Molecular Imprinted Ag-LaFeO3 for Highly Selective Detection of Benzene
Zhang YM, Chen MP, Zhang DM, Zhao XB, Zhu ZQ, Zhang J* and Liu QJ*
School of Materials Science and Engineering, Yunnan Key Laboratory for Micro/nano Materials & Technology, Yunnan University, China
- *Corresponding Author:
- Zhang J
School of Materials Science and Engineering
Yunnan Key Laboratory for Micro/Nano Materials & Technology
Yunnan University
650091 Kunming, China
Tel: +86-871-65033814
E-mail: zhj@ynu.edu.cn
Liu QJ
School of Materials Science and Engineering
Yunnan Key Laboratory for Micro/Nano Materials & Technology
Yunnan University
650091 Kunming, China
Tel: +86-871-65033814
E-mail: qjliu@ynu.edu.cn
Received Date: November 09, 2017; Accepted Date: January 05, 2018; Published Date: January 12, 2018
Citation: Zhang YM, Chen MP, Zhang DM, Zhao XB, Zhu ZQ, et al. (2018) Molecular Imprinted Ag-LaFeO3 for Highly Selective Detection of Benzene. J Mol Sci. 2:1.
Abstract
Sensitivity is an essential parameter to gas sensor for the multiple pollutants reality of the current situation. Selective recognition of a certain gas is a research hotspot. In this paper, molecular imprint and design are adopted to solve this hot problem. A highly selective benzene sensor was developed based on molecular imprinting technique (MIT). Organic/inorganic combined MIT was for the first time used to recognize small organic molecule by our group. Ag-LaFeO3 was used as crosslinker, benzene used as template, and then the molecular imprinted Ag- LaFeO3 (MI-AL) with a small dimension was achieved. The structure of the MI-AL is orthogonal perovskite. And then the MI-ALs was printed onto an alumina tube. Subsequently, a high selectivity molecular imprinting benzene gas sensor was obtained. At 125°C, the response to 1 ppm benzene based on the sensor is 18.5, and the response is lower than 5 to the other test gases. The response time and recovery time are 55 s and 40 s, respectively.
Keywords
Ag-LaFeO3; Molecular imprinting technique; Benzene sensor; High selectivity
Introduction
As people spend most time indoors, indoor air quality has become an important health factor. Many different compounds contribute to the problem of potential health issues caused by the air inside buildings. Benzene is a poisonous volatile organic compound, which can be emitted from daily-life items, construction materials, and various chemical processes [1,2]. Since benzene is extremely detrimental to human health [3,4], the accurate detection of benzene in very low concentration is of great importance. In addition, it has been known that benzene is a recognized biomarker for leukemia [5,6]. So it is very necessary to monitor the concentration of benzene timely and accurately. The main testing methods include spectrophotometry, chromatography, fluorescence, electrochemical and gas sensor. Gas sensors based on semiconducting oxides are thought to be the most effective means to monitor the gases because they are small, low cost and easy to use [7,8]. In this paper, Ag-LaFeO3 has been selected because of its good thermostability [9], controllable structure [10], and our own experience in this field [11,12].
Molecular imprinting is an effective means to prepare specific binding sites for given molecules in appropriate solid matrices. In this approach, the shape and functionality of a template can be transcribed onto microporous materials. The configuration of the functional groups in the template may be memorized within the matrix. Today, this field has become dominated by the use of organic macromolecular for chromatographic separation, enzyme-mimicking catalysts, chemical sensors, and biosensors [13-18]. Hawari et al. [19] demonstrated an e-nose sensor that could detect Limonene volatiles using MIP as the sensing material. The MIPs were prepared by spin-coating on an interdigitated electrode (IDE), with the properties of the sensor estimated by capacitance. The results showed that the sensor responds to Limonene volatile gases. Hawari et al. [20] also use IDE structure to fabricate a sensor for detecting Alpha Pinene volatile gases by using MIP. The results showed that the IDE–MIP sensor exhibited high sensitivity and selectivity responses towards alpha pinene when compared to non-imprinted polymers. Lieberzeit et al. [21] examined a formaldehyde gas sensor using MIPs. A co-polymer thin film was prepared with styrene, MAA and EGDMA, before the thin film was coated with QCM to form a sensor. The sensor exhibits a detection limit of 500 ppb formaldehyde in dry air. The device possesses great selectivity to formaldehyde. Alizadeh et al. [22] combined the imprinted polymeric particles with graphene to produce a nanocomposite chemo-resistor gas sensor. The results showed that the sensor can recognize acetonitrile specifically. Jha et al. [23] designed a sensor array with volatile acids imprinted with 3-element QCM for the recognition of the odorous organic acids, which are propanoic acid, hexanoic acid and octanoic acid. Each element in QCM is in charge of detecting the odor of one acid and they all show good selectivity. Jha et al. [24] also developed a QCM sensor array to identify primary aldehydes in human body odor. While, organic/inorganic combined MIT was for the first time used to recognize small organic molecules by our group, such as methanol [25] and formaldehyde [26]. The results show that MIT could improve the selectivity of as prepared sensors.
In this paper, the target gas molecules benzene was used as template molecule, formaldehyde as functional monomer, azodiisobutyronitrile (AIBN) as initiator, Ag-LaFeO3 as crosslinker. Organic/inorganic combination is very popular in which the inorganic part is benefit to build a stable structure for template recognition and carrier transportation, and the organic part is good for the gas adsorption.
Experimental
Preparation of MI-ALs
Based on our previous experiments [11], the sample Ag-LaFeO3 with Ag: La=1:99 mole ratio (Ag-LaFeO3) was found to be the most promising gas sensing material with low operating temperature. In this study, Ag-LaFeO3 was further modified with MIT. Benzene as template mixed with formaldehyde (FA, functional monomer) was treated by ultrasonic concussion for 30 min, and stand for 8 h as solution A. x= FA : Ag-LaFeO3=3:10, 4:10, 5:10 and 6:10, molar ratio. Then, AIBN was dissolved in 10 mL benzene and mixed with solution A and Ag-LaFeO3 according to the ratio x. The final mixture was treated by ultrasonic concussion for 30 min, stirred at 60°C for 12 h and dried. The MI-AL was finally prepared.
Fabrication of gas sensor
The prepared MI-AL was further mixed with distilled water and ground to form a paste, which was subsequently printed onto an alumina tube. There are two Au electrodes placed at the both end sides of the tube. The length of the alumina tube is 4 mm and the diameter is 1.2 mm. In order to improve their stability and repeatability, the gas sensors were aged at 150°C for 170 h in air.
Characterization
The X-ray Diffraction (XRD) patterns were obtained for the phase identification with a D/max23 diffractometer using Cu Kα1 radiation (λ=1.54056 Å), where the diffracted X-ray intensities were recorded as a function of 2θ. The accelerating voltage was 35 kV and the applied current was 25 mA, and the sample was scanned from 10 ° to 90 ° (2θ) in steps of 0.02 °. The particle morphology of the sample was tested by transmission electron microscope (TEM, JEM-2100).
Results and Discussion
Gas sensing properties of the MI-AL with x=4:10 is better than those with x=3:10, 5:10 and 6:10. In this paper, we mainly discuss the MI-AL with x=4:10.
Structure and micro-morphology characterization
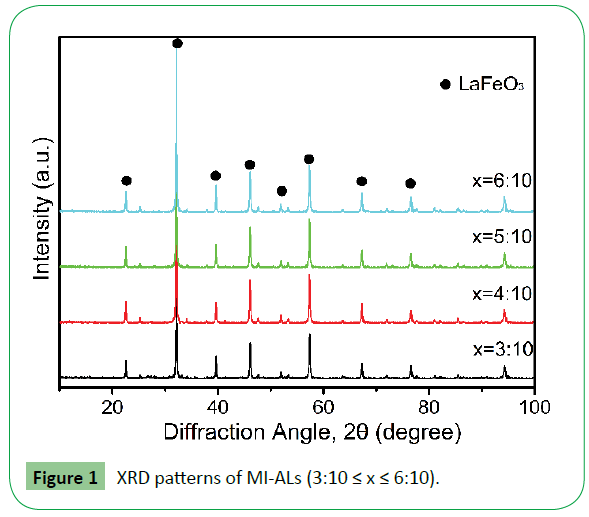
X-ray powder diffraction patterns of MI-ALs are showed in Figure 1.
The patterns indicate that the structure of MI-ALs is orthogonal perovskite which include only one phase of LaFeO3, indicating that the MI-ALs are with high purity and crystallinity. The average crystallite size was estimated by means of Scherrer formula (D=kλ/βCosθ, k=1, where λ is the wavelength of X-ray, θ is the diffraction angle, and β is the true half-peak width.) through measuring the half-peak widths of the lines in the pattern. The average crystallite size for the MI-ALs with x=3:10, 4:10, 5:10 and 6:10 are about 42 nm, 39 nm, 44 nm and 41 nm, respectively.
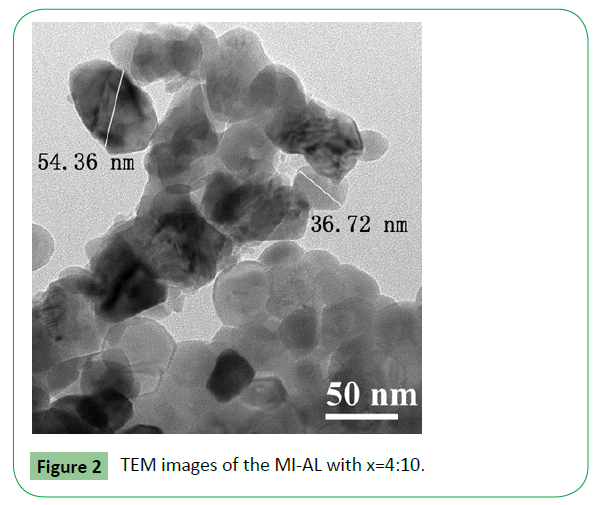
Transmission electron micrographs for the MI-AL with x=4:10 is shown in Figure 2. For this sample, there are many spherical shaped particles. The particle size was in the range of 20 nm- 70 nm. Thus the specific surface area of the compound is large, which can adsorb benzene more easily and enhance the response. Comparing with the XRD results, TEM images reveal that there were various sizes of particles in the MI-ALs. The big particles are composed of small crystallites.
Gas-sensing properties
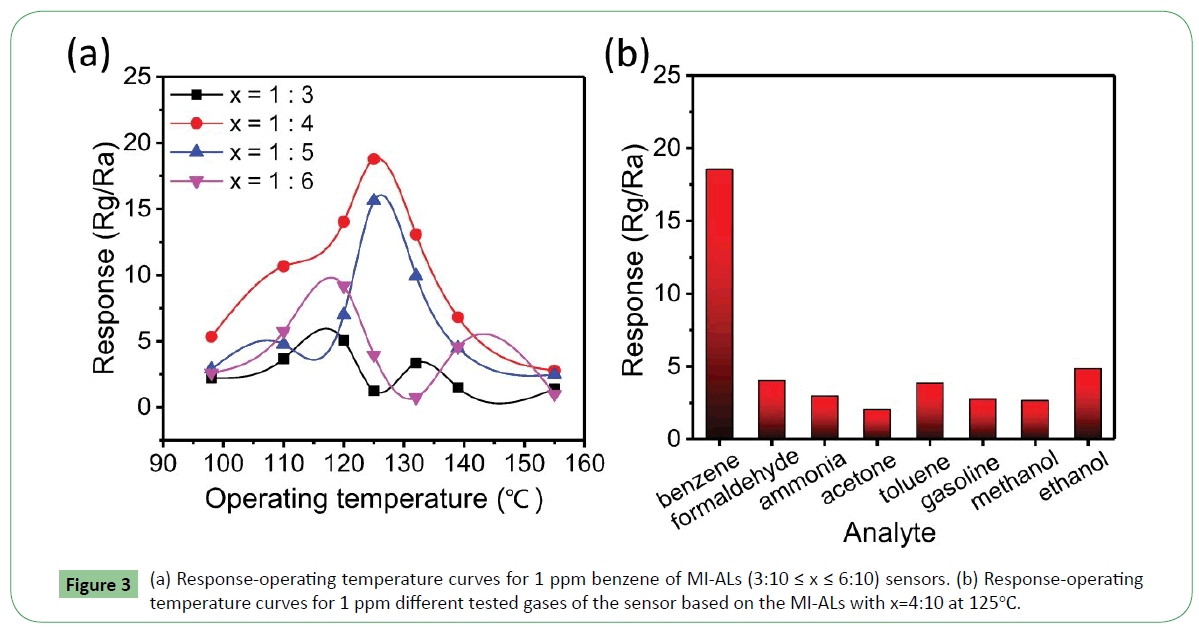
The relationship between response and operating temperature of the sensors based on MI-ALs (3:10 ≤ x ≤ 6:10) to 1 ppm benzene gas were depicted in Figure 3a. The gas response β was defined as the ratio of the electrical resistance in gas (Rg) to that in air (Ra). As it shows, the sensor with x=4:10 exhibit higher response to benzene gas at the operating temperature of 125°C. If the amount of the functional monomer (FA) is lower (x=3:10), the interaction between the template molecule and functional monomer is weak, and specific recognition sites in the MI-ALs for benzene are reduced, which result in bad gas sensing properties. On the contrary, if the amount of the functional monomer is higher (5:10 ≤ x ≤ 6:10), FA molecules will form poly-FA via intermolecular hydrogen bond interaction, which lead to poor gas sensing properties. Taking the conception of sensing properties and cost conservation into consideration, the sensor with x=4:10 is chosen as an optimal element to do further study. Figure 3b shows the response to 1 ppm benzene, formaldehyde, ammonia, acetone, toluene, gasoline, methanol and ethanol based on the MI-ALs with x=4:10. It can be seen from the figure that the response to 1 ppm benzene is 18.5, while the response to the other gases are all lower than 5, which indicate that the MI-ALs with x=4:10 has very good selectivity to benzene, even compared to toluene of which the molecular structure is very similar with benzene.
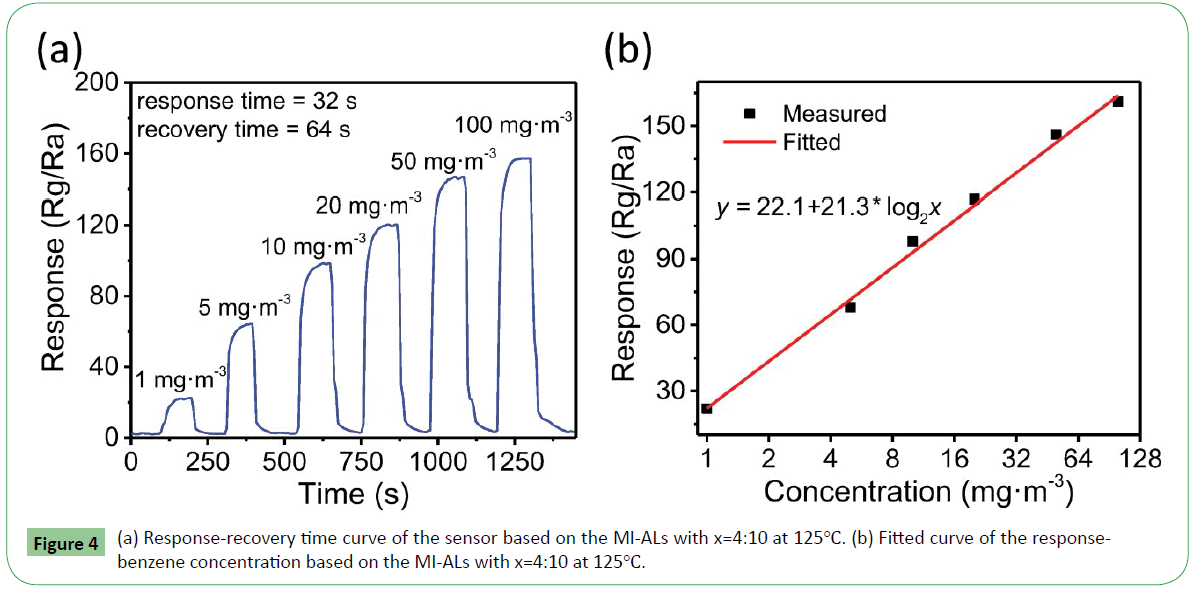
The response and recovery times are defined as the time required reaching 90% response (recovery) when gas is in (out) [27]. Figure 4a presents a response-recovery characteristic for the sensor based on the MI-ALs with x=4:10. As seen in Fig. 5, the resistance of the sensor increases sharply when benzene flow is in but returns to its original state while the gas flow is out although with some delay. When benzene is in, the sensor begins to response which takes 55 s and when benzene is out, the sensor begins to recover which takes 40 s. After the sensor has recovered about 90%, it became slower. The reason is when the concentration of benzene on the surface of the materials is very low, the interaction between this part of benzene and sensor is chemisorption [28]; it is very difficult for the benzene to desorb from the surface [29,30]. The response and recovery time are about 55 s and 40 s, respectively. Then the relationship of response-benzene concentration is fitted, the results are showed in Figure 4b. It can be seen from Figure 4b that the response to benzene is near linear to the concentration of benzene. Thus, the sensor based on the MI-ALs with x=4:10 could be applied to real-time detection of benzene.
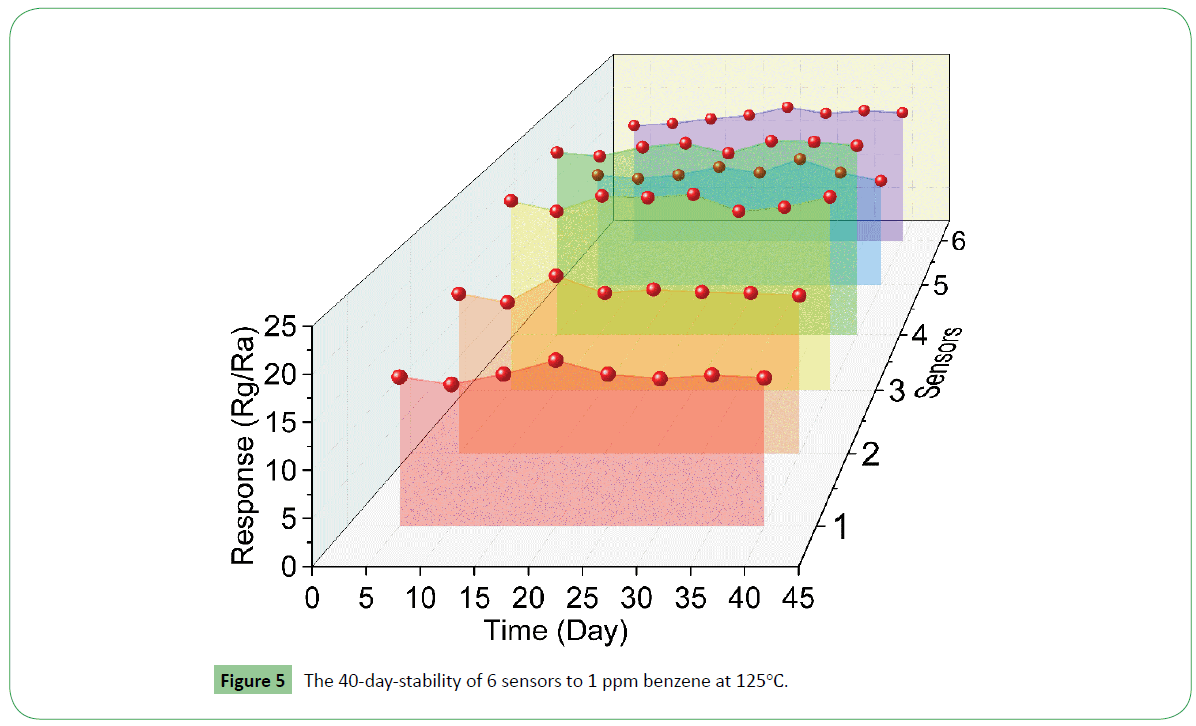
Reproducibility, reusability and stability are another three important properties for a gas sensor. Hence three repeated experiments of MI-ALs with x=4:10 were performed and get sample-1, sample-2 and sample-3. Then fabricated sensor-1 and sensor-2 from sample-1, sensor-3 and sensor-4 from sample-2, sensor-5 and sensor-6 from sample-3. The 40-day-stability of the six sensors were tested. It can be seen from Figure 5 that all six sensors possess almost equal response (15-19) to 1 ppm benzene, showing the good reproducibility. It also can be seen that the sensors keep a certain response tested every five days, indicating that the sensors based on the MI-ALs with x=4:10 possess good reusability and stability to 1 ppm benzene.
Gas-sensing mechanism
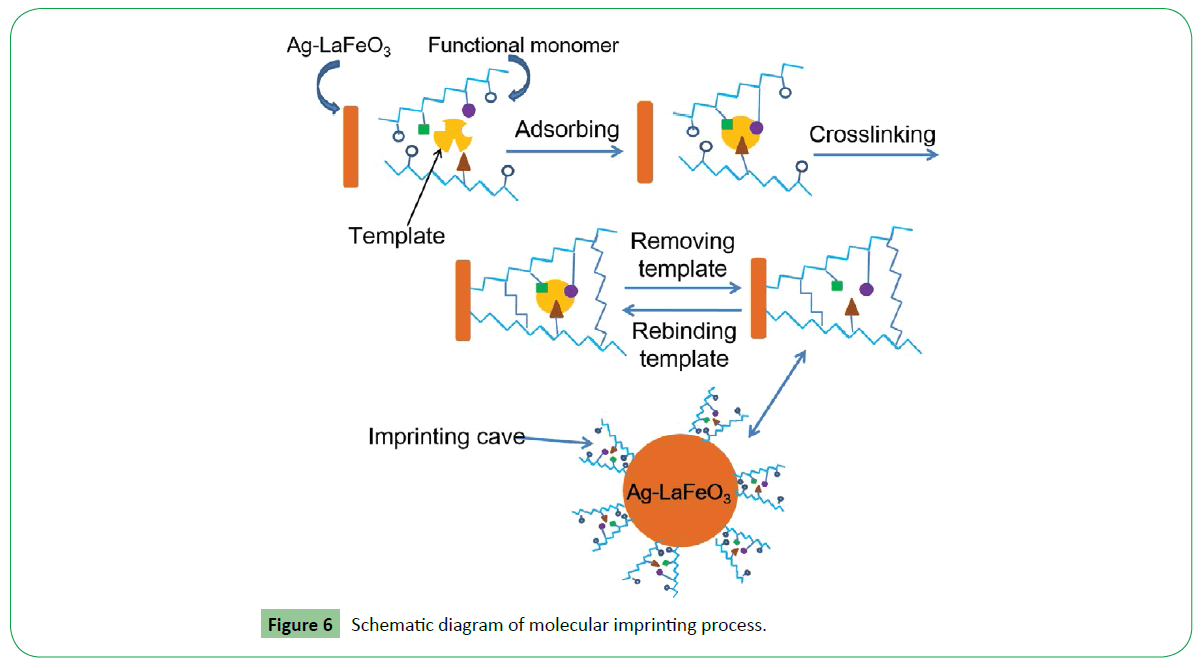
As a typical p-type semiconductor in air, LaFeO3 detect gases based on the changes of the resistance before and after being exposed to the test gas [11]. After Ag-LaFeO3 polymerized with FA, the gas sensing mechanism is similar to that of LaFeO3, because LaFeO3 is the only phase in MI-ALs Figure 1, XRD patterns). The mechanism of the specific recognition of benzene is illustrated in Figure 6. A number of recognition cavities complementary to benzene in shape, size and chemical functionality can selectively adsorb benzene so the sensor selectivity can be improved obviously. As illustrated by Figure 6, when the template (benzene) is mixed with functional monomers (FA), benzene is allowed to interact via hydrogen-bond with FA, and then a benzene-FA complex is formed [31]. The resulting complex is subsequently copolymerized with a large excess of crosslinker. Finally, after the removal of the template, recognition cavities complementary to benzene molecules were formed on the surface of Ag-LaFeO3, which have much accessible sites, high recognition and binding ability for benzene and result in the improvement of the selectivity of the sensor.
Conclusions
The selectivity of Ag-LaFeO3 can be improved by using molecular imprinting method. The optimal amount of functional monomer is x=4:10. The structure of MI-ALs is of orthogonal perovskite and the average grain size of the MI-ALs with x=4:10 is about 39 nm. The MI-ALs has good selectivity for low concentration benzene. To 1 ppm benzene, the response is 18.5 at the operating temperature of 125°C, and the response time and recovery time are 55 s and 40 s, respectively. These finding may present a new feasible way for exploring modified LaFeO3-based sensing materials. With different template and functional monomer, we can prepare sensing materials with special recognition cite to different target gases just based LaFeO3. Namely, the selectivity of LaFeO3 could be modulated by MIT; it is an interesting and promising finding.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 51402257 and 51562038), Yunnan Basic Applied Research Project, China (No. 2017FB086).
References
- Ge C, Xie C, Zeng D, Cai S (2007) Formaldehyde- benzene-, and xylene-sensing characterizations of Zn-W-O nanocomposite ceramics. J Am Ceram Soc 90: 3263-3267.
- Marbrook M, Hawkins P (2002) Benzene sensing using thin films of titanium dioxide operating at room temperatures. Sensors 2: 374-382.
- Wang L, Wang S, Xu M, Hu X, Zhang H, et al. (2013) A Au-functionalized ZnO nanowire gas sensor for detection of benzene and toluene. Phys Chem Chem Phys 15: 17179-17186.
- Kheirbek I, Johnson S, Ross Z, Pezeshki G, Ito K, et al. (2012) Spatial variability in levels of benzene, formaldehyde, and total benzene, toluene, ethylbenzene and xylenes in new york city: a land-use regression study Environ Health 11: 51.
- McHale CM, Zhang L, Smith MT (2012) Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis 33: 240-252.
- Sonawane B, Bayliss D, Valcovic L, Chen C, Rodan B, et al. (2000) Carcinogenic effects of benzene a status update and research needs to improve risk assessment: U. S. EPA perspective. J Toxicol Environ Health A 61: 471-472
- Yu C, Crump D (1998) A review of the emission of VOCs from polymeric materials used in buildings. Build Environ 33: 357-374.
- Barsan N, Koziej D, Weimar U (2007) Metal oxide-based gas sensor research: How to?. Sens Actuators B 121: 18-35.
- Wetchakun K, Samerjai T, Tamaekong N, Liewhiran C, Siriwong C, et al. (2011) Semiconducting Metal Oxides as Sensors for Environmentally Hazardous Gases. Sens Actuators B 160: 580-591.
- Natile MM, Ponzoni A, Concina I, Glisenti A (2014) Chemical Tuning versus Microstructure Features in Solid-State Gas Sensors: LaFe1-xGaxO3. Case Study Chem Mater 26: 1505-1513.
- Zhang Y.M, Lin YT, Chen JL, Zhang J, Zhu ZQ, et al. (2014) A High Sensitivity Gas Sensor for Formaldehyde Based on Silver Doped Lanthanum Ferrite. Sens Actuators B 190: 171-176.
- Zhang YM, Zhang J, Chen JL, Zhu ZQ, Liu QJ, et al. (2014) Improvement of Response to Formaldehyde at Ag-LaFeO3 Based Gas Sensors Through Incorporation of SWCNTs. Sens Actuators B 195: 509-514.
- Bhakta S, Dixit CK, Bist I, Macharia J, Shen M, et al. (2017) Albumin removal from human serum using surface nanopockets on silica-coated magnetic nanoparticles. Chem Commun 53: 9254-9257.
- Uzun L, Turner APF (2016) Molecularly-imprinted polymer sensors: realising their Potential. Biosens Bioelectron 76: 131-144.
- Chen K, He R, Luo X, Qin P, Tan L, et al. (2017) A fluorescent glycosyl-imprinted polymer for pH and temperature regulated sensing of target glycopeptide antibiotic. Biosens Bioelectron 94: 609-615.
- Cieplak M, Szwabinska K, Sosnowska M, Bikram KCC, Borowicz P, et al. (2015) Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens Bioelectron 74: 960-966.
- Iskierko Z, Sharma PS, Bartold K, Pietrzyk-Le A, Noworyta K, et al. (2016) Molecularly imprinted polymers for separating and sensing of macromolecular compounds and microorganisms. Biotechnol Adv 34: 30-46.
- Liu W, Ma Y, Sun G, Wang S, Deng J, et al. (2017) Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens Bioelectron 92: 305-312.
- Hawari HF, Samsudin NM, Ahmad MN, Shakaff AYM, Ghani SA, et al. (2012) Recognition of Limonene Volatile Using Interdigitated Electrode Molecular Imprinted Polymer Sensor, 2012 Third International Conference on Intelligent Systems Modelling and Simulation: 723-726.
- Hawari HF, Samsudin NM, Md Shakaff AY, Ghani SA, Ahmad MN, Wahab Y, Hashim U (2013) Development of Interdigitated Electrode Molecular Imprinted Polymer Sensor for Monitoring Alpha Pinene Emissions from Mango Fruit. Procedia Eng 53: 197-202.
- Hussain M, Kotova K, Lieberzeit PA (2016) Molecularly Imprinted Polymer Nanoparticles for Formaldehyde Sensing with QCM. Sensors 16: 1011-1011.
- Alizadeh T, Hamedsoltani L (2014) Graphene/graphite/molecularly imprinted polymer nanocomposite as the highly selective gas sensor for nitrobenzene vapor recognition. J Environ Chem Eng 2: 1514-1526.
- Jha SK, Liu C, Hayashi K (2014) Molecular imprinted polyacrylic acids based QCM sensor array for recognition of organic acids in body odor. Sens Actuators B 204: 74-87.
- Jha SK, Hayashi KA (2015) Quick responding quartz crystal microbalance sensor array based on molecular imprinted polyacrylic acids coating for selective identification of aldehydes in body odor. Talanta 134: 105-105.
- Zhu Q, Zhang YM, Zhang J, Zhu ZQ, Liu QJ (2015) A new and high response gas sensor for methanol using molecularly imprinted technique. Sens Actuators B 207: 398-403.
- Zhang YM, Liu QJ, Zhang J, Zhu Q, Zhu ZQ (2014) A highly sensitive and selective formaldehyde gas sensor using a molecular imprinting technique based on Ag–LaFeO3. J Mater Chem C 2: 10067-10072.
- Mai LQ, Xui L, Gaoi Q, Hani CH, Hui B, et al. (2010) Single β-AgVO3 nanowire H2S sensor. Nano Lett 10: 2604-2608.
- Bejaoui A, Guerin J, Aguir K (2013) Modeling of a p-type resistive gas sensor in the presence of a reducing gas. Sens Actuators B 181: 340-347.
- Maric S, Lörgen J, Herrmann U, Schramm U, Bargon J (2004) A new method to reduce the desorption time of a QCM sensor, using a halogen spot for heating, Sens Actuators B 101: 265-267.
- Zhou J, Gu YD, Hu YF, Mai WJ, Yeh PH, et al. (2009) Gigantic enhancement in response and reset time of ZnO UV nanosensor by utilizing Schottky contact and surface functionalization. Appl Phys Lett 94: 191103.
- Gao BJ, Lu JH, Chen ZP, Guo JF (2009) Preparation and Recognition Performance of Cholic Acid-Imprinted Material Prepared with Novel Surface-Imprinting Technique. Polymer 50: 3275-3284.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences