Time PCR system (applied biosystems, USA). RNA analysis reagents (SYBR green master mix) were from Bio-Rad, USA. We used the comparative ΔΔ cycle threshold method to quantify μ-opioid receptor (oprm1), Toll-like receptor 4 (Tlr4), MrgprD and MrgprX2 gene expression.
Target gene expression levels in the test samples were normalized to the endogenous reference Glyceraldehyde-3- Phosphate Dehydrogenase (GAPDH) levels and reported as the fold difference relative to GAPDH gene expression in untreated baseline control. All assays were performed in duplicates for n=6 sample.
Environment behind obesity
Several scientists believe that obesity is more influenced by environmental factors like high food consumption, high sweetened beverages, less activity outdoors, television watching etc., instead of biological ones.
Even, nowadays due to social globalization, we are everyday influenced by the interesting posters and pictures of food items which offers high calorific value and fat content. Furthermore, our environment has been sliding to more towards obesogenic environment and encouraging us to eat more unhealthy stuffs.
Today’s time is completely opposite from the past, where human used to engage themselves in outdoor activities which were regulating the expenditure and intake of energy in a more proper way.
Results
Morphine enhanced the proliferation of MTLn3 breast cancer cell line in vitro
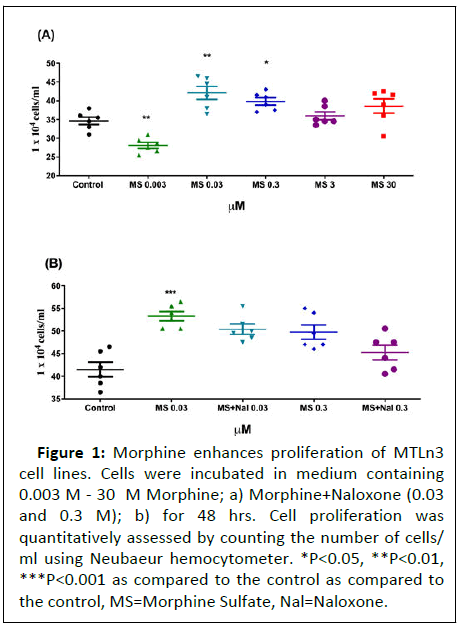
To determine if morphine promotes MTLn3 cell proliferation, a dose response effect was studied by incubating the cells with doses ranging between (0.003 M-3M) for a period of 48 hours. A significant increase in cell proliferation was observed at 0.03 and 0.3 M Morphine, with the effect subsiding at higher doses (Figure 1).
Figure 1: Morphine enhances proliferation of MTLn3
cell lines. Cells were incubated in medium containing
0.003 M - 30 M Morphine; a) Morphine+Naloxone (0.03
and 0.3 M); b) for 48 hrs. Cell proliferation was
quantitatively assessed by counting the number of cells/
ml using Neubaeur hemocytometer. *P<0.05, **P<0.01,
***P<0.001 as compared to the control as compared to
the control, MS=Morphine Sulfate, Nal=Naloxone.
Proliferative effects of morphine were not altered by equimolar concentrations of the opiate antagonist naloxone
To determine whether the proliferative effects of morphine was μ-opioid receptor mediated, the MTLn3 cells were treated with a combination of morphine+naloxone at equimolar concentrations. Two different concentrations of naloxone (0.03 and 0.3) M were used based on the proliferative effect observed with morphine on MTLn3 cells. Interestingly, naloxone did not alter morphine-induced proliferation of MTLn3 cells at either of the concentrations used suggesting that the enhanced proliferation by morphine was independent of r dioipo-eceptor in Figure 1.
Fentanyl showed no effect on MTLn3 breast cancer cell proliferation
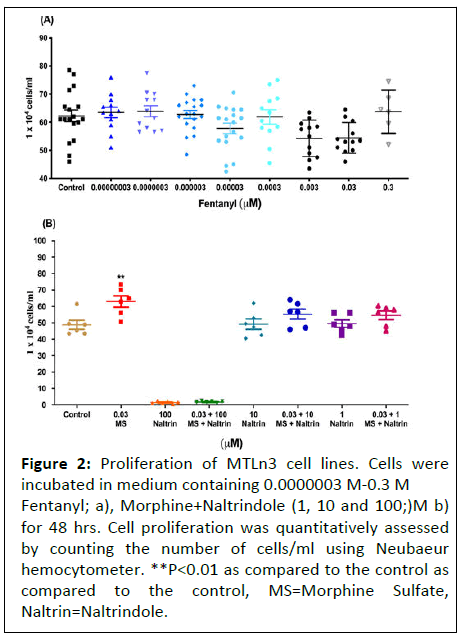
In order to confirm the absence of participation of dioiporeceptor in mediating cancer cell proliferation, we assessed the cells with another highly potent dioipo- receptor agonist, fentanyl, at doses ranging from (0.03 pM-0.3 μM) on proliferation. No changes were observed in the total number of cells following exposure to fentanyl for 48 hours as compared to the control (Figure 2).
Figure 2: Proliferation of MTLn3 cell lines. Cells were incubated in medium containing 0.0000003 M-0.3 M Fentanyl; a), Morphine+Naltrindole (1, 10 and 100;)M b) for 48 hrs. Cell proliferation was quantitatively assessed by counting the number of cells/ml using Neubaeur hemocytometer. **P<0.01 as compared to the control as compared to the control, MS=Morphine Sulfate, Naltrin=Naltrindole.
Proliferative effects of morphine on MTLn3 cells are independent of delta opioid receptor
To determine whether delta-opioid receptor played any role in morphine induced proliferative effects on MTLn3 cancer cells, we incubated the cells with naltrindole (delta receptor antagonist) at 1, 10 and 100 μM concentrations along with morphine.
No significant inhibitory effect was noticed, suggesting that morphine mediated effects were independent of delta opioid receptor.
Potent and biased μ-opioid receptor agonists do not enhance the proliferation of MTLn3 cancer cells
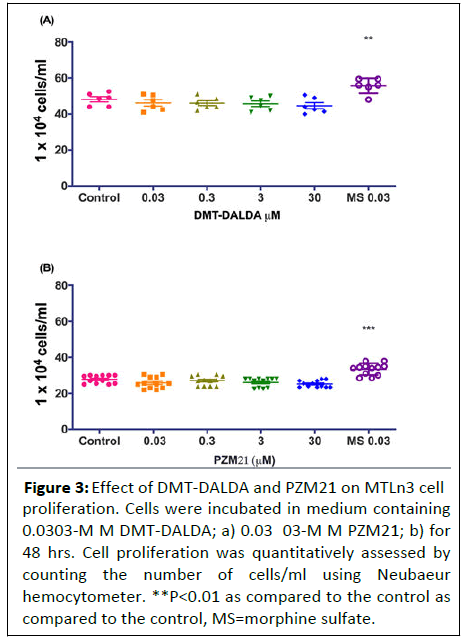
Further, in order to confirm the above finding that proliferative effects of morphine may not be μ-opioid mediated, we treated the MTLn3 cells with highly selective μ-opioid agonists.
DMT-DALDA (0.3-30 μM) at equimolar concentrations as that of morphine did not have an effect on MTLn3 cell proliferation (Figure 3).
Figure 3: Effect of DMT-DALDA and PZM21 on MTLn3 cell proliferation. Cells were incubated in medium containing 0.0303-M M DMT-DALDA; a) 0.03 03-M M PZM21; b) for 48 hrs. Cell proliferation was quantitatively assessed by counting the number of cells/ml using Neubaeur hemocytometer. **P<0.01 as compared to the control as compared to the control, MS=morphine sulfate.
MTLn3 cancer cells expressed MrgprX2 and TLR4 but lacks μ-opioid receptor
As the above findings suggests that the proliferative effects of morphine were independent of u-opioid receptors and MRGs might play a role in morphine mediated tumor growth, it was imperative to determine the expression of these receptors in MTLn3 cancer cells.
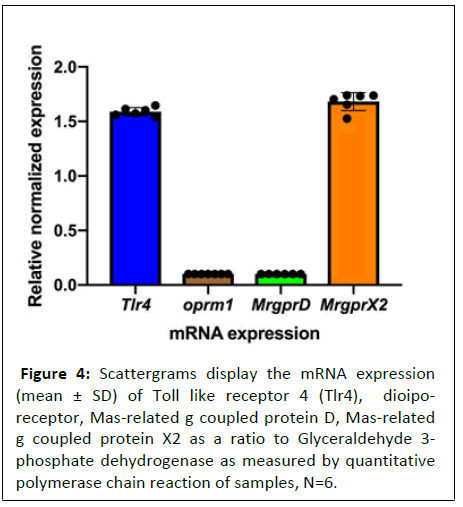
Using qPCR, we determined the expression of μ- opioid receptor OPRM1 and two MRG receptors (MrgprX2 and MrgprD). Previous studies have shown that morphine may also act on toll-like receptor 4 (TLR4) and therefore, we also determined expression of TLR4 in MTLn3 cancer cells using qPCR [22,23]. As shown in figure 4, the MTLn3 cells expressed both MrgprX2 and Tlr4. No presence of OPRM1 or MrgprD message was observed (Figure 4).
Figure 4: Scattergrams display the mRNA expression (mean ± SD) of Toll like receptor 4 (Tlr4), dioiporeceptor,
Mas-related g coupled protein D, Mas-related
g coupled protein X2 as a ratio to Glyceraldehyde 3-
phosphate dehydrogenase as measured by quantitative
polymerase chain reaction of samples, N=6.
Morphine has been shown to initiate survivalpromoting signaling, enhancing cancer growth and metastases. Indirectly, opiates may exert a suppressive effect on immune cell function, such as decreased mitogenic activity of lymphocytes and other inflammatory cells or serve to enhance angiogenesis.
Discussion
Emerging evidence suggests that systemic morphine, though beneficial in reducing cancer pain, may enhance the tumor growth and metastasis. The present study under ex vivo conditions demonstrated that;
• Exposure of breast cancer cells to morphine indeed enhances tumor proliferation in a concentration-dependent fashion and, for the first time
• Provides insights into the possible MRG-related mechanism by which morphine may exert these effects.
Cancer pain and morphine
Cancer and therapeutic interventions (chemotherapy and radiation) to treat cancer are frequently associated with debilitating pain, which severely impairs quality of life. Moreover, it has been suggested that stress, secondary to uncontrolled pain, can have deleterious effect upon immune function, and thereby enhance tumor growth [24,25]. Reduction of pain, as with oral opiates, has been considered by some to reduce tumor growth [26-30].
Morphine and tumorigenesis
In contrast to the preceding comments on the salutary effects of opiates on tumor growth, pre-clinical and clinical studies are now pointing at a potential role of morphine on tumor growth and metastasis. The direct involvement being that morphine may not affect tumorigenesis but may promote tumor growth and metastasis [31]. In retrospective reviews, it has been suggested that cancer patients whose pain is managed perioperatively with morphine suffered worse outcomes when compared with patients in which non opioid regional anesthetic management was employed, supporting speculation of a promotion by opiates of tumor progression and relapse [32]. Similarly, an association was noted between opioid use and shorter survival in patients with advanced cancer [33]. A retrospective assessment of anesthetic management during radical prostatectomy revealed a statistically significant reduction in cancer recurrence in patients receiving epidural analgesia.
In the present in vitro study, we demonstrated a direct effect of morphine on cell proliferation which peaked at 0.03-0.3 μM concentration. We would note that these concentrations are lower than those reported in human plasma of cancer patient on oral morphine [34-36].
In the present studies, as in previous reports, at higher concentrations, a reduction in cell proliferation was observed and suggests that extended exposure to high opioid concentrations may suppress tumor growth, whereas clinically relevant use of opioids may promote cancer growth.
Involvement of μ-opioid receptor
With the emerging studies of morphine promoting tumor progression and recurrence, it is frequently not clear if these tumor enhancing effects are uniformly representative of an opiate receptor interaction. Some, but not all, of the above effects are prevented by naloxone or naltrexone or mu receptor inactivation [37,38]. In the present study, based on the characteristics of opiate receptor pharmacology, cell proliferation induced by morphine was not opiate receptor mediated;
• Agonist pharmacology, in the present work, morphine, but not the potent mu agonists fentanyl, DMT-DALDA or the biased mu opiate agonist PZM21, resulted in a concentration dependent proliferation.
• Antagonist actions naloxone, a pan opiate receptor antagonist, at equimolar concentration as morphine in this study did not alter the proliferative effects of morphine. Further, naltrindole, a delta opioid antagonist, was without effect. This pharmacological profile excludes the opiate receptor in mediating morphine evoked MTLn3 cell proliferation.
Non canonical signaling in the pro proliferative actions of morphine
Given that the ex vivo effects of morphine in this study were not opiate receptor mediated, two possibilities exist based on current thinking suggesting non-canonical signaling by morphine: Toll Like Receptor 4 (TLR4) and/or Mas Receptor G protein (MRGs) [39]. In this regard, we indeed found TLR4 and MrgprX2 receptor mRNA present in the MTLn3 cells. Regarding TLR4, morphine and several other opioids are reported to act as TLR4 ligands. Importantly, this activation is reported to be reversed in a non-stereospecific fashion by naloxone/naltrexone [40]. However, as noted, the pro-proliferative effects of morphine were not reversed by naloxone, excluding a role for both an opiate and the TLR4 receptor. MRGs were originally identified as a family of orphan G protein coupled receptor belonging to a subfamily encoded by Mas-Related Genes (MRGs) [41-44]. MRG genes are expressed in a variety of cell types, including neurons (dorsal root ganglion) blood vessels, fibroblasts and mast cells, and as shown in the present work, this rat syngenic cell line [45,46]. As noted, morphine has been shown to activate MRGs, and this activation results in mast cell degranulation. In recent work we found that MRG signaling by morphine led to the proliferation of fibroblasts and the formation of intrathecal masses after the chronic infusion of morphine. In the Presto Tango assay system identifying activation of MRGs, it has been shown that morphine but not PZM21, DMT-DALDA or fentanyl is a potent MRG agonist [47-49]. Accordingly, consistent with above commentary, PZM21 and DMT-DALDA did not produce such fibroblast-rich intrathecal masses. The present work with the several agonists suggests a parallel pharmacology in the proliferation of these MRGs expressing tumor cells by some (morphine) but not all mu agonists (PZM21, DMT DALDA and fentanyl), as reported here.
Conclusion
The present implicating MRGs in proliferation may not generalize to all tumor lines. Further, the implications here are that preclinical models employing the MTLn3 line may show an enhanced metastasis when prepared with this cell line in the presence of chronic systemic morphine. Of note if the MRG signaling appears relevant, it should be stressed that many agents can activate MRG. Simple consideration of drugs which can degranulate mast cells (antibiotics, muscle relaxants) as well as a variety of endogenous molecule (RFamide neuropeptides), Bovine Adrenal Medulla 22 (BAM22) peptide, cortistatin and Proadrenomedullin N-Terminal Peptide (PAMP) have been identified as endogenous ligands for various members of the MRG family. The above background raises several issues;
• There is little doubt that control of pain must be achieved for humanitarian reasons and to avoid the potential consequences of pain-evoked changes in system function that may exacerbate tumor growth;
• One would hypothesize that if, like in ex studies, morphine systemically does enhance tumor growth, high systemic levels of morphine should be avoided;
• This raises the potential importance of controlling pain by the use of neuraxially delivered agents, given the low systemic exposure observed after the spinal delivery of equi-analgesic doses of intrathecal morphine, the incidence of tumor growth would be less after intrathecal delivery. In previous work, we have shown that equi-analgesic doses of morphine given systemically or intrathecally resulted in pronounced suppression of mitogenic activity of circulating lymphocytes by the former, but not the later. It would be speculative to suggest that these results indicate that intrathecal morphine might not promote tumor activity as when given at higher systemic doses;
• Finally, the work with the biased ligands suggest that development of analgesic including opiates should take into consideration their potential effects upon unwanted facilitation of tumor growth and metastasizes.
References
- Van den Beuken-van Everdingen MH (2007) Prevalence of pain in patients with cancer: A systematic review of the past 40 years. Ann Oncol 18: 1437-49.
- Mantyh PW (2006) Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 7: 797-809.
- Mercadante S, Fulfaro F (2005) World Health Organization guidelines for cancer pain: A reappraisal. Annal Oncol 16: 132-135.
- Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI (2006) Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis?. J Am Soc Anesthesiol 105: 660-664.
- Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, et al. (2008) Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. J Am Soc Anesthesiol 109: 180-187.
- Heaney A, Buggy DJ (2012) Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis?. Br J Anaesth 109: 17-28.
- Afsharimani B, Cabot P, Parat MO (2011) Morphine and tumor growth and metastasis. Cancer Metastasis Rev 30: 225-238.
- Hamra JG, Yaksh TL (1996) Equianalgesic doses of subcutaneous but not intrathecal morphine alter phenotypic expression of cell surface markers and mitogen-induced proliferation in rat lymphocytes. J Am Soc Anesthesiol 85: 355-365.
- Roy S, Loh HH (1996) Effects of opioids on the immune system. Neurochem Res 21: 1375-1386.
- Fecho K, Lysle DT (1999) Phenotypic analysis of splenocyte subsets following acute morphine treatment in the rat. Cell Immunol 195: 137-146.
- Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, et al. (2002) Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 62: 4491-4498.
- Singleton PA, Lingen MW, Fekete MJ, Garcia JGN, Moss J (2006) Methylnaltrexone inhibits opiate and VEGF-induced angiogenesis: role of receptor transactivation. Microvascular Res 72: 3-11.
- Ustun F, Durmus-Altun G, Altaner S, Tuncbilek N, Uzal C, et al. (2011) Evaluation of morphine effect on tumour angiogenesis in mouse breast tumour model, EATC. Med Oncol 28: 1264-1272.
- Bimonte S, Barbieri A, Rea D, Palma G, Luciano A, et al. (2015) Morphine promotes tumor angiogenesis and increases breast cancer progression. Bio Med Res Int 2015.
- Gach K, Wyrębska A, Fichna J, Janecka A (2011) The role of morphine in regulation of cancer cell growth. Naunyn-Schmiedeberg's archives of pharmacology 384: 221-230.
- King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, et al. (2007) Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain 132: 154-168.
- Nguyen J, Luk K, Vang D, Soto W, Vincent L, et al. (2014) Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth 113: 4-13.
- Doornebal CW, Vrijland K, Hau CS, Coffelt SB, Ciampricotti M, et al. (2015) Morphine does not facilitate breast cancer progression in two preclinical mouse models for human invasive lobular and HER2+ breast cancer. Pain 156: 1424-1432.
- Koodie L, Yuan H, Pumper JA, Yu H, Charboneau R, et al. (2014) Morphine inhibits migration of tumor-infiltrating leukocytes and suppresses angiogenesis associated with tumor growth in mice. Am J Pathol 184: 1073-1084.
- Chen P, Wang D, Li M, Zhang Y, Quirion R, et al. (2010) Modulation of sensory neuron specific receptors in the development of morphine tolerance and its neurochemical mechanisms. J Neurosci Res 88: 2952-2963.
- Yaksh TL, Eddinger KA, Kokubu S, Wang Z, DiNardo A, et al. (2019) Mast cell degranulation and fibroblast activation in the morphine-induced spinal mass: role of mas-related G protein-coupled receptor signaling. Anesthesiol 131: 132-147.
- Hutchinson MR, Lewis SS, Coats BD, Rezvani N, Zhang Y, et al. (2010) Possible involvement of toll-like receptor 4/myeloid differentiation factor-2 activity of opioid inactive isomers causes spinal proinflammation and related behavioral consequences. Neuroscience 167: 880-893.
- Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, et al. (2010) Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1β. Neuroscience 165: 569-583.
- Page GG, Beneliyahu S, Liebeskind JC (1994) The role of LGL/NK cells in surgery-induced promotion of metastasis and its attenuation by morphine. Brain Behav Immun 8: 241-250.
- Page GG, Blakely WP, Ben-Eliyahu S (2001) Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 90: 191-199.
- Simon RH, Arbo TE (1986) Morphine increases metastatic tumor growth. Brain Res Bull 16: 363-367.
- Yeager MP, Colacchio TA (1991) Effect of morphine on growth of metastatic colon cancer in vivo. Arch Surg 126: 454-456.
- Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, et al. (2002) Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol 441: 185-191.
- Franchi S, Panerai AE, Sacerdote P (2007) Buprenorphine ameliorates the effect of surgery on hypothalamus–pituitary–adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 21: 767-774.
- Bar-Yosef S, Melamed R, Page GG, Shakhar G, Shakhar K, et al. (2001) Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. J Am So Anesthesiol 94: 1066-1073.
- Lin X, Wang YJ, Li Q, Hou YY, Hong MH, et al. (2009) Chronic high dose morphine treatment promotes SH SY5Y cell apoptosis via c Jun N terminal kinase mediated activation of mitochondria dependent pathway. FEBS J 276: 2022-2036.
- Maher DP, White PF (2016) Proposed mechanisms for association between opioid usage and cancer recurrence after surgery. J Clin Anesth 28: 36-40.
- Zylla D, Steele G, Shapiro A, Richter S, Gupta P (2018) Impact of opioid use on health care utilization and survival in patients with newly diagnosed stage IV malignancies. Supportive Care Cancer 26: 2259-2266.
- Gretton SK, Ross JR, Rutter D, Sato H, Droney JM, et al. (2013) Plasma morphine and metabolite concentrations are associated with clinical effects of morphine in cancer patients. J Pain Symptom Manage 45: 670-680.
- Klepstad P, Borchgrevink PC, Dale O, Zahlsen K, Aamo T, et al. (2003) Routine drug monitoring of serum concentrations of morphine, morphine-3-glucuronide and morphine-6-glucuronide do not predict clinical observations in cancer patients. Palliat Med 17: 679-687.
- Quigley C, Joel S, Patel N, Baksh A, Slevin M (2003) Plasma concentrations of morphine, morphine-6-glucuronide and morphine-3-glucuronide and their relationship with analgesia and side effects in patients with cancer-related pain. Palliat Med 17: 185-190.
- Mathew B, Lennon FE, Siegler JH, Mirzapoiazova T, Mambetsariev N, et al (2011) Novel role of the mu opioid receptor in lung cancer progression: a laboratory study. Anesth Analg 112 : 558.
- Lennon FE, Mirzapoiazova T, Mambetsariev B, Poroyko VA, Salgia R, et al. (2014) The Mu opioid receptor promotes opioid and growth factor-induced proliferation, migration and Epithelial Mesenchymal Transition (EMT) in human lung cancer. PloS One 9: e91577.
- Lansu K, Karpiak J, Liu J, Huang XP, McCorvy JD, et al. (2017) In silico design of novel probes for the atypical opioid receptor MRGPRX2. Nat Chem Biol 13: 529-536.
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, et al. (2007) Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci World J 7: 98-111.
- Choi SS, Lahn BT (2003) Adaptive evolution of MRG, a neuron-specific gene family implicated in nociception. Genome Res 13: 2252-2259.
- Dong X, Han SK, Zylka MJ, Simon MI, Anderson DJ (2001) A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619-632.
- Lembo P, Grazzini E, Groblewski T, O'Donnell D, et al. (2002) Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat Neurosci 5: 201-209.
- Grazzini E, Puma C, Roy MO, Yu XH, O'Donnell D, et al. (2004) Sensory neuron-specific receptor activation elicits central and peripheral nociceptive effects in rats. Proc Natl Acad Sci 101: 7175-7180.
- Allia E, Tarabra E, Volante M, Cerrato M, Ghigo E, et al. (2005) Expression of cortistatin and MrgX2, a specific cortistatin receptor, in human neuroendocrine tissues and related tumours. J Pathol 207: 336-345.
- Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, et al. (2006) Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349: 1322-1328.
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, et al. (2002) Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Gαq/11 pathway. Proc Natl Acad Sci 99: 14740-14745.
- Robas N, Mead E, Fidock M (2003) MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J Biol Chem 278: 44400-44404.
- Kamohara M, Matsuo A, Takasaki J, Kohda M, Matsumoto M, et al. (2005) Identification of MrgX2 as a human G-protein-coupled receptor for proadrenomedullin N-terminal peptides. Biochem Biophys Res Commun 330: 1146-1152.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences