Mitigative Effects of Antioxidants in Lead Toxicity
Ibrahim Yusuf Lamidi and Isaac Oluwatobi Akefe*
1Department of Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria
2Department of Physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria
- *corresponding Author:
- Isaac Oluwatobi Akefe
Department of Physiology, Faculty of Veterinary Medicine
Ahmadu Bello University, Zaria, Nigeria
Tel: +2348034986335
E-mail: akefeisaac@yahoo.com
Received Date: 14 October 2017; Accepted Date: 16 October 2017; Published Date: 27 October 2017
Citation: Lamidi IY, Akefe IO (2017) Mitigate Effects of Antioxidants in Lead Toxicity. Res Rep Toxi Vol.1 No.1:3
Abstract
Lead is a toxic metal pollutant persisting in nature as oxides or salts. It poses as an occupational and environmental hazard, adversely affecting multiple systems such as the hematopoietic, hepatic, nervous, and renal systems in humans and animals. Lead toxicity occurs via oxidative damage, and chelating agents conventionally used for treatment exert detrimental effects and are incapable of alleviating some toxic outcomes of lead. This finding has engendered the need for potentially safe alternative therapy, preferably possessing both chelating and antioxidant activities. Some antioxidants chelate metallic pollutants such as lead and scavenge reactive oxygen species released in excess during lead-induced oxidative damage. Several studies have affirmed the appreciable function of antioxidants singly or in combination in ameliorating lead-induced perturbations, thus offering great promise in augmenting health status and welfare of man as well as animals.
Keywords
Antioxidants; Toxicity; Oxidative stress; Health
Introduction
Lead has been affirmed as a principal lethal metal pollutant to both humans and other living organisms. Occupational and environmental exposures to this toxic metal remain a global health problem [1]. This ubiquitous pollutant has been detected in biological systems predominantly in environmental and industrial areas. It persists in nature as oxides or salts and lingers in environmental soil, water, dust and in manufactured products, comprising lead [2]. Since ancient times, anthropogenic use of lead has been on the increase with each passing century with that of the 21st century surpassing the sum of preceding eras [3]. Plants are usually exposed to lead through their water uptake, while man and animals through their consumption of such plants [4].
Environmental lead accesses the body via inhalation of airborne contaminated dust or ingestion of food and water into the digestive tract. Once absorbed, lead diffuses swiftly via the bloodstream to diverse systems and organs including the liver, kidneys, brain and to well calcify tissues including bones and teeth [5]. Complete regulation and aversion of exposure to lead is yet to be accomplished. So far, no such level of lead has been ascribed beneficial to the body likewise, no “safe” exposure level has been specified [6]. Nevertheless, lead levels of 10 μg/dL (equivalent to 0.48 μmol/L) in blood or higher has been confirmed to be toxic and induces cognitive impairments, hypertension, neurological disorders and other lead-induced perturbations [7]. Lead intoxication is an insidious risk, capable of triggering irrevocable health consequences. It interferes with physiological processes and alters systemic functions; resulting in grave disorders [8]. Blood lead levels of about 40– 60 μg/dL following occupational exposures result in acute and more commonly chronic toxicity. If untreated it becomes severe; characterized by persistent nausea, encephalopathy, fatigue, frenzy, spasms and coma [9].
Lead-induced oxidative damage has been proposed as one of the important mechanisms of lead-related pathologies [7]. In addition, chelating agents exert detrimental effects and are incapable of alleviating some toxic effects of lead [10]. In recent times, several studies have been focused on sourcing for alternatives and potentially safe treatments for lead toxicity. Consequently, to mitigate adverse effects of lead toxicity, natural compounds with both chelating and antioxidant activities are considered as good candidates [11].
The aim of this review is to elaborate on the mechanisms of lead-induced toxicity, harmful consequences of lead in various body systems, short comings of the current therapeutic measures, and the prized function of antioxidants in ameliorating lead-induced toxicity.
Mechanism of Lead Toxicity
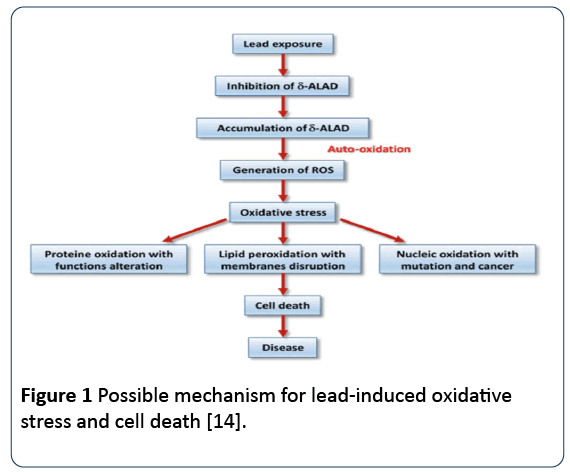
Lead toxicity induces oxidative stress through generation of ROS (superoxide radicals, hydroxyl radicals). The ROS result from detrimental tissue responses which impair metabolism and ravage cellular structural components [12]. Lead damages corpuscular materials, modifies cell genetics, and induces oxidative damage. It instigates increase yield of free radicals and decreases availability of endogenous anti-oxidant reserves (glutathione, glutathione peroxidase, superoxide dismutase, catalase), involved in scavenging the ROS generated in the lead-exposed individuals. In addition, it impairs activation of enzymes and competitively impedes absorption of trace mineral. It attaches to sulfhydryl proteins, interrupts the synthesis of structural proteins, modifies homeostasis of calcium, heightens lipid peroxidation, decrease saturated fatty acid, and increases cell membrane fatty acid contents [13] (Figure 1).
Figure 1: Possible mechanism for lead-induced oxidative stress and cell death [14].
Lead Effects on Health
Effects on nervous system
In comparison to other systems, the nervous system is extremely sensitive and is most paramount in lead inducedtoxicity. Both the central and peripheral nervous systems (CNS) are distressed following exposure to lead. In adults, the effects on the peripheral nervous system are more pronounced while the effect on the central nervous system is more prominent in children [15]. Fetuses and neonates are notably vulnerable to the neural effects of lead as the developing system takes up a higher proportion of lead. The quota of systemic circulating lead accessing nervous organs of children is considerably superior in relation to adults [16]. Encephalopathy sometimes result following lead exposure with major symptoms including gloominess, irritability, attention deficit, migraine, muscular tremor, amnesia and delusions. More intense indications are seen following prolonged exposures and include hallucination, incoordination, seizures, paralysis, loss of consciousness and ataxia [17].
Mustafa and Hussein [18] reported that rats administered lead acetate to showed several negative neurological indications; decrease in vitality, muscle-mass weakness, tremor and abnormal gait. Children may appear distracted, overactive and irritable, even at low lead level exposure. Neonates with higher lead levels may exhibit delayed growth, impaired cognition, loss of short-term memory and hearing. At higher levels, lead can cause irreversible brain damage and mortality [19].
Renal effects
Renal dysfunction ensues predominantly at high levels of lead exposure (60 μg/dL) but damage at lower levels have been stated (~10 μg/dL) [20]. Lead-induced renal functional aberration is of two types: Chronic nephropathy and acute nephropathy. Acute nephropathy is characterized primarily by impairment in the functional mechanism of tubular transport, and structurally by the manifestation of degenerative alterations in the tubular epithelium alongside the manifestation of nuclear inclusion bodies, comprising lead protein complexes. It does not trigger protein appearance in urine, but may instigate aberrant excretion of glucose, phosphates and amino acids, a condition known as Fanconi's syndrome. On the other hand, chronic nephropathy is more severe and may result in irreversible physiological and anatomical changes. It is characterized by glomerular and tubulo-interstitial alterations, give rise to renal break-down, hypertension and hyperuricaemia [21].
The foremost site of lead storage is bones [22] where it is stored in two compartments; the interchangeable pool at the bone surface and the non- interchangeable pool hidden in the cortical aspect of the bone.
Lead can easily gain entrance into plasma from the interchangeable pool; but can leave the non-interchangeable pool to the surface especially, when bone is vigorously being re-absorbed [7]. Lead exposure is known to reduce bone density in adults, thereby increasing the risk of osteoporosis [23]. Al-Naimi et al. [24] reported that dosing of lead acetate at 75 mg/kg body weight for 20 and 40 days caused mild hyperplasia of hemopoietic tissue with propagation of megakaryocytes and the incidence of thin trabecular of hardened cartilage enclosed by a thin bone layer. The blocks of mineralized cartilage resulting from impaired resorption of osteoclasts are extensive and further project into the metaphyseal marrow cavity in contrast to normal bones [24].
Reproductive health effects
Lead triggers a number of detrimental effects on the reproductive systems in both male and female. Common effects recorded in male include: reduced libido, abnormal sperm motility, chromosomal mutilation, sterility, abnormal prostatic function and fluctuations in serum testosterone [25,26]. However females are more prone to infertility, miscarriages, pre-eclampsia, pregnancy hypertension, premature membrane rupture, and impulsive delivery [25]. Direct effect of lead on embryonical stages of the fetus during gestation period has also been documented [27].
Effects on the hematopoietic system
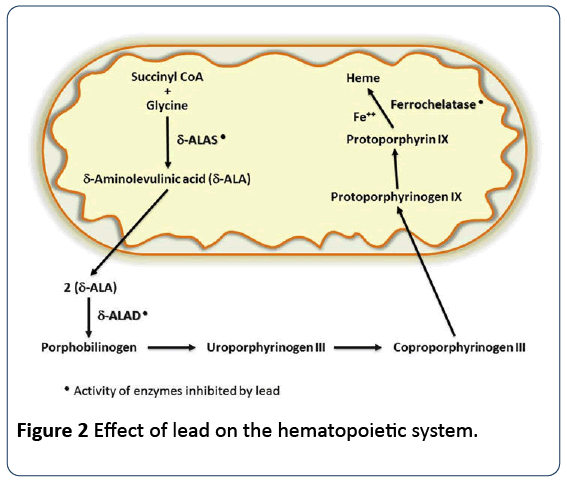
Lead precisely affects the hematopoietic system via inhibition of synthesis of hemoglobin by restricting essential enzymes, required in the heme synthesis pathway. It also lowers the life-span of circulating erythrocytes by augmenting the fragility of cell membranes. The combined effects of the two activities lead to anemia [28]. Anemia occurring on account of lead poisoning can be of two types: hemolytic anemia, which is related with sudden high level lead exposure; and frank anemia, occurring when the blood lead level is substantially elevated for a lingering period of time [29]. Lead considerably affects the heme synthesis pathway in a dosedependent fashion by down-regulating three key enzymes, essential for synthesis of heme. Delta-aminolevulinic acid dehydratase (ALAD), which is a cytosolic enzyme that catalyzes the organization of porphobilinogen from δ-aminolevulinic acid (ALA); aminolevulinicacid synthetase (ALAS), which is a mitochondrial enzyme catalyzing formation of aminolevulinic acid (ALA); and ultimately, mitochondrial enzyme ferrochelatase which catalyses the attachment of iron with protoporphyrin to form heme [30].
Lead impedes the three afore-mentioned vital enzymes of this pathway with its effect on ALAD more prominent and this is used clinically to measure the degree of lead toxicity. Inhibition of ALAD results in the accrual of aminolevulinic acid, evident in plasma and urine at levels less than 10 μg/dL. Although inhibition of ALAD is first noticeable at blood lead levels of 10–20 μg/dL, heme biosynthesis does not reduce until the function of ALAD is inhibited by 80–90%, which occurs at a much higher blood lead concentration of about 55 μg/dL [31] (Figure 2).
One of the initially observed hematological effects of lead poisoning is the basophilic stippling’s of erythrocytes which is potential biomarker for detection of lead toxicity. This aggregates as products of degradation of ribonucleic acid [7]. Other noticeable hematological changes, associated with lead induced-t xicity include; anemia characterized by anisocytosis and RBC lipo-peroxidation leading to increased RBC fragility [32,33] thrombocytopenia and leucopenia [4,34].
Cardiovascular effects
Chronic and acute lead poisoning has been documented to cause cardiac and vascular damages with possibly fatal consequences, including hypertension and circulatory disorders [35]. Low-level lead exposure contributes to hypertension in exposed animals and humans [36]. Other key disorders include ischaemic coronary heart ailment, cerebrovascular disturbances and peripheral vascular disease. Although indication of causal affiliation of lead exposure and hypertension has been documented, it applies only in cases of cardiovascular sequels of lead toxicity [35].
Effects on the liver
Disruption of the normal anatomical organisation of hepatic lobules, loss of the distinctive cord-like structure of functional liver cells, hyperchromatic hepatocytes with occasional vacuolations and congestion of sinusoids occur in lead poisoning [37]. Abdel Moneim [1] reported that at histology, lead acetate induced focal hepatic necrosis, accompanied by dilated blood sinusoids and congested central veins, infiltration of acute inflammatory cells, mainly in the central zone, derangement of hepatocyte cords with pyknotic and karyolitic nuclei, vacuolization of hepatocytes and fatty change (steatosis), which include the intracellular accumulation of fats.
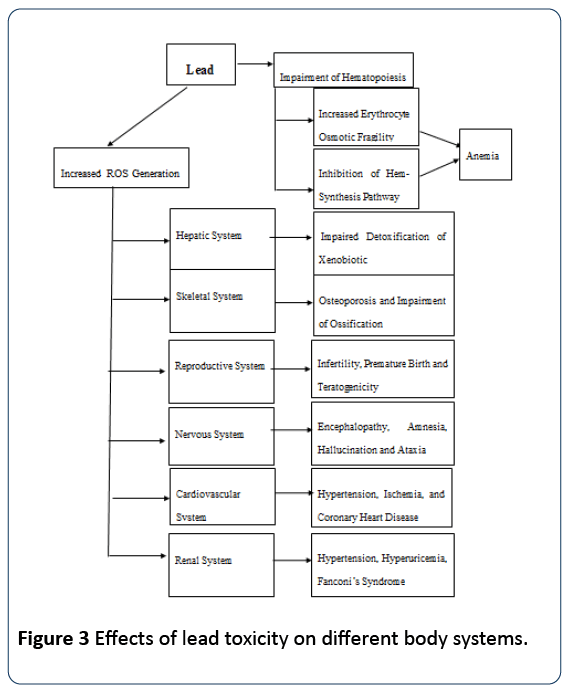
Significant increase in free radical generation, activities of liver transaminase (alanine aminotransferase and aspartate aminotranferase) and total bilirubin in experimental leadinduced toxicity in male Wistar rats was reported by Abdel Moneim [1] and Shatha et al. [2]. The elevated plasma ALT and AST activity was complemented with high liver microsomal membrane fluidity, ROS production, and variation in the hepatocyte histogram [2] (Figure 3).
Role of antioxidants in mitigating lead toxicity
Treatment of lead poisoning is classically by chelation therapy. There are however documented evidences of synthetic chelators' side-effects. The commonly used lead chelators include dimercaptosuccinic acid (DMSA), CaNa2EDTA, dimercaprol (BAL), unithiol (DMPS) and Dpenicillamine (DPA). Ethylene di-amine tetra acetic acid is reported to have side-effects including renal toxicity, cardiac problems due to hypocalcaemia; Penicillamine has side-effects like abdominal pain, skin lesions, alopecia, stomatitis, glossitis, luecopenia, thrombocytopenia, enuresis; Succimer's sideeffects include nausea, vomiting, diarrhoea, skin rash, and DMSA has side-effects like nausea, vomiting, papular rash, pruritis [38].
Lead-induced oxidative damage is one of the important mechanisms of lead-related pathologies [7]. It has been reported that chelating agents may produce a toxic potential themselves, and also are incapable of alleviating some toxic effects of lead [10]. Therefore, sourcing for potentially safe alternatives for deterrence and remedy of lead toxicity is important. Natural compounds such as Zn, lysine, arginine, vitamin C and E, antox, alpha lipoic acid, and thiol containing compounds which possess both chelating and antioxidant activities are superior entrants employed in managing lead toxicity.
Zinc: Zinc is one of the most abundant and essential trace elements found in the body. It performs numerous physiological and biochemical functions. Basically up to 10% of the entire mammalian cell proteins require Zn for their metabolism, structural and functional modification [39-41]. Zn is needed for the function of over 300 enzymes, and participates in many metabolic and enzymatic functions [42]. Zinc, a divalent cation with diverse biochemical and physiological roles, plays major neuro modulatory functions in the central nervous system.
Zinc has been shown to reduce the poisonous effects of lead in rat testes via an antioxidant mechanism [43]. One of the few mechanisms for the protection provided by zinc includes ability to stabilize lipid membrane and protects lipid peroxidation by free radicals, thereby protecting the tissues [44]. Adhikari et al. [45] reported that zinc protects against lead-induced cell death in testes of young rats. A similar effect was also reported by [46]. Zinc also augments the quality of semen retrieved from rats exposed to lead toxicity [47]. In addition, the reproductive potential of male portan rats exposed to various levels of lead was improved with regard to zinc administration [48].
L-arginine: L-arginine contributes too many important and diverse biochemical reactions associated with normal physiology of an organism [49]. It is classified as “semiessential” or “conditionally essential” amino acid as it is extracted from the diet as a supplement to synthesis in mammals and humans. This amino acid is a nitric oxide precursor and a competent antioxidant agent in reducing the extent of lipid peroxidation in serum of lead-pre-exposed rats [50]. L-arginine under lead intoxication modulates antioxidant enzyme activity by increasing the activity of glutathione system, especially in animals with low resistance to hypoxia. It possesses both preventive and therapeutic effects against lead intoxication [51].
Vitamin C: Vitamin C also known as ascorbic acid is a watersoluble vitamin required for physiological growth and development. It is needed for collagen synthesis which is necessary for the formation of tendons, ligaments, skin, and blood vessels. Vitamin C aids in wound healing and scar tissue formation. It restores and sustains bones, teeth and cartilage acting as a major antioxidant [52]. Vitamin C is a potent antioxidant which decreases oxygen, nitrogen, and sulfurcentered radicals [53]. Studies on rats treated with lead manifested lowered levels of iron, resulting in impairment of heme formation. Administration of 500 mg/day of vitamin C for one month in battery-manufacturing workers has been shown to reduce malondialdehyde concentration and nitrite levels improving antioxidant status including erythrocyte osmotic fragility and activities of endogenous antioxidant enzymes by scavenging the ROS generated due to high blood lead levels [52].
Studies have proven that with increase in blood lead level, the erythrocyte count, hematocrit and hemoglobin level of Rattus norvegicus (albino rats) drastically decreased [33]. However, most of the effects of lead on hematology weasameliorated by the treatment of vitamin C. Vitamin C treatment were successful in increasing the hemoglobin and hematocrit to normal levels [33].
Vitamin E: Studies have also demonstrated that vitamin E may help to reduce lead-induced hepatotoxicity of leadexposed rats, and to ameliorate the oxidative stress status in the brain of rats [54]. Vitamin E scavenges ROS by readily donating electrons to ROS in the body during oxidative reactions, and become oxidized in the process. Consequently, the scavenging of ROS reduces lipid peroxidation and oxidative stress that may cause tissue injury [55].
Vitamin E is effective in the treatment of lead-induced toxicity, while a combination of vitamins E and C produce synergistic and additive effects [54]. The administration of vitamins E significantly decreases malondialdehyde and nitrogen oxide concentrations in the cerebral cortex of rats. It also significantly increases the brain glutathione levels and the activities of superoxide dismutase and catalase, when compared with the lead only group [54], indicating the capability of the vitamin to decrease the ROS-induced negative effects, prompted by lead in the body. Intake of vitamin E in lead-exposed fish prevents the accumulation of lead in tissues, and enhances the growth factor of fish [56]. Seung et al. [57] reported that vitamin E provides fortification against oxidative stress, caused by lead toxicity. Ping et al. [58] demonstrated that lead exposure decreases the defense capacity of an animal, and that ascorbic acid greatly enhances the prophylactic potential for removal of lead from tissue. Studies have proved that vitamin E reverses the anemia triggered by a decrease in erythrocyte count, Hematocrit and Hemoglobin level of lead-exposed Rattus norvegicus (albino rats) [33]. Conversely, rats that received vitamin, C and E synergism gave the lowest blood lead and proved more efficient than vitamin C or E in all of the studied parameters despite the same total international unit dose used [33].
Antox: Lead is relentlessly neurotoxic and antox combination therapy reduces the ensuing damage, due to its potential to neutralise ROS generated by lead. The body generates enzymatic antioxidants but does not synthesis the antioxidants including vitamins E and C and flavonoids which shield the body sites that the enzymatic antioxidants cannot access [59]. Preston [60] reported that selenium, betacarotene which is a precursor to vitamin A, vitamins E and C are relevant in the overall cellular antioxidant defence mechanism. Antox contains three auxiliary vitamins A, C and E, with selenium which is critical for normal physiologic processes. The protective effect of the vitamins represents potent antioxidants and ROS-scavenging activities [61] selenid complexes. Selenium provides fortification against ROSinduced cell damage [62].
Alpha-lipoic acid: Alpha-lipoic acid (α-LA) is a biological molecule which functions in cellular acyl group transfer active ties. It is a natural antioxidant agent obtained from many food types. This antioxidant possesses the ability to mop-up ROS, in both lipophilic and hydrophilic media [63]. In addition, α-LA stimulates regeneration of endogenous antioxidants, such vitamins C and E, alongside reduced gluthathione activity [64]. Moreover, α-LA inhibits function and synthesis of inducible nitricoxide synthase (iNOS) [65] and scavenges free radicals [66]. It also exhibits a neuroprotective effect inhibiting apoptosis; and this has been attributed to its potential to protect neurones against ROS-mediated DNA damage via inhibition of ROS formation [67].
Studies have shown that α-LA protect animals from lead acetate (PbA)-induced seizures and mortality. In addition, pretreatment with α-LA decreased the lipid peroxidation, caused by lead acetate, as indicated by a decrease in hippocampal malondialdehyde concentratons. The increase in hippocampal NO levels, induced by lead acetate was reduced significantly in α-LA treated groups in a dose-dependent manner. An effect attributed to its potential to inhibit iNOS. α- LA was reported to inhibit brian iNOS induced insults [68]. Moreover, both hippocampal GSH level and GSH-PX activity increased in α-LA-treated animals in relation to lead acetatetreated animals. This finding reflects the capability of α-LA to restore the lead acetate depressed antioxidant defense mechanisms. Both α-LA and dihydrolipoic acid, have ROSscavenging property [69]. The di-thiol group in α-LA is utilised in the synthesis of endogenous antioxidants, such as glutathione, vitamin E, and vitamin C [63]. In addition, α-LA may increase GSH concentration by augmenting cysteine uptake, which is a rate-limiting step in GSH synthesis [66]. Thus, α-LA antagonises depletion of intracellular GSH. Moreover it can neutraliz ROS, in fatty and aqueous cellular regions. In contrast to vitamin E, vitamin C functions as an antioxidant in both its reduced and oxidised form [70]. Furthermore, α-LA readily crosses the blood-brain barrier, attaining high concentrations and exerting its effects [67]. α-LA also antagonises decrease in of GSH-dependent antioxidant enzymes [69]. This discovery was also reported as an increase in hippocampal GSH-Px activity, depressed by lead-autoxicity [70]. The decrease in the level of lipid peroxidative products and elevation in GSH level and GSH-Px activity in brain cells, in response to α-LA administration is indicative of enhanced ROSscavenging and improved detoxification of lipid hydroperoxides and hydrogen peroxide [70].
Quercetin: Quercetin (3,5,7,3',4'-pentahydroxyflavon) is potent flavonoids that protects against degradation by free radicals such as reactive nitrogen and oxygen species, which are generated during metabolism, or induced by exogenous damages [71]. Quercetin is abundant in food, including green leafy vegetables, fruit and beverages [72]. Quercetin at 50 mg/kg bodyweight was found to have protective effect in rats intoxicated with lead, and this response was reflected on the blood and testis. Results of the research suggest potential preventive or therapeutic applications of quercetin for individuals subjected to lead environmental pollution [73].
Thiol-containing compounds (Methionine, NAcetylcysteine and Homocysteine): The thiol-containing compounds are known to exert antioxidant features by binding to lead at their –SH group. They serve as useful component for the effective treatment of lead poisoning. Methionine is a precursor for glutathione, which defends the body cells from oxidative stress by scavenging ROS and also plays a fundamental function in detoxification [74,75]. The amino acid, methionine has been documented to chelate lead and eliminate it from tissues [76]. N-Acetylcysteine also possesses antioxidant potential against lead, including oxidative damage via stimulation of glutathione synthesis; maintaining intracellular glutathione concentrations and scavenging ROS [77]. In addition, N-Acetylcysteine has also been reported to possess lead chelating ability [78]. Homocysteine is a thiol compound formed by demethylation of methionine, and appears at moderately high levels and is a known independent risk factor for atherosclerosis and increased vascular dysfunction [79]. Conversely, some authors believe that homocysteine contains a thiol group and displays its antioxidant effect in cellular systems at micromolar concentrations [80]. Studies have proven that administration of sulphur-containing antioxidant molecules exerts protective functions by scavenging ROS engendered secondarily by lead, including those produced by xenobiotic-induced lipid peroxidation, or by the decrease in vitamin levels levels and antioxidant enzyme activities [81]. Recent study on a group of work force occupationally exposed to lead revealed that, lead exposed workers treated with N-acetylcysteine (NAC) demonstrated a significant decline in their blood lead levels. In addition, all groups that received NAC had drastically elevated activity of glutamate dehydrogenase. It was further reported that treatment with NAC normalised the level of homocysteine and decreased oxidative stress. It was therefore concluded that NAC may be recommended as an alternative therapy for chronic lead toxicity in humans [82].
Herbal antioxidants: Herbal antioxidants have been reported to play a role in ameliorating lead-induced toxicity. Bacopa monnieri (Brahmi) is a versatile, indigenous medicinal herb with a wide spectrum of pharmacological properties [83]. B. monnieri has been shown to exert antioxidant effects through the chelating of metal ions, breaking oxidative chain reaction, improving the activities of antioxidative defence enzymes and scavenging the free radicals [84]. B. monnieri also confers protection against reduction in ATPase activity. Ethanolic extract of B. monnieri contains a mixture of alkaloids, steroids and saponins [83]. A number of reports suggested that hepatoprotective and antioxidant properties of B. monnieri were attributed predominantly due to the manifestation of its characteristic active constituent dammarane type triterpenoid saponin called “Bacopside-A” [85].
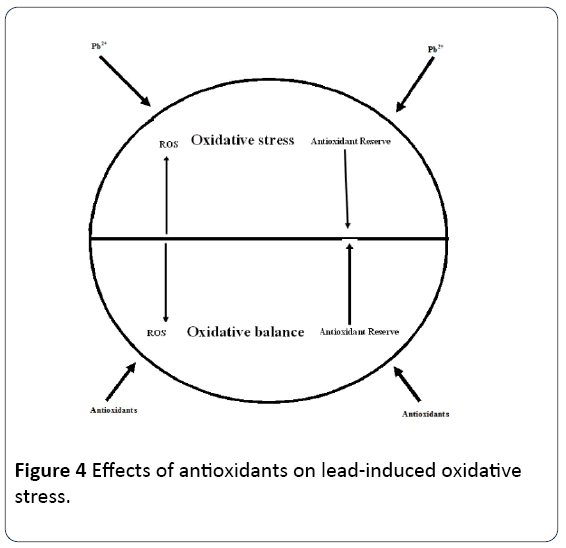
In general, phenolic and flavonoid components present in the extract may provide strong antioxidant property to plant by acting as ROS scavengers, hydrogen donors, reducing agents, singlet oxygen quenchers and metal chelators, [86]. Thus, anti-oxidative potential of this herb is beneficial in reducing lead-mediated oxidative stress, and ameliorating significantly lipid peroxidation by enhancing the pro-oxidant/ antioxidant equilibrium of the cells. The amelioration subsequently helps in maintenance of protein levels as well as provides protection against alteration in enzyme activities in the liver cultures of goats. The administration of ethanol extract, B. monieri to lead exposed cultures act as therapeutic eliminators of heavy metal, and significantly exert protective effects against adverse effects of lead intoxication due to their well-known antioxidant and hepatoprotective properties. Thus, this herbal treatment approach may play pivotal role in alleviating the lead toxicity [86]. Moringa oleifera is another herb reported to have potent antioxidant activity. A study was conducted to estimate the protective role of aqueous extract of Moringa oleifera leaves (Lam) on lead-induced damage on hepatocytes and hepatic reticular fibres in rats. Results of the study revealed that lead treatment intensified markers of hepatic damage (ALT, AST and ALP) and decreased catalase activities. Histological examinations reveal alterations of hepatic structure including hepatocytic vacuolations, sinuosoidal congestion and loss of reticular fibres subsequent to lead therapy. Treatment with Moringa oleifera averted and is reversed lead-induced hepatic damage. Omotoso et al. [87] reported that Moringa oleifera leaf extract exhibits a potent capacity to prevent hepatotoxicity induced by lead, partly due to its chemical constituents which has hepato-protective properties [87]. Jawhar et al. [88] evaluated the effects of Berberis vulgaris on oxidative stress and liver injury in leadintoxicated mice. Lead treated group showed considerable alteration of serum alanine-aminotransferase (ALT), aspartateaminotransferase (AST), total cholesterol (TC), total bilirubin (TB), catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD) and diminished glutathione (GSH). Histological assessment of lead-intoxicated mice liver revealed alterations in hepatocytes and focal necrosis. Berberis vulgaris treatment significantly prevented lead accumulation, increased ALT, AST, TC, and TB, inhibited lipid peroxidation and protein carbonyls (PCO) formation. Additionally, Berberis vulgaris extract normalized the antioxidant enzymes (CAT, SOD and GPx), GSH and architecture of liver tissues [88] (Figure 4).
Curcumin, a potent antioxidant and nutritive ingredient, common for its therapeutic potentials and possesses a variety of beneficial effects against diverse adverse effects. Ankit et al. [89], carried out a research to evaluate the mitigative effects of curcumin (3.87 μM, i.e., 1.43 μg/ml) against varying doses of lead acetate (10-6 M, i.e., 0.379 μg/ml and 10-4 M, i.e., 37.9 μg/ml, durations: 24 h and 69 h evoked genotoxicity and oxidative stress in peripheral blood lymphocyte cultures (PBLC) of humans. The result of the study revealed that lead acetate exhibited dose and duration-dependent rise in both genotoxicity and oxidative stress while curcumin, when added along with lead acetate, showed significant mitigation of genotoxic and oxidative stress-related indices. The study signified that, due to change in antioxidant defense system, the adverse genotoxic effect of lead becomes evident. Curcumin therefore, protects chromatin substance against lead-mediated genotoxicity by stabilizing the activity of antioxidant defense structure [89].
Similarly, a research was conducted to evaluate beneficial effects of garlic on learning and memory deficits and brain tissue damages induced by lead exposure during juvenile rat growth. Results of the study revealed that treatment with garlic attenuates the learning and memory impairments due to lead exposure during juvenile rat growth and this was attributed to the ability of garlic to protect the brain against oxidative damage as well the lowering effects of brain lead content [90] (Figure 4).
Conclusion
There is sufficient evidence establishing antioxidants as superior therapy to conventionally-used chelating agents, which exert detrimental effects and are incompetent in alleviating some toxic outcomes of lead. The major shortcoming of chelating agents is the fact that, some of them induce loss of essential minerals and redistribute lead to the brain. They are also incapable of removing lead from intracellular site thus leading to hepatotoxicity, nephrotoxicity, poor clinical recovery and pro-oxidants effects manifested as headache, nausea, and increased blood pressure levels. This presents evidences suggest the need for novel strategies to ameliorate lead-induced toxicity. Antioxidants possess both chelating and ROS scavenging capacity enabling elimination of lead from intracellular sites and blood stream. Contrary to the use of chelating agents which exert rebound effects, antioxidants interventions can be initiated even when the patient is still exposed to the source of lead thus offering a greater promise for abating lead-induced toxicity while enhancing welfare status and health of man and animals. Molecular mechanisms underlying the beneficial activities of antioxidants alongside their optimum dose and treatment duration for lead-induced toxicity should be further studied.
Acknowledgement
We appreciate the efforts of Professor J.O. Ayo of Department of physiology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria for thoroughly going through the manuscript and making corrections before submission.
References
- MoneimAE (2016) Indigoferaoblongifoliaprevents lead acetate-induced hepatotoxicity, oxidative stress, fibrosis and apoptosis in rats. PLoS ONE 11(7): e0158965.
- Shatha HK, NazarmutebZA, Moayad MU (2016)The effect of penicillamine in reducing the toxic effects of lead acetate on some blood parameters, liver functions and testicular tissue in male rats. Int J Pharm Pharm Res5: 22-40.
- El-NekeetyM, El-KadyK, Soliman MS, Hassan NS, Abdel-Wahab MA(2009)Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food ChemToxicol 47: 2209-2215.
- Hedayati A, DarabitabarF (2017)Lethal and sub-lethal impacts of lead on some hematological, biochemical and immunological indices in Caspian roach.Pollut 3: 21-27.
- Alya A, Ines BD, Montassar L, Najoua GH, Saloua EF (2015)Oxidative stress, biochemical alterations and hyperlipidaemia in female rats induced by lead chronic toxicity during puberty and post-puberty periods. Iran J Basic Med Sci 18: 1034-1043.
- Flora G, Gupta D, Tiwari A (2012)Toxicity of lead: A review with recent updates. InterdiscipToxicol 5: 47–58.
- Patrick L (2006)Lead toxicity, a review of the literature. Part 1: Exposure, evaluation, and treatment. Altern Med Rev 11: 2–22.
- Kalia K, Flora SJ (2005)Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 47: 1–21.
- Pearce JM (2007) Burton’s line in lead poisoning. EurNeurol 57: 118–119.
- Ajayi GO, AdeniyiTT, Babayemi DO (2009)Hepatoprotective and some haematological effects of Allium sativum and vitamin C in lead exposed Wistarrats.Int J Med MedSci 1: 64-67.
- Bokara KK, BrownE, McCormick R, Yallapragada P, Rajanna S (2008) Lead-induced increase in antioxidant enzymes and lipid peroxidation products in developing rat brain. Biometals 21: 9-16.
- Carocci A, Catalano A, Lauria G, Sinicropi MS, Genchi G(2016) Lead toxicity, antioxidant defense and environment. Rev Environ ContamToxicol. 238: 45-67.
- Foyer CH, Noctor G (2002)Oxygen processing in photosynthesis: Regulation and signaling. New Phytol 146: 359–388.
- Malecka A, Jarmuszkiewicz W,Tomaszewska B( 2001) Antioxidant defense to lead stress in sub cellular compartments of pea root cells. ActaBiochimicaPolinica48: 687-698.
- Brent JA (2006) Review of Medical Toxicology. ClinToxicol 44: 355–355.
- Needleman H (2004)Lead poisoning. Annu Rev Med 55: 209–222.
- Flora S, Flora G, Saxena G (2006) Environmental occurrence, health effects and management of lead poisoning. In: Cascas SB,Sordo J (eds). Lead chemistry, analytical aspects environmental impacts and health effects. ElsevierPublication, Netherland, pp.158-228.
- Mustafa HN, Hussein AM (2016) Does allicin combinedwith vitamin B-complex have superior potentials than a-tocopherol alone in ameliorating lead acetate-induced Purkinje cell alterations in rats? An immunohistochemicaland ultrastructuralstudy. Folia Morphol 75: 76-86.
- Cleveland LM, Minter ML, Cobb KA, Scott AA, German VF (2008). Lead hazards for pregnant women and children: Part 1: Immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two-part article details how exposure happens, whom it affects, and the harm it can do. American Journal of Nursing 108: 40–49.
- Grant LD (2008) Lead and compounds. Environmental toxicants. John Wiley & SonsInc.,Hoboken, New Jersey, USA.pp. 757–809.
- Rastogi SK (2008) Renal effects of environmental and occupational lead exposure. Indian J Occup Environ Med 12: 103–106.
- Renner R(2010) Exposure on tap: Drinking water as an overlooked source of lead. Environmental Health Perspective 118: 68–74.
- Khalil N, Faulkner KA, Greenspan SL, Cauley JA (2014) Associations between bone mineral density, grip strength, and lead body burden in older men. J Am GeriatrSoc 62: 141–146.
- Al-Naimi RA, Abdulhadi D, Zahroon OS, Al-Taae EH (2011)Toxicopathologicalstudy of lead acetate poisoning in growing rats and the protactiveeffect of crystien or calicium. J Vet Sci 4: 26-39.
- Flora S, Pachauri V, Saxena G (2011) Arsenic, cadmium and lead.In: Jerrold S. Meyer, Brian J (eds),Reproductive and Developmental Toxicology. Elsevier, Amsterdam, TheNetherlands, pp.415–438.
- Hanan MA (2015) Protective effect of quercetin and or zinc against lead toxicity on rat testes. Global J Pharmacol 9: 366-376.
- Saleh HA, El-Aziz GA,El-Fark MM, El-Gohary M (2009) Effect of maternal lead exposure on craniofacial ossification in rat fetuses and the role of antioxidant therapy. Anat His Em 38: 392–399.
- Guidotti TL, McNamara J, Moses MS (2008)The interpretation of trace element analysis in body fluids. Indian J Med Res 128: 524–532.
- Vij AG (2009) Hemopoietic, hemostatic and mutagenic effects of lead and possible prevention by zinc and vitamin C. Al-AmeenJ MedSci 2: 27–36.
- Piomelli S (2002) Childhood lead poisoning. PediatrClin North Am 49: 1285–1304.
- Ahamed M, Verma S, SiddiquiMK (2005) Environmental exposure to lead and its correlation with biochemical indices in children. Sci Total Environ346: 48–55.
- Saeed AA (2015)Haemato-biochemical changes induced by lead intoxication in male and female albino mice. Int J Recent Sci Res6: 3999-4004.
- Xhyrel JJ, Nikko LG, Marlon CP (2016) Relative antioxidant efficacy of a-tocopherol and ascorbic acid on blood lead, haemoglobin and haematocrit level of lead-exposed Rattusnorvegicus (albino rat). Der Pharmacia Lettre 8: 169-179.
- Ambali SF, Angani M, Shittu M, Kawu MU (2011) Haematological changes induced by subchronic co-administration of chlorpyrifos and lead in Wistar rats: Alleviating effect of vitamin C. Der Pharmacia Sinica2: 276-284.
- Navas-Acien A, Guallar E, SilbergeldEK, Rothenberg SJ (2007) Lead exposure and cardiovascular disease: A systematic review. Environ Health Perspect 115: 472–482.
- Agency for Toxic Substances and Disease Registry (2005) Toxicological profile for lead. (Draft for Public Comment), U.S. Department of Health and Human Services, Public Health Service.Atlanta, GA, USA. pp.43–59.
- BukolaRO, Adeleke AA, OmamuyovwiMI, Stephen OA ( 2015)Lead-induced damage on hepatocytes and hepatic reticular fibres in rats: Protective role of aqueous extract of Moringaoleifera leaves (lam).J Biosci Med 3: 27-35.
- Thuppil V, Tannir S (2013) Treating lead toxicity: Possibilities beyond synthetic chelation. JKIMSU 2: 4-31.
- Andreini C, Banci L, Bertini I, Rosato A (2006) Counting the zinc-proteins encoded in the human genome. J Proteome Res 5: 196-201.
- Prasad AS (2013)Discovery of human zinc deficiency: Its impact on human health and disease. AdvNutr 4: 176-190.
- Prasad AS (2014) Impact of the discovery of human zinc deficiency on health. J Trace Elem Med Biol 28: 357-363.
- Andreini C, Bertini I (2012) A bioinformatics view of zinc enzymes. J InorgBiochem 111: 150-156.
- Claveria C, Corbella R, Martin D, Diaz C (2000) Protective effects of zinc on cadmium toxicity in rodents. Biol Trace Elem Res 75: 1-9.
- Xu L, Sun H, Wang S, Song L, Chang H(2005) The roles of metal lothionein on cadmium-induced testes damages in Sprague-Dawley rats. Environ ToxicolPharmacol20: 83-87.
- AdhikariN, Sinha N, Narayan R, Saxena DK (2001) Lead-induced cell death in testes of young rats. J ApplToxicol 21: 275-277.
- Batra N, Nehru B, Bansal MP (2001) Influence of lead and zinc on rat male reproduction at biochemical and histopathological levels.J ApplToxicol 21: 507-512.
- Rafique M, Naheed K, Khalida P, Anjum N (2010) The effects of lead and zinc on the quality of semen of albino rats. J Coll Physicians Surg Pak 19: 510-513.
- Batra N, Nehru B, Bansal MP (2004) Reproductive potential of male portan rats exposed to various levels of lead with regard to zinc status. Br. J. Nutr 91: 387-391.
- Alderton WK,Cooper CE, Knowles RG (2001) Nitric oxide: Structure, function and inhibition. Biochem J357: 593–615.
- Hsu PC, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicol 180: 33–44.
- Tkachenko H, Kurhalyuk N, Khabrovska L, Kaminski P (2007) Effect of L-arginine on lead induced oxidative stress in the blood of rats with different resistance to hypoxia.Pol JFood NutrSci 57: 387–394.
- Ganesh HG, Arun JP, Jyotsna AP, Mandakini SK, Ajit S (2016) Biochemical effects of lead exposure on oxidative stress and antioxidant status of battery manufacturing workers of Western Maharashtra, India. J Basic ClinPhysiolPharmacol 27: 141-146.
- Niki E (1991) Action of ascorbic acid as a scavenger of active and stable oxygen radicals. Am J ClinNutr 54: 1119-1124.
- Ebuehi OA, Ogedegbe RA, EbuehiOM (2012) Oral administration of vitamin C and vitamin E ameliorateslead-induced hepatotoxicity and oxidative stress in the rat brain. Nig Q J Hosp Med 22: 85-90.
- Obianime AW, Aprioku JS (2009) Comparative and interactive studies of aqueous leaf extracts of Ocimumgratissimum Linn. (Lamiaeceae), Vitamins C and E on the basal serum phosphatase levels of male guinea-pigs. West Afr J Pharmacol Drug Res 24: 1-7
- Abdalla AE (2009) The role of antioxidant (vitamin E) in the control of lead (Pb) pollution and enhancement of growth within Nile tilapia (Oreochromisniloticus). Int J Appl Res Vet M 7: 97.
- Senug H, Min-Won B, Hui-Young L, Dong-Jae K, Yi-Rang N (2007) Arsenite-induced apoptosis is prevented by antioxidants in zebra fish liver cell line. Toxicol In vitro21: 870-877.
- Ping-Chi H, Ming-Yie L, Chao-Chin H, Lih-Yuh C, Yueliang G (1998) Effects of vitamin E and/ or C on reactive oxygen species-related lead toxicity in the rat. Toxicol 128: 169 – 179.
- Haggag AM, Abu-Taleb AM, El-Abiad NM (2002) The role of Antox (Vitamins A, E, C and Selenium) in carbon tyetracholride-induced hepatotoxicity in rats. Arab JNuclSciAppl35: 339-345.
- Preston AM (1991) Cigarette smoking-nutritional implications. Prog Food NutrSci15: 183-217.
- Hawazzen AL, Al-Rawi M (2007) Effect of antox on paraquat-induced histological and biochemical changes in kidney of albino rats. J App. Sci 3: 988 – 993.
- Santamaria A, Sanchez A, Roman B (2003) Protective effects of antioxidant selenium on quinalinic acid induces neurotoxicity in rat. JNeurochem86: 479–488.
- Bast A, Haenen GR (2003) Lipoic acid: A multifunctional antioxidant. Biofactors 17: 207-213.
- Maczurek A, Hage K, Kenklies M, Sharman M, Martins R (2008) Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer’s disease. Adv. Drug Deliv. Rev60: 1463-1470.
- Gurer H, Ozgunes H, Oztezcan S, Ercal N (1999) Antioxidant role of alpha-lipoic acid in lead toxicity. Free RadicBiolMed 27: 75-81.
- Han D,Handelman G, Marcocci L, Sen CK, Roy S (1997) Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors 6: 321-338.
- Jia Z, Zhu H, Vitto MJ, Misra BR, Li Y (2009) Alpha-lipoic acid potently inhibits peroxynitrite-mediated DNA strand breakage and hydroxyl radical formation: implications for the neuroprotective effects of alpha-lipoic acid. Mol Cell Biochem 323: 131-138.
- Kiemer AK, Müller C, Vollmar AM (2002)Inhibition of LPS-induced nitric oxide and TNF-alpha production by alpha-lipoic acid in rat Kupffer cells and in RAW 264.7 murine macrophages. ?Immunol. Cell Bio 80: 550-557.
- Shay PK, Moreau RF,Smith EJ,Hagen TM (2008) Is alpha-lipoic acid a scavenger of reactive oxygen species in vivo? Evidence for its initiation of stress signaling pathways that promote endogenous antioxidant capacity.IUBMB 60: 362-366.
- Abdel-Wahab BA, Metwally ME (2014) Protective effect of alpha lipoic acidagainst lead-induced hippocampal neurotoxicity and neuronal oxidative Stress in rats. Austin J PharmacolTher2: 8.
- De Groot H (1994) Reactive oxygen species in tissue injury. Hepatogastroenterol 41: 328-332.
- Beecher GR (2003) Overview of dietary flavonoids: Nomenclature, occurrence and intake.J. Nutr133: 3248-3254.
- Al-Omair MA, Sedky A, Ali A, Elsawy H (2017) Ameliorative potentials of quercetin against lead-induced haematological and testicular alterations in albino rats. Chin J Physiol 60: 131-138.
- Reed DJ (1990) Glutathione: Toxicological Implications. Annu Rev PharmacolToxicol30: 603-631
- Reed JC, Orrenius S (1997) Meeting Report: Dateline Edinburgh. Cell Death & Differ 4: 254-258.
- Patra RC, SwarupD, Dwivedi SK (2001) Antioxidant effects of alpha tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicol162: 81-8.
- Ercal N, Luo X, Matthews RH, Armstrong DW (1996) In vitro study of the metabolic effects of D-amino acids. Chirality 8: 24–29
- Aruoma OI, Halliwell B, Hoey BM, Butler J (1989)The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med6: 593-597.
- Refsum H (2001) Folate, vitamin B12 and homocysteine in relation to birth defects and pregnancy outcome. Br J Nutr 85: S109-113.
- Zappacosta B, Mordente A, Persichilli S, Minucci A, Carlino P (2001) Is homocysteine a pro-oxidant? Free Radic Res 35: 499-505.
- Caylak E, AytekinM, Halifeoglu I (2008) Antioxidant effects of methionine, a-lipoic acid, N acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. ExpToxicolPathol 60: 289-294.
- Kasperczyk S, Dobrakowski M, Kasperczyk A, Romuk E,Rykaczewska-Czerwinska M (2015) Effect of N-acetylcysteineadministrationonhomocysteine level, oxidative damage to proteins, and levelsof iron (Fe) and Fe-related proteins in lead-exposed workers. ToxicolInd Health 32: 1-9.
- JyotiA, Sharma D (2006) Neuroprotective role of Bacopamonniera extract against aluminium-induced oxidative stress in the hippocampus of rat brain. Neurotoxicol 27: 451-457.
- Russo A,Izzo AA, BorrelliF, Renis M, Vanella A (2003) Free radical scavenging capacity and protective effect of Bacopamonniera L. on DNA damage. Phytother Res 17: 870-875.
- Sumathi T, Nongbri A (2008) Hepatoprotective effect of bacoside-A, a major constituent of Bacopamonniera (Linn). Phytomed 15: 901-905.
- Shah FC, Jain NK (2016) Ameliorative action of synthetic and herbal antioxidants on lead induced hepatotoxicity: An in vitro study. Asian J Pharm Clin Res 9: 364-370.
- Omotoso BR, Abiodun AA, Ijomone OM, Adewole SO (2015) Lead-induced damage on hepatocytes and hepatic reticular fibres in rats; protective role of aqueous extract of moringaoleifera leaves (Lam). J Biosci Med 3: 27-35.
- Jawhar L,Jaouad E, Hamadi F, Yassine C, HananeG (2017) Berberis vulgaris effects on oxidative stress and liver injury in lead-intoxicated miceJ Complement Integr Med 14: 1-7.
- Ankit N, Ambar P, Naumita S, Shiva C, Alpesh P (2017) Ameliorative effects of curcumin against lead induced toxicity in human peripheral blood lymphocytes culture. Drug ChemToxicol 1: 1-8.
- Simagol G, Mahmoud H, Azadeh F, Fatemeh A, Akram S (2017) Beneficial effects of garlic on learning and memory deficits and brain tissue damages induced by lead exposure during juvenile rat growth is comparable to the effect of ascorbic acid. Drug ChemToxicol 40: 206-214.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences