ISSN : 0976-8505
Der Chemica Sinica
Metal Complexes of Nicotine: A Group of Negligible Compounds
Chemistry Department, Faculty of Science, King Khalid University, Abha 9004, Kingdom of Saudi Arabia Applied Research Sector, Egyptian Organization for Biological Products and Vaccines (VACSERA Holding Company), 51 Wezaret El-Zeraa St., Agouza, Giza, Egypt
- Corresponding Author:
- Ahmed E Fazary
Chemistry Department, Faculty of Science
King Khalid University, Abha 9004, Kingdom of Saudi Arabia
Applied Research Sector, Egyptian Organization for Biological Products and Vaccines (VACSERA Holding Company)
51 Wezaret El-Zeraa St., Agouza, Giza, Egypt
Abstract
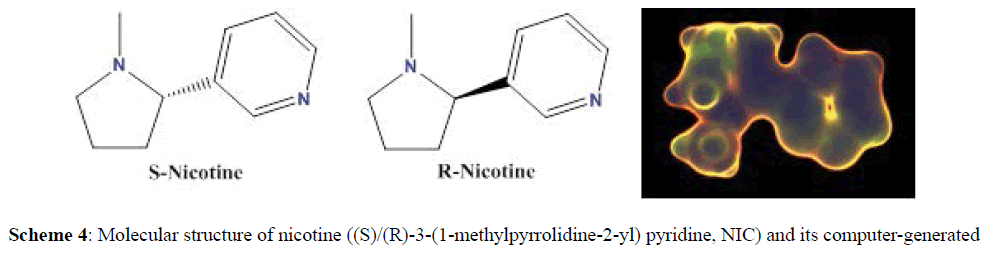
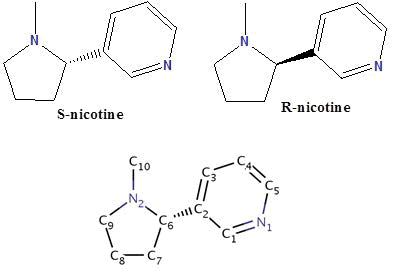
Nicotine (NIC) is an oxygenic alkaloid compound exists in two forms R and S, and consists of a pyridine ring, substituted at the 3-position with an N-methyl-pyrrolidine ring. Nicotine is extracted mostly from the Nicotiana tabacum plant, and although it was made in the roots and accumulates in smaller amounts in leaves of edible plants of the nightshade family called “Solanaceae’’ such as tomatoes, potatoes, green peppers, and coca plants. Biochemically, it’s parasympathomimetic, stimulant drug, and is a nicotinic acetylcholine receptor agonist. It functions as an antiherbivore, insecticide, imidacloprid, and recently chromatin-modifying enzymes inhibitor. Nicotine was considered to be one of the most biologically important compounds, for which information about their metal ions complexation properties are very rare in the literature. However, recently it gains much attention from many bioinorganic chemists over the world. In the present mini review article, I described the research done during the last few decades about the solid chemistry of nicotine and studies concerning a number of crystal structures of nicotine complexes. Also, I reported about the rare research work done previously about the protonation and complexation equilibria studies of nicotine in which the data of the different equilibrium constants and stability constants of nicotine would be valued in metal based drug research.
Graphical Abstract: Molecular Structure of Nicotine ((S)/(R)-3-(1-Methylpyrrolidine-2-yl) pyridine, NIC).
Keywords
Nicotine; Metal complexes; Solution equilibrium; Crystal structures
Introduction
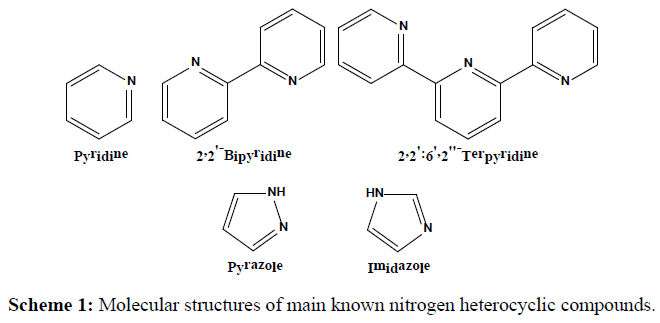
In coordination and bioinorganic chemistry, one of the most important modules of bio ligands is the nitrogen heterocyclic compounds [1]. The most widely studied of these compounds are sex membered heterocycles such as nitrogen pyridine, 2,2’-bipyridine and 2,2’:6’,2”-terpyridine [1,2] (Scheme 1). Other related ligands that have been extensively employed in inorganic chemistry are the five membered nitrogen heterocycles such as pyrazole and imidazole compounds [1,2] (Scheme 1).
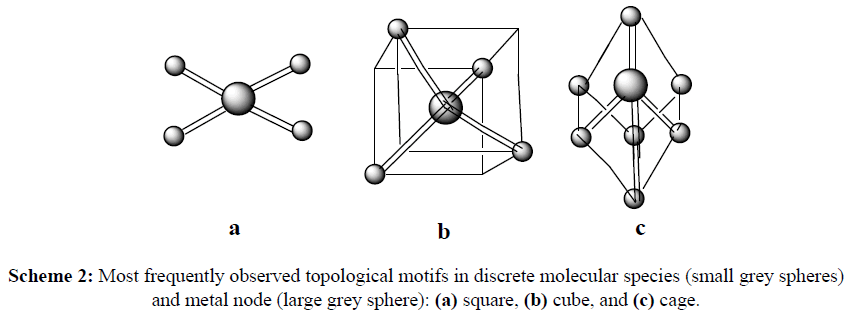
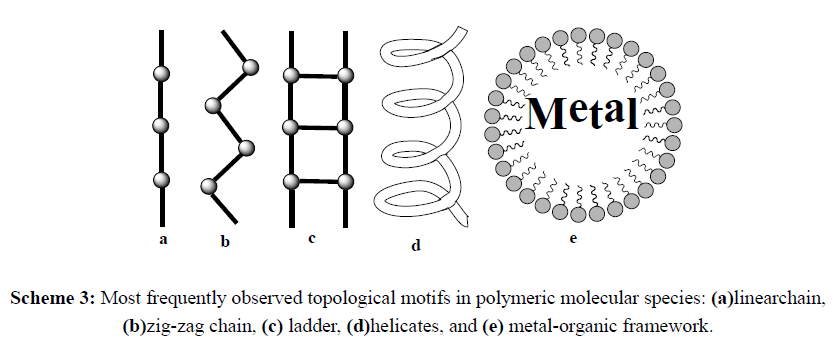
Due to the interactions between the μ* antibonding orbitals of the nitrogen ligand and the d-orbitals of a metal ion, strong coordinate bonds are mostly formed, resulting in a stable metal complexes [3]. The incorporation of two or more of these donor ligands into a single molecule can result in species which can bridge between two metal centres, chelate to a single metal centre, or a combination of both these motifs. Chelation to a metal centre increases the stability of the complex, and so is desirable for inclusion into the design of bio ligands. The bridging of metal centres by a ligand can result in connection between the metal ion centres, as well as resulting in higher order molecular architectures including discrete molecular species, such as molecular squares, cubes and cages (Scheme 2), and polymeric molecular species (Scheme 3) such as chains, ladders, helicates and metal-organic frameworks [4].
Our concern in this article is nicotine which consists of a pyridine ring, substituted at the 3-position with an N-methylpyrrolidine ring (Scheme 4) [5]. Nicotine is extracted from the Nicotiana tabacum plant (Figure 1), although it is found in smaller amounts amongst other plants of the Solanaceae family [5].
Bioinorganic Chemistry of Nicotine Metal Complexes
Previous work on the bioinorganic chemistry of nicotine has been limited to a few studies, and only a few number of crystal structures of nicotine complexes have been reported. Original work on the solid chemistry of nicotine metal complexes was carried out to investigate the synthesis of various nicotine metal salts called “double salts” [6]. Most of these complexes have been synthesized and their three-dimensional structures were elucidated using single crystal X-ray crystallography technique.
By looking to the literature, we found only a few numbers of studies about the organic and inorganic chemistry of nicotine and its derivatives. Most of the organic chemistry studies reports about the synthesis of new substituted nicotine derivatives and analogues due to their pharmacological applications as potential chiral coordination ligands [7-19]. Three crystal structures of metal complexes of nicotine had been previously [20-23]. In these reports, the first crystal structure of a mercuric complex of nicotine was determined [23] describing a structure in which nicotine acts as a bridging ligand between tetrahedral Hg(II) centres, forming a one-dimensional coordination polymer. Then, a detailed crystal structures of a complexes in which four nicotine molecules are coordinated to copper [24], silver(I) [25] and zirconium [26] were investigated. In these structures nicotine acts a monodentate ligand, coordinated via the pyridine nitrogen. Followed by a report about three nicotine complexes, each one of them consist of a lanthanide metal bonded to three indenyl ligands and a single, pyridine linked, nicotine molecule [27]. These previous X-ray crystal structures indicates that only two of the possible three bonding modes of nicotine have been observed; coordination of the pyridine nitrogen, and coordination of both the pyridine and pyrrolidinenitrogens, where the nicotine molecule acts as a bridging ligand.
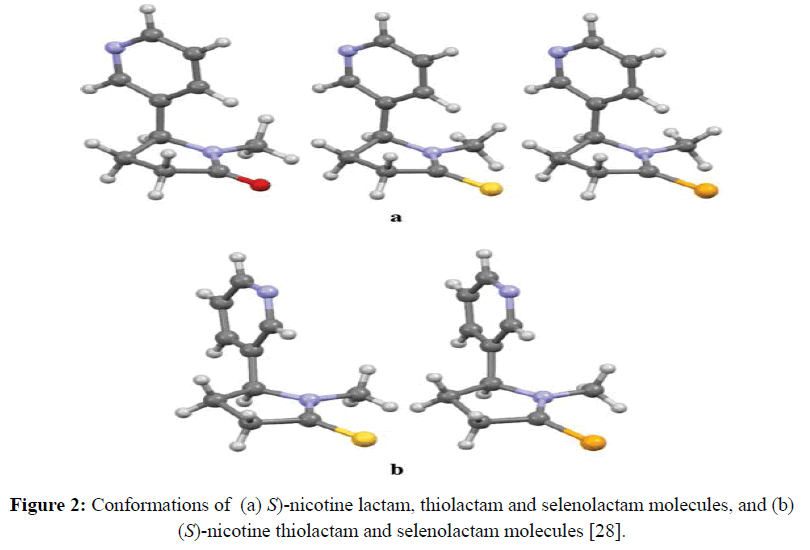
Reactions of zinc(II) chloride with (S)-(-)-nicotine yielded new metal complex, which was characterized by elemental analysis, infrared, single crystal X-ray diffraction 13C and 1H NMR spectral methods [28]. Quantum chemical calculations indicate that in the isolated state the anti-conformation of the uncoordinated chalcogen nicotine lactams is slightly more preferred energetically and it does not undergo substantial changes upon coordination to the metal center (Figure 2). The preference for the syn conformation in crystals of thio- and selenolactams of nicotine might be ascribed to their involvement in columnar stacking interactions, which do not operate in the isolated state [28]. This study has provided useful information regarding functionalization of (S)-(-)-nicotine molecule as a mixed-donor-atom ligand to enforce formation of 1D coordination polymers [28].
Figure 2: Conformations of (a) S)-nicotine lactam, thiolactam and selenolactam molecules, and (b) (S)-nicotine thiolactam and selenolactam molecules [28].
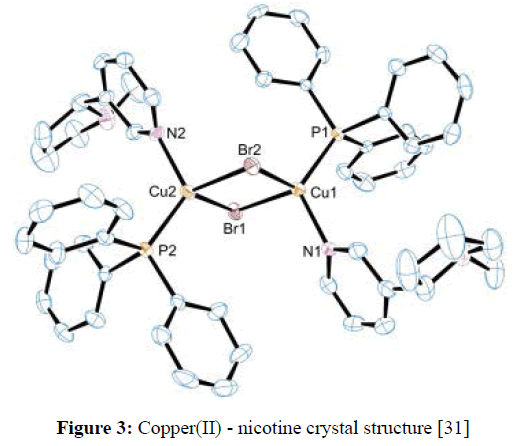
A metal complex derivative from a coumarin bearing a porphyrin unit was spectroscopically characterized and its sensing ability towards the alkaloids nicotine was evaluated [29]. Both solid state and solution chemistry of copper(II)-nicotine complexes have been reported [30,31]. The quantum-mechanical calculations helped elucidate the experimental data as they provided some information on the energetic of the possible interaction modes of Cu(II) with nicotine (Figure 3). The studies showed that nicotine acts as a monodentate ligand utilizing for this purpose the pyridine nitrogen atom [30,31]. A number of chiral methacrylate copper (II), zinc (II), metal complexes of (S)-(-)- nicotine have been studied and their X-ray crystal structures established to investigate the possibility of chirality being transferred from the respective metal complexes to the backbone of the derived copolymers, the chiral metal complex template having previously been cleaved and removed [32].
Figure 3: Copper(II) - nicotine crystal structure [31]
The monoclinic crystal structure of tetraaquabis(ethylisonicotinate)cobalt(II) disaccharinate complex has been determined by X-ray diffraction analysis at room temperature in three mutually perpendicular planes [33]. This complex presents a slightly distorted octahedral environment, with equatorially coordinated water molecules and axially pyridine N-bound ethylisonicotinate ligands [33]. The magnetic environments of the complex have been identified by electron paramagnetic resonance technique. The cyclic voltammogram of the studied complex investigated in dimethylformamide solution exhibits only metal centerdelectroactivity in the potential range -1.0-1.25 V versus Ag/AgCl reference electrode [33]. Mono and bis-zinc(II)-centered salphen derivatives are studied as efficient adsorption materials for nicotine derivatives using X-ray analysis and UV-vis, NMR spectroscopic methods, as well the high binding constants of the supramolecular complexes based on nicotine were additionally determined. X-ray analyses [34]. The influence of Pt(IV) metal complexes involving substituted nicotinamides and isonicotinamides on the activity of cyclic adenosine monophosphate (cAMP) phosphodiesterase was studied, whereas substituted nicotine amides are inhibitors of enzymatic cAMP phosphodiesterase activity [35].
Three copper(II) coordination polymers involving nicotinato anion as a bridging ligand and 2,2′-bipyridine or dicyanamide as coligands [36]. The copper metal-nicotine ligand association in these complexes is very similar and generates tubelike coordination polymers, which are constructed from pairs of copper atoms connected through two carboxylato groups from two nicotinate ligands [36]. Each pyridyl moiety from the nicotine-ligand coordinates at the apical position of the copper ions from another pair, resulting in chains running along the crystallographic c axis [36]. The cryomagnetic investigation of the three copper(II) coordination polymers reveals weak antiferromagnetic interactions mediated by the nicotinato ligand [36]. Anumber of tridentate monoanion or tridentate dianionacetoacetanilide isonicotinylhydrazone and its metal chelates were synthesized and characterized mainly by elemental analysis; conductivity measurements; and electronic, infrared, and nuclear magnetic resonance spectral studies [36].
These compounds were found to exhibited anticancer activity and revealed that they are active against pathogenic fungal strains, and the chelates were found to be more active than the ligand [37]. A self-assembled structural analysis of binuclear square pyramidal new 3-D neutral copper(II)-multidentatenicotinato organic framework coordination polymer was reported [38]. A family of three ferrimagnetic manganese(II) complexes involving N,N′-dimethylnicotinium ligand were synthesized and characterized [39]. The use of high temperature expansion of the partition function and molecular mean field approach showing that these compounds adopt a two-dimensional structure [39].
Novel metal La(II), Pr(III), and Nd(IV) complexes of nicotine were synthesized and their chemical structures were exhaustively characterized using X-ray diffraction, circular dichroism, and nuclear magnetic resonance spectroscopic methods [40]. The chiral carbon atom of nicotine lies approximately twice as far from the metal centre than the chiral sulfur atom of the methyl tolylsulfoxide ligand in the previously reported lanthanums-methyl tolylsulfoxide complexes [40]. A strong intramolecular antiferromagnetic dodecylnicotinatebis-adducts of binuclear copper carboxylates were synthesized and their crystal structure, thermal behavior and magnetic properties were studied [41]. The dimer is centrosymmetric with the copper(II) ions in a square-pyramidal coordination with four O-alkyl O atoms in the basal plane and the nicotine N atom at apical positions, and the copper(II) ions are bridged by four O-alkyl carboxylate groups [42]. The stoichiometric complexation of palladium(II) or mercury(II) ions with 6-Pyridylnicotine and bis-6,6'- nicotine were also studied [42].
Conformational analysis and steric effect of nicotine-transition metal complexes models were used to rationalize the structure and some stereospecific reactions that these complexes undergo [43]. Two different steric models are presented which correlate the rate of N-alkylation of alkyl-substituted pyridines and quinolines as well as acyclic amines and cyclic nonaromatic amines using molecular mechanics/molecular orbital derived steric energies [44]. The stereoselectivities of methylation of conformationally mobile nicotine analogs are examined with regard to steric and electronic effects. The Curtin-Hammett principle and the Winstein-Holness equation allow quantitative analysis of the kinetics [43]. Zinc(II) complexation with pure nicotine was used in a simple, economical and quick method for extraction of pure nicotine from Nicotiana tabacum and subsequent removal of the metal as ZnS [44].
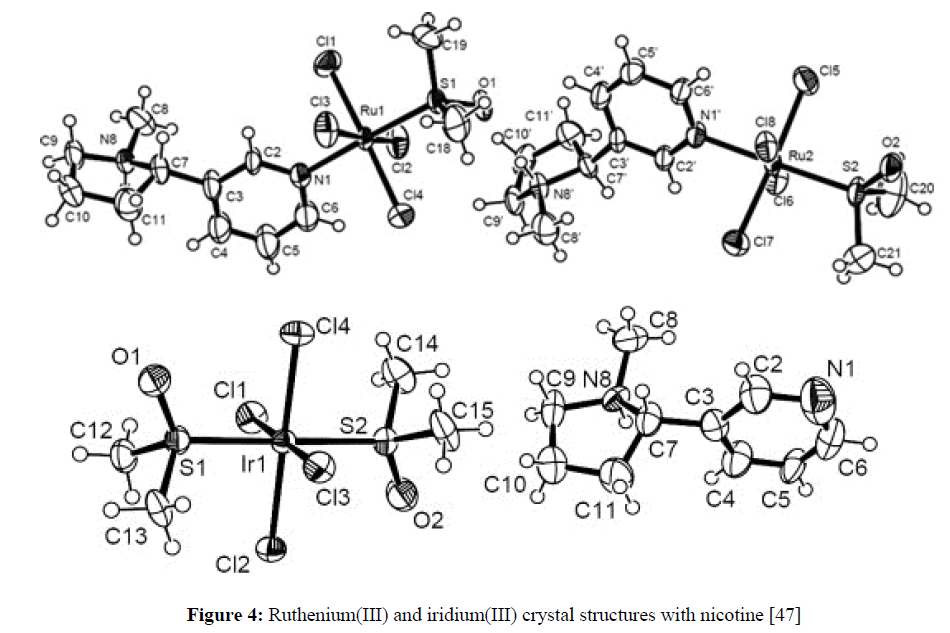
The effect of various Lewis acids such as SbCl5, SnCl4, SbCl3, AlI3, FeCl3, AlBr3, AlCl3, TiCl4, ZnCl2, HgCl2, CdCl2 on the optical rotatory power of nicotine has been meausred in dimethylformamide or in toluene [45]. The increment of optical rotation of nicotine by the action of most Lewis acids and the difference between the specific rotation of free nicotine and that of nicotine co-ordinated to Lewis acid decreases [45]. A number of complex metal-nicotine species were studied and estimated. The compounds have been characterized, and their plausible structures have been suggested for all these complexes based on the elemental analyses, magnetic measurements, X-ray crystal, electron spin resonance and infrared spectral studies (Figure 4) [6,7,46,47].
Figure 4: Ruthenium(III) and iridium(III) crystal structures with nicotine [47]
Solution Chemistry of Nicotine Metal Complexes
Recently, in July 2016, it has been reported about the chemical investigation of the interaction mechanisms of nicotine with some divalent metal ions such as Pb2+, Fe2+, and Cu2+. The complex formation equilibria between nicotine and these metal ions have been investigated at room temperature, in solutions using UV/Vis spectrophotometric and potentiometric techniques. The protolysis constants of nicotine have been determined and evaluated under the same experimental conditions. Using the specific ion interaction theory, the conditional acid constants of nicotine have been deduced in zero ionic strength solutions. The experimental results of the graphical and numerical methods adopted indicated the formation of predominating nicotine mononuclear complexes.
Lastly, the protonation and metal complex formation equilibrium constants of nicotine with trivalent and divalent metal ions have been investigated in water solutions using pH-potentiometric and cyclic voltammetry techniques. Also, the dissociation constants of NIC and the equilibrium constants of its binary complexes with the studied metal ions in water solutions were observed at different temperatures. From the experimental stability constants of different metal-nicotine complex species, the concentration distribution diagrams of the various metal ions - nicotine complex species in solutions were also estimated using HySS 2009 software.
Conclusion and Recommendations
It was shown that divalent and trivalent metal ions complexes of nicotine were stable in both solution and solid forms. The observed order of stability of the nicotine complex systems is trivalent metal complex ions>divalent metal complex ions. The formation of the binary NIC complex was found to be spontaneous and exothermic. The stability constants and synthesis of the complexes between nicotine and essential metal ions were studied to investigate the complexation behavior of these systems as it could mimic many biological interactions of the. The complexes appear to be superior in biological properties to ligand alone. The complexes were found to increase the cytotoxic, antioxidant, and antimicrobial activity of the pure ligand which is probably related to the enhancement of hydrophobicity of the synthesized compounds. The stability constant determination was focused on mono-nuclear (single metal) complexes. Further investigation involving poly-nuclear complexes is suggested in order to understand the distribution of complexes in physiological pH condition. In this review, the nicotine ligand is monomer. The used of polymer ligand will be interesting and their macromolecular complexes might be able to improve more biological properties.
Acknowledgement
This work was supported by King Abdulaziz City for Science and Technology (KACST), Kingdom of Saudi Arabia, under Grant MS-35-73.
References
- Natori Y, Imahori T, Yoshimura Y (2016) Development of stereoselective synthesis of biologically active nitrogen-heterocyclic compounds: Applications for syntheses of natural product and organocatalyst. J Synth Org Chem74: 335-349.
- Khan MF, Alam MM, Verma G, Akhtar W, Akhter M, et al. (2016) The therapeutic voyage of pyrazole and its analogs: A review.Eur J Med Chem 120: 170-201.
- Power PP (2012) Stable two-coordinate, open-shell (d 1-d 9) transition metal complexes. Chem Rev 112: 3482-3507.
- Smith CR (1953) Complex metal-nicotine compounds.J Am Chem Soc 75: 2010-2012.
- Schmidt B, Neitemeier V (1998) 6-Pyridylnicotine-A New Chiral 2,2’-Bipyridine. Synthesis 1998: 42.
- Wagner FF, Comins DL (2007) Recent advances in the synthesis of nicotine and its derivatives. Tetrahedron63: 8065-8082.
- Seeman JI, Chavdarian CG, Secor HV (1985) Synthesis of the enantiomers of nornicotine. J Org Chem50: 5419-5421.
- Seeman JI, Chavdarian CG, Kornfeld RA, Naworal JD (1985) Nicotine chemistry. The addition of organolithium reagents to (-)-nicotine. Tetrahedron 41: 595-602.
- Seeman JI, Secor HV, Chavdarian CG, Sanders EB, Bassfield RL, et al. (1981) Steric and conformational effects in nicotine chemistry. J Org Chem46: 3040-3048.
- Secor HV, Chavdarian CG, Seeman JI (1981) The radical and organometallic methylation of nicotine and nicotine n-oxide. Tetrahedron Lett22: 3151-3154.
- Acheson RM, Ferris MJ, Critchley SR,Watkin DJ (1980) Identification of the product from nicotine and sulphur as bis-1-methyl-2-(3-pyridyl) pyrrol-3-yl disulphide. J Chem Soc 2: 326-329.
- Acheson RM, Ferris MJ, Sinclair NM (1980) Transformations involving the pyrrolidine ring of nicotine. J Chem Soc1: 579-585.
- Cosford NDP, Bleicher L, Herbaut A, McCallum JS, Vernier JM, et al. (1996) (S)-(-)-5-ethynyl-3-(1-methyl-2-pyrrolidinyl)pyridine maleate (SIB- 1508Y): A novel anti-Parkinsonian agent with selectivity for neuronal nicotinic acetylcholine receptors. J Med Chem39: 3235-3237.
- Seeman JI, Clawson LE, Secor HV (1985) Nicotine chemistry. The addition of alkyl radicals to (S)-(-)-nicotine: Synthesis of optically active 6-alkylnicotines. Synthesis10: 953-955.
- Sanders EB, DeBardeleben JF, Osdene TS (1975) Nicotine chemistry. 5′-Cyanonicotine. J Org Chem 40: 2848-2849.
- FévrierFC, SmithED, Comins DL (2005) Regioselective C-2 and C-6 substitution of (S)-nicotine and nicotine derivatives. Org Lett7: 5457-5460.
- Hoffmann HMR, Frackenpohl J (2004) Recent advances in Cinchona alkaloid chemistry. Eur J Org Chem21: 4293-4312.
- Damaj MI, Glassco W, Dukat M, Martin BR (1999) Pharmacological characterization of nicotine-induced seizures in mice.J Pharm Exp Ther 291: 1284-1291.
- Flammia D, Dukat M, Damaj MI, Martin B, Glennon RA (1999) Lobeline: Structure-affinity investigation of nicotinic acetylcholinergic receptor binding. J Med Chem42: 3726-3731.
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, et al. (1998) Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharm Exp Ther284: 1058-1065.
- Imad Damaj M, Glassco W, Dukat M, May EL, Glennon RA, et al. (1996) Pharmacology of novel nicotinic analogs. Drug Dev Res38: 177-187.
- Roduit JP, Wellig A, Kiener A (1997) Renewable functionalized pyridines derived from microbial metabolites of the alkaloid (5)-nicotine. Heterocycles45: 1687-1702.
- Comins DL, King LS, Smith ED, Février FC (2005) Synthesis of C-4 substituted nicotine derivatives via an N-acylpyridinium salt of (S)-nicotine. Org Lett 7: 5059-5062.
- Taylor EC, Boyer NE (1959)Pyridine-1-oxides IV Nicotine-1-oxide, nicotine-1′-oxide, and nicotine-1,1′-dioxide. J Org Chem24: 275-277.
- Youssif S (2001) Recent trends in the chemistry of pyridine N-oxide. Arkivoc1: 242-268.
- Malczewska-Jaskóła K, Jankowski W, Warzajtis B, Jasiewicz B, Hoffmann M, et al. (2015) Chalcogenated (S)-(-)-nicotine derivatives as chiral linkers for 1D coordination polymers. Polyhedron 100: 404-411.
- Santos CIM, Oliveira E, Santos HM, Menezes JCJMD, Faustino MAF, et al. (2015) Untangling interactions of a zinc(ii) complex containing a coumarin-porphyrin unit with alkaloids in water solutions: A photophysical study. Photochem Photobiol Sci 14: 757-764.
- Jasiewicz B, Hoffmann M, Ga̧sowska A, Jastrza̧b R, Malczewska-Jaskóła K (2014) Spectroscopic, potentiometric and quantum-mechanical studies of S-(-)-nicotine complexes with Cu(II) ion. Acta Chim Slov 61: 137-144.
- Hirtenlehner C, Monkowius U (2012) Syntheses, crystal structures and blue luminescence of Cu2X 2(Ph3P)2[(-)-nicotine]2 (X = Br, I).Inorg Chem Commun 15: 109-112.
- Jana S, Cormack PAG, Kennedy AR, Sherrington DC (2009) Synthesis of main chain chiral methacrylate copolymers via chirality transfer from polymerizable chiral metal complexes.J Mater Chem19: 3427-3442.
- Uçar I, Karabulut B, Bulut A, Büyükgüngör O (2008) Crystal structure and EPR studies of mixed ligand complex of cobalt(II) with saccharin and ethylisonicotine.Spectrochim Acta Mol Biomol Spectrosc71: 1239-1245.
- Escudero-Adán EC, Benet-Buchholz J, Kleij AW (2008) Supramolecular adsorption of alkaloids by metallosalphen complexes. Inorg Chem 47: 4256-4263.
- Tatyanenko LV, Bogdanov GN, Dobrokhotova OV, Fadeev MA, Fedorov BS (2006) Modulation of reactivity of cyclic adenosine monophosphate phosphodiesterase under the effect of pyridinecarboxylic acid derivatives and Pt(IV) metal complexes on their basis. Biomed Khim 52: 174-179.
- Madalan AM, Paraschiv C, Sutter JP, Schmidtmann M, Müller A, et al. (2005) Construction of tube and ladderlike copper(II) coordination polymers based on the nicotinato tecton. Cryst Growth Des 5: 707-711.
- Deepa KP, Aravindakshan KK (2004) Synthesis, characterization, and antifungal studies of transition metal complexes of ω-bromoacetoacetanilide isonicotinylhydrazone. Appl Biochem Biotechnol118: 283-292.
- Lu JY, Babb AM (2001) A new 3-D neutral framework coordination polymer constructed via square pyramidal binuclear Cu(II) and nicotinato ligand. InorgChemCommun 4: 716-718.
- Colin JC, Journaux Y (2001)A family of bimetallic CuIIMnII molecular based magnets containing chiral cations: Synthesis and magnetic properties. C R AcadSci4: 207-213.
- Guan J, Fischer RD (2001) Tris(indenyl)lanthanoid complexes (Ln = La, Pr, Nd) containing either (S)-(-)-nicotine or two simpler pyridine bases. Eur J InorgChem10: 2497-2508.
- Rusjan M, Chaia Z, Piro OE, Guillon D, Cukiernik FD (2000) Synthesis, structure and magnetic properties of tetrakis-μ-carboxylato-bis(dodecylnicotinato)dicopper(II) complexes; crystal and molecular structure of the decyl carboxylate derivative.ActaCryst B56: 666-672.
- Schmidt B, Neitemeier V (1998) 6-Pyridylnicotine and bis-6,6'-nicotine - new chiral 2,2'-bipyridines.Synthesis1: 42-44.
- Seeman JI (1987) Recent studies on conformational analysis and steric effects. Pure ApplChem59: 1661-1672.
- Christy M, Muhammad Y, Nasir A (1986) Isolation of nicotine from leaves of from NicotianaTabacum by complexation method. Research and Industry 31: 236-237.
- Tomita A, Ochiai E, Hirai H, Makishima S (1967) The effect of lewis acids on the optical rotation of nicotine. J InorgNuclChem29: 105-112.
- Maurya RC, Shukla R, Gupta DC, Shukla RK, Anandam N, et al. (1986) Synthesis and characterization of some new cyanonitrosyl complexes of chromium(I) with nicotine and related ligands. Synth React Inorg M16: 1243-1252.
- Albertí FM, Fiol JJ, García-Raso A, Torres M, Terrón A, et al. (2010) Ruthenium(III) and iridium(III) complexes with nicotine. Polyhedron29: 34-41.
- Manfredi C, Vero S, Vasca E, Perrotta D, Trifuoggi M, et al. (2016) On the interaction between Nicotine and Metal(II) ions in aqueous solutions. J Solution Chem45: 971-989.
- FazaryAE, Ju YH, Fawy KF, Al-Shihri AS, Bani-Fwaz MZ, et al. (2017) Nicotine-Metal ion interactions in solutions: Potentiometric, cyclic voltammetry investigations and quantum chemical calculations. J ChemThermodyn112: 283-292.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences