ISSN : 0976-8505
Der Chemica Sinica
Matrix Solid Phase Dispersion Extraction and Screening of Phytochemicals from Dioscorea steriscus Tubers of Mashonaland Central Province, Zimbabwe
Masimba Tapera1* and Sharon Machacha2
1Department of Physical Sciences, Harare Polytechnic, Harare, Zimbabwe
2Department of Research and Specialist Services, Gvt laboratories, Harare, Zimbabwe
Abstract
Dioscorea steriscus is a common tuberous, perennial plant widely distributed in Zimbabwe and Southern Africa. The plant tubers are consumed by several Zimbabweans as either relish or vegetable and are also used to prepare folk remedies for several ailments. Phytochemicals were extracted from Dioscorea steriscus tubers using matrix solid phase dispersion (MSPD) extraction technique with three different solvents namely methanol, ethanol and ethyl acetate. Appropriate laboratory screening tests to determine the presence eight different phytochemicals were performed. In the methanol extract, all phytochemicals tested for were present; in the ethanol extract all were present except for glycosides and saponins. Saponins, tannins, steroids and glycosides were absent in the ethyl acetate extract. Total phenolic content (TPC) was found to be 78.23, 73.02 and 69.34 mgGAE/g fresh weight in the three extracts. Due to the presence of substantial amounts of phenolic compounds, Dioscorea steriscus was found to have potential applications in food, cosmetic, plastic and pharmaceutical industries.
Keywords
Natural products; Dioscorea steriscus; Phytochemicals; Phenolic compounds; Total phenolic content
Introduction
Phytochemicals are non-nutritive plant chemicals that have protective or disease preventive properties. It is wellknown that plants produce these chemicals to protect themselves, but recent researches demonstrate that they can also protect humans against diseases [1]. There are many phytochemicals and each works differently. Phenolic compounds are an important group of phytochemicals [2]. These compounds are broadly distributed in the plant kingdom and are the most abundant secondary metabolites in plants. There are many structures of phenolic compounds currently known, ranging from simple molecules such as phenolic acids to highly polymerized substances such as tannins [3]. Phenolics possess various properties such as antioxidant, ant apoptosis, anti-aging, anti-carcinogen, anti-inflammation, anti-atherosclerosis, cardiovascular protection, improvement of the endothelial function, as well as inhibition of angiogenesis and cell proliferation activity [1]. Phenolic compounds are useful as dietary antioxidants to serve as a protective factor against oxidative cellular damage. Phenolic antioxidants are substances that act as terminators for free radicals [4]. Oxidation is an essential metabolic process that results in energy production. Oxidants are compounds produced by normal metabolism in the body, and if not controlled, can cause extensive damage [5]. Oxidative damage in body cells is associated with the generation of reactive oxygen species (ROS). ROS have the capability to induce changes of genetic material directly by oxidizing specific DNA-bases or indirectly by oxidizing proteins or lipids important in maintaining the function of individual cells [6]. The produced functional damage can trigger events such as mutagenesis, carcinogenesis and aging [7]. Under normal physiological conditions, the production of ROS in cells is counterbalanced by antioxidants [8]. An adequate dietary supplement of antioxidants is therefore essential for maintaining good health. There is an increase in cases of diseases like cancer, neurodegenerative disorders and inflammatory disorders, heart disease and Alzheimer’s disease [5,9]. It is increasingly being realized that many of the modern human diseases are due to oxidative stress initiated by overproduction of ROS [10]. Some studies have shown that phytochemicals from plant materials significantly contribute to the reduction of chronic and degenerative diseases as cited by Oliveira et al. [5]. Various indigenous vegetables and herbs in Zimbabwe containing phytochemical compounds are used as food and folk-medicines [11]. The beneficial effects derived from phytochemical compounds especially phenolic compounds have been attributed to their antioxidant activity. Phenolic compounds from plant origin are more beneficial in reducing ROS levels due to synergistic actions of wide range of biomolecules such as phenols, flavonoids, vitamin C, vitamin E and phytomicronutrients [5]. There is need to find new sources of natural antioxidants which are safer than their synthetic counterparts [12]. This study therefore focused on a plant that is used as food and for treatment of several ailments, to establish its phytochemical composition. The study helps in identifying phytochemicals present in the plant and possibly providing a substitute to dietary intake of synthetic antioxidants which are genotoxic and carcinogenic at high concentrations. The study is also useful in establishing potential commercial uses of the plant as a source of active ingredients in the cosmetic and pharmaceutical industries. Several researches have been carried out in which the phenolic composition of different Dioscorea species have been investigated. Dioscorea steriscus belongs to the family Dioscoraceae and is found among the common indigenous vegetables in Zimbabwe. It is widely distributed in the Zimbabwean forests and its tubers are consumed in most Zimbabwean provinces. It has been routinely used as food and medicine. Among the variety of phenolic compounds, phenolic acids have attracted considerable interest in the past few years due to their many potential health benefits [13]. According to Chipurura et al. [14], wild vegetables are good sources of phenolic compounds, which can be useful for the prevention of cardiovascular and other chronic diseases. He also suggested that studies on its mechanism of action may yield a potential compound against oxidative stress. According to Alagammal et al. [15], phenolic compounds are the significant contributors to the antioxidant activity in plant extracts. Sheikh et al. [16] screened phytochemicals from D. pentaphylla, D. alata, D. oppositifolia, D. bulbifera, D. glabra and D. pubera of Meghalaya, India to validate the ethnobotanical importance of the tubers. Roy et al. [17] also screened phytochemicals from D. villosa and revealed the presence of phenol, tannins, flavonoids, saponins, alkaloids and gylosides. The data obtained by Narkhede et al. [10] clearly demonstrated that D. alata tubers are a potential source of natural antioxidants and may be a good candidate for pharmaceutical plant based products. Considerable quantities of hydroxybenzoic acid, hydroxycinnamic acid and their derivatives were found in extracts of D. hispida by Theerasin et al. [6]. Dioscoraceae tubers are used as medicines in oriental countries to prevent diarrhoea and diabetes [18]. Of all these publications on Dioscorea species, no single work has focussed on phytochemical composition of D. steriscus. Phytochemical information on one Dioscorea species is not enough to represent all other species. Plant phytochemical composition differs depending on the environment in which the plant grows the plant family and the plant species. During normal plant development, growth is not constant and chemical composition can be expected to vary [19]. According to Warring et al. [19], the chemistry of plant species can differ significantly even when the plants are grown at constant relative growth rates, depending upon availability of nutrients and light. The chemical composition of wild plant species differs with rates at which the plants grow [20]. Dzomba et al. [21] only revealed that Dioscorea steriscus tuber extracts possess anti-obesity and anticancer activity. The present study reveals information on phytochemical composition of Dioscorea steriscus tubers.
Materials and Methods
Chemicals
All chemicals and reagents used in the experiments were analytical reagent grade procured from Skylabs, South Africa and Sigma Aldrich, Germany.
Plant Material
Dioscorea steriscus (Manyanya) tubers were purchased from the local vegetable vendors at the Bindura Green Market in Mashona land central province. The plant branch and a tuber were taken to Harare national Herbarium for authentication. The tubers were washed using clean water to remove dirt, weighed, and stored in a refrigerator at a temperature of -5ºC.
Extraction of Phytochemicals
Extraction of phytochemicals was performed using matrix solid phase dispersion extraction technique according to Karasova et al. [22] with some modifications. Fresh Dioscorea steriscus tubers were sliced into tiny pieces using a scalpel blade. 2 g of the sliced tubers was mixed with 4 g of clean sand in a porcelain mortar and then ground using a pestle until homogeneity was achieved. Nine other separate sets of mortar and pestles were used to mix and homogenise the sliced tubers with clean sand using the same mass proportions. A spatula was used to transfer the ground mixture from the mortar into a 10 mL syringe (up to half-full) in which a filter frit was placed at the bottom as an MSPD sorbent support. Another filter frit was placed at the top of the mixture and the mixture was gently pressed using a plunger to compact it and eliminate voids. The plunger was pulled out and 10.0 ml of elution solvent was slowly added to the mixture avoiding overflowing. Three elution solvents namely, methanol, ethanol, ethyl acetate were used on fresh mixtures separately. Each time a pipette bulb was used to gently apply pressure onto the top frit and the syringe was left to drip into a small receiving test tube. The sorbent-sample mixture was washed with 5.0 ml of the solvent each time.
Phytochemical screening
Chemical tests were performed on methanol, ethanol and ethyl acetate extracts of Dioscorea steriscus tubers. Standard procedures to identify the constituents were used as described by Uddin et al. [23] and Firdouse et al. [24].
Test for flavonoids
About 2 ml of the extracts were mixed with concentrated sulphuric acid (1 ml) and 0.5 g of Magnesium separately. A pink or red coloration that appears on standing (3 min) indicates the presence of flavonoids.
Test tannins
About 1 ml of each of the extracts was added in 2 ml of water in a test tube. 2 to 3 drops of diluted ferric chloride solution was added and observed for green to blue-green coloration.
Test for saponins
The extracts (2 ml) were shaken with an equal volume of water. A copious lather formation indicates the presents of saponins.
Test for alkaloids
The extracts (2 ml) were mixed with a little amount of dilute sulphuric acid and Mayer’s reagent. Formation of a white precipitate indicates the presence of alkaloids.
Test for phenol
To 2 ml of the test extracts, a few drops of ferric chloride solution were added. Bluish green or red colour indicates the presence of phenol.
Test for terpernoids and steroids
The extracts, about 2 ml were separately shaken with chloroform (2 ml) followed by the addition of concentrated sulphuric acid, a reddish brown coloration of the interface indicates the presence of terpenoids.
Test for steroids
Each of the extracts (2 ml) was dissolved in 2 ml of acetic anhydride and 2 ml of concentrated sulphuric acid was added. A colour change from violet to blue or green indicates the presence of steroids.
Tests for glycosides
To the extracts (1 ml), 5% hydrochloric acid (1 ml) was added. The mixture was boiled in a water bath and then filtered. The filtrate was shaken with an equal volume of chloroform and kept to stand for 5 min. The lower chloroform was shaken with half of its volume with dilute ammonia. The formation of rose pink to red colour of the ammoniacal layer gives indication of glycosides.
Total phenolic content (TPC) determination
TPC was determined by spectrophotometry, using Gallic acid as standard, according to the method cited by Oliveira et al. [5] and described by Roesler et al. [4]. Briefly, 1.0 m; of the sample extract was transferred to separate tubes containing 5.0 ml of a 1/10 dilution of Folin-Ciocalteu’s reagent in water. Then, 4.0 ml of sodium carbonate solution (7.5% w/v) was added. The tubes were then allowed to stand at room temperature for 60 min before absorbance at 765 nm was measured against water. The TPC was expressed as Gallic acid equivalents (GAE) in mg/g fresh material. The concentration of phenolics in samples was derived from a standard curve of Gallic acid.
Results
Total phenolic content
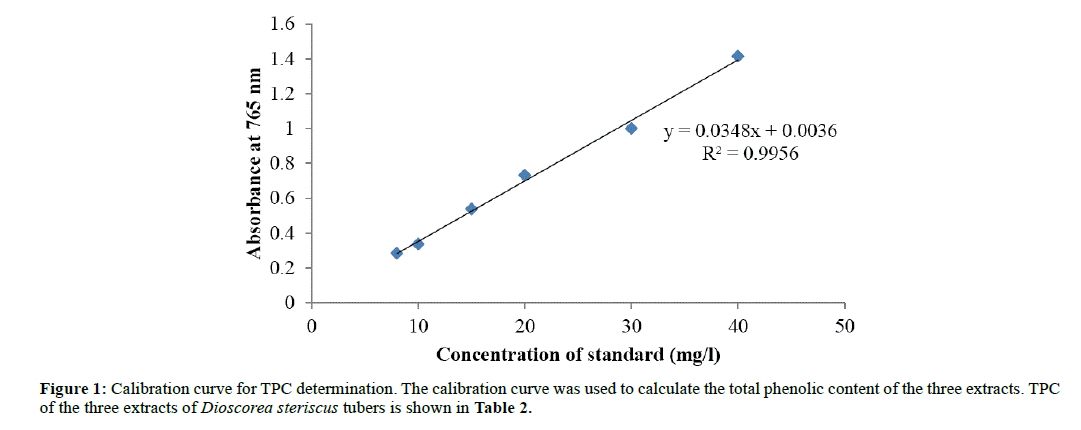
Gallic acid was used as a standard. A calibration curve was obtained with concentrations of the standards in milligrams per liter (X-axis) against their average absorbencies at 765 nm wavelength (Y-axis) (Table 1).
| Phytochemical | Methanol extract | Ethanol extract | Ethyl acetate extract |
|---|---|---|---|
| Flavonoids | + | + | + |
| Terpenoids | + | + | + |
| Saponins | + | - | - |
| Alkaloids | + | + | + |
| Polyphenols | + | + | + |
| Tannins | + | + | - |
| Steroids | + | + | - |
| Glycosides | + | - | - |
Table 1: Phytochemical screening results. + indicate present, - indicate absent
The calibration curve was used to calculate the total phenolic content of the three extracts (Figure 1). TPC of the three extracts of Dioscorea steriscus tubers is shown in Table 2.
| Extract | Absorbance at 765 nm wavelength | Total phenolic content (mg GAE/g extract) |
|---|---|---|
| Methanol | 0.68150 | 78,23 |
| Ethanol | 0.636174 | 73.02 |
| Ethyl Acetate | 0.604158 | 69.34 |
Table 2: Total phenolic content (TPC) of the extracts. mgGAE/g=Milligrams Gallic acid equivalent per gram
Discussion
Phytochemical screening was conducted with appropriate tests carried out to determine the presence of flavanoids, terpenoids, saponins, alkaloids, polyphenols, tannins, steroids, and glycosides. In the methanol extract, all phytochemicals tested were present whilst in the ethanol extract all were present except for glycosides and saponins. In the ethyl acetate extract, saponins, tannins, steroids and glycosides were absent. Methanol has proved to be a better solvent for extracting phytochemicals compared to ethanol and ethyl acetate. This is because methanol is more polar than both ethanol and ethyl acetate. TPC for methanol, ethanol and ethyl acetate extracts was determined by Folin- Ciocalteau assay using Gallic acid as a standard phenolic compound. A linear calibration curve of Gallic acid for TPC, in the concentration range of 8 mg/l-40 mg/l with coefficient of determination (R2) value of 0.995, was obtained (Figure 1). The regression equation was found to be y=0.034x+0.003. The calibration curve was used to calculate the total phenolic content of the three extracts (Table 2). The total phenolic content of Dioscorea steriscus tubers was found to be 78.23 mg in methanol extract, 73.02 mg in ethanol extract, and 69.34 mg in ethyl acetate extract. The highest TPC was obtained in the methanol extract sample and the lowest TPC was obtained in ethyl acetate extract sample. This is because methanol has high polarity; more compounds can dissolve in it and hence extract more phenolic compounds.
Conclusion
Dioscorea steriscus tubers were screened for phytochemicals using simple laboratory tests. Eight groups of phytochemicals were screened for in three extracts. It was clearly established that Dioscorea steriscus tubers have high concentrations of phenolic compounds. The availability of significant amounts of phenolic compounds in Dioscorea steriscus tubers that may impart medicinal potential for alleviation of body disorders is clear evidence that the plant could be an additional and superior source of natural antioxidants. Due to the presence of substantial amounts of phenolic compounds, Dioscorea steriscus tuber has potential applications in food, cosmetic, plastic and pharmaceutical industries. There is need for preservation of Dioscorea steriscus plants through cultivation and domestication.
Acknowledgements
Authors appreciate research support from Harare Polytechnic College. Authors also appreciate the help from Mr. Matebesi, the Course Coordinator, Horticulture at Harare Polytechnic College.
References
- Joshi RK (2011) Qualitative analysis of phenolic constituents from leaves of Anaphalis contorta. Int J Nat Prod Res 2: 23-25.
- Bonoli M, Marconi E, Caboni MF (2009) Free and bound phenolic compounds in barley flours, Evaluation of the extraction capability of different solvent mixtures and pressurized liquid methods by micellar electrokinetic chromatography and spectrophotometry. J Chromatogr A 1057: 1-12.
- Dai J, Mumper RJ (2010) Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules 15: 7313-7352.
- Roesler R, Malta LG, Carrasco LC, Holanda RB, Sousa CAS, et al. (2007) Antioxidant activity of cerrado fruits. Food Science and Technology (Campinas) 27: 53-60.
- Oliveira CS, Maciel LF, Miranda MS, Bispo ES (2011) Phenolic compounds, flavonoids and antioxidant activity in different cocoa samples from organic and conventional cultivation. British Food J 113: 1094-1102.
- Theerasin S, Baker AT (2009) Analysis and identification of phenolic compounds in Dioscorea hispida Dennst. As J Food Ag Ind 2: 547-560.
- Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44: 239-267.
- Willcox JK, Ash SL, Catignani GL (2004) Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 44: 275-295.
- Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4: 118-126.
- Narkhede A, Jaskaran G, Kaja T, Divya S, Elangbam S, et al. (2013) Total polyphenolic content and free radical quenching potential of Dioscorea alata tubers. Int J Pharm Pharm Sci 2: 1475-1491.
- Muchuweti M, Kasiamhuru A, Benhura N, Chipurura B, Amuna P, et al. (2009) Assessment of the nutritional value of wild leafy vegetables consumed in the Buhera district of Zimbabwe: A preliminary study. J Acta Horticulture 806: 323-330.
- Dzomba P, Togarepi E, Mahamadi C (2012) In-vitro assessment of antioxidant potential and phenolic capacity of Temnocalyx obovatus leaves. African J Biochem Res 6: 98-104.
- Aberoumand S, Deokule S (2008) Comparison of Phenolic Compounds of Some Edible Plants of Iran and India. J Nutr 7: 582-585.
- Chipurura B, Muchuweti M, Kasiyamhuru A (2013) Wild Leafy Vegetables Consumed in Buhera District of Zimbabwe and Their Phenolic Compounds Content. J Ecol Food Nutr 2: 178-189.
- Alagammal M, Paulpriya K, Mohan VR (2013) Anticancer activity of Ethanol extract of Polygala javana. Research Journal of Recent Sciences 2: 18-22.
- Sheikh N, Kumar Y, Misra AK, Pfoze L (2013) Phytochemical screening to validate the ethnobotanical importance of root tubers of Dioscorea species of Meghalaya, North East India. J Med Plant Sci 1: 62-69.
- Roy A, Geetha RV (2013) Preliminary phytochemical screening of the ethanolic extract of dioscorea villosa tubers and estimation of diosgenin by HPTLC technique. Int J Drug Dev Res 5: 377-381.
- Sakthidevi G, Mohan VR (2013) Total phenolic, flavonoid contents and in vitro antioxidant activity of dioscorea alata tuber. J Pharm Sci Res 5: 115-119.
- Waring RH, McDonald AJS, Larson S, Ericson T, Wiren A, et al. (1985) Differences in chemical composition of plants grown at constant relative growth rates with stable mineral nutrition. J Oecologia 66: 157-160
- Poorter H, Bergkotte M (1992) Chemical composition of 24 wild species differing in relative growth rate. Plant Cell Environ 15: 221-229.
- Dzomba P, C. Musekiwa C (2014) Anti-obesity and antioxidant activity of dietary flavonoids from Dioscorea steriscus tubers. Journal of Coastal Life Medicine 6: 465-470.
- Karasova G, Brandsteterova E, Lachova M (2003) Matrix solid phase dispersion as an effective preparation method for food sanples and plants before HPLC analysis. Czech J Food Sci 6: 219-234.
- Uddin G, Rauf A, Siddiqui BS, Shah SQ, (2011) Preliminary comparative phytochemical screening of Diospyros lotus Stewart. Middle-East. J Sci Res 1: 78-81.
- Firdouse S, Alam P (2011) Phytochemical investigation of extract of Amorphophallus campanulatus tubers. Int J Phytomed 3: 32-35.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences