Market Analysis@ Cellular Therapies
Demitrios Dhronopoulos
Associate Professor, University of Nottingham, UK, Email: Demitrios.dhronopoulos@nottingham.ac.uk
The market of cell medical aid in terms of revenues is $3 B and its world market is poised to grow at a CAGR of twenty three.27% at intervals the amount 2017-2021. The market of somatic cell medical aid is predicted to succeed in $ one hundred forty five.8 M by 2021, growing at a CAGR of eleven.0% throughout the forecast amount 2016- 2021.The worldwide somatic cell medical aid market on the premise of the mode of treatment is split into allogeneic and autologous somatic cell medical aid.
The factor medical aid market in spite of presenting few marketable merchandise and being emerging in terms of revenue generation holds extreme growth potential. In line with a brand new estimation administrated in an exceedingly latest study, the worldwide factor medical aid trade has the potential to become a multi-million dollar trade by the tip of 2018 as new merchandise, particularly those within the advanced stage of clinical studies or with unfinished approvals could enter the market to reinforce the expansion.
Importance and scope of cell therapy:
Cell medical aid deals with the administration of live whole cells or maturation of a selected cell population in a very patient for treatment of a malady. By administrating an oversized range of effector cells has benefited an oversized range of cancer patients, transplant patients with unresolved infections and patients with chemicals destroyed stem cells in eyes. Factor medical aid on the opposite hand is expressed as Associate in Nursing experimental treatment that involves introduction of genetic material into the cells so as to correct the defective genes. Retroviruses and adenoviruses are among the foremost usually used infectious agent vectors in trials for treating diseases beneath factor medical aid. As an example, some patients have a severe combined immunological disorder malady (SCID) however sadly don't have an acceptable donor for the bone marrow. Many dozens of patients are treated with each combined cell and factor medical aid approach. A lot of evidences for wider clinical applications for each cell and factor medical aid are expected as a lot of cell and factor medical aid studies progress from run to clinical test.
Market Value on Cell and sequence Therapy:
The global vegetative cell medical care market is predicted to succeed in $330 M by 2020, at a CAGR of 39.5% from 2015 to 2020. The worldwide vegetative cell market in 2015 is predicted to be dominated by North America, followed by Asia-Pacific and Europe. However, Asia-Pacific market is predicted to surpass North America by 2020, thanks to the high variety of on-going analysis activities. Autologous vegetative cell and Non-Stem Cell primarily based Therapies market is presently calculable as $650 M, as well as merchandise of all application areas. This market is anticipated to succeed in $2.2 B by 2017, growing at a CAGR of twenty first. ACT has already been flourishing within the treatment of glandular carcinoma, skin burns, cosmetic surgeries, skin substitutes; wound healing, pressure ulcers, etc. Products factory-made by the trade connected cell medical care and its market price. international markets for stem cells by BCC analysis (2012), cited by Ministry of Food and Drug Safety, foreseen the worldwide market size of vegetative cell medical care product would be $6.6 B in 2016 and grow 11.7% on the average each year. Currently, US's vegetative cell medical care product occupying the largest market share amounts to $1.3 B and Europe's is $872 M. US's vegetative cell medical care product market is foreseen to possess a mean annual rate of 11.5% ($2.3 B in 2016), and Europe's is ten.9% ($1.5 B in 2016). Till Lammas Day, 2013, 3 vegetative cell therapies has non inheritable an acquisition allow for domestic use in Korea: Hearticellgram-AMI by FCB- Pham cell (the world's initial vegetative cell medical care product), Cardizem by Medipost and Cupistem by Anterogen. And also, twenty four medicines square measure undergoing run until Gregorian calendar month one, 2013.
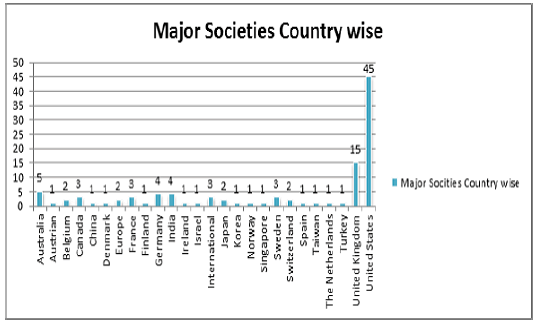
Top Major Societies Associated with Cell and Gene Therapy
• British Society and Gene and Cell Therapy
• American Society of Gene and Cell Therapy (ASGCT)
• Australasian Gene Therapy Society (AGTS)
• Austrian Network for Gene Therapy
• British Society of Gene Therapy (BSGT)
• European Society of Gene and Cell Therapy (ESGCT)
• Finnish Gene Therapy Society (FGTS)
• German Gene Therapy Society (DGGT)
• International Society for Cancer Gene Therapy (ISCGT)
• Irish Society for Gene & Cell Therapy (ISGCT)
• Israeli Society of Gene & Cell Therapy (ISGCT)
• Japan Society of Gene Therapy (JSGT)
• Korean Society of Gene and Cell Therapy (KSGCT)
• Netherlands Society of Gene and Cell Therapy (NVGCT)
• Société Francophone de ThérapieCellulaire et Génique (SFTCG)
• Spanish Society of Gene and Cell Therapy (SETGyC)
• Swedish Society for Gene and Cell Therapy (SSGCT)
• Turkish Society of Gene & Cell Therapy (TSGCT)
• List of University in Europe:
• Finland- A.I. Virtanen Institute for Molecular Sciences
• Finland-Cancer Gene Therapy Group University of Helsinki
• Israel-The Goldyne Savad Institute of Gene Therapy, Hadassah, Jerusalem
• Italy- San Raffaele Telethon Institute for Gene Therapy (HSR-TIGET), Milan
• Italy-TIGEM-Telethon Institute of Genetics and Medicine, Napoli
• Netherlands- Oncogenomics VUmc, Amsterdam
• Netherlands-Pharmaceutical Gene Modulation, Groningen
• Sweden- Molecular Medicine and Gene Therapy, Lund University
• Turkey-Human Gene and Cell Therapy Center, Akdeniz University Hospitals and Clinics
Societies Associated with Cellular Therapies
Market Value on Cell and Gene Therapy:
The global stem cell therapy market is expected to reach $330 million by 2020, at a CAGR of 39.5% from 2015 to 2020.
The global stem cell market in 2015is expected to be dominated by North America, followed by Asia-Pacific and Europe. However, Asia-Pacific market is expected to surpass North America by 2020, owing to the high number of on-going research activities.
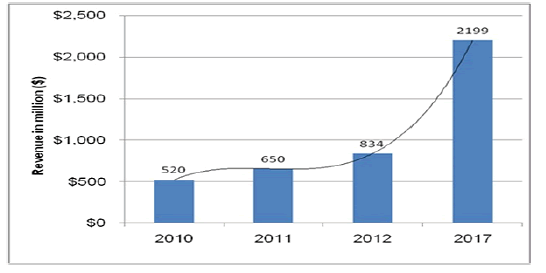
Autologous Stem Cell and Non-Stem Cell Based Therapies market is currently estimated as $650 million, including products of all application areas. This market is anticipated to reach $2.2 billion by 2017, growing at a CAGR of 21%. ACT has already been successful in the treatment of prostate cancer, skin burns, cosmetic surgeries, skin substitutes, wound healing, pressure ulcers, etc.
The markets for gene therapy are difficult to estimate as there is only one approved gene therapy product and it is marketed in China since 2004. Gene therapy markets are estimated for the years 2014-2024. The estimates are based on epidemiology of diseases to be treated with gene therapy, the portion of those who will be eligible for these treatments, competing technologies and the technical developments anticipated in the next decades.
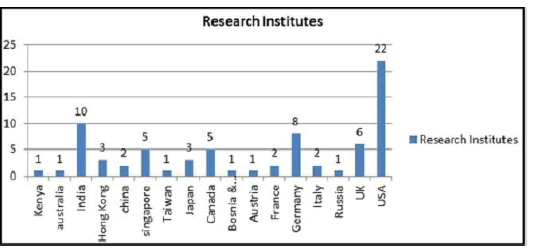
List of Research Institutes
Products manufactured by the industry related cell therapy and its market Value:
Global markets for stem cells by BCC Research (2012), cited by Ministry of Food and Drug Safety, predicted the global market size of stem cell therapy product would be 6.6 billion dollars in 2016 and grow 11.7% on average every year. Currently, US's stem cell therapy product occupying the biggest market share amounts to 1.3 billion dollars and Europe's is 872 million dollars. US's stem cell therapy product market is predicted to have an average annual growth rate of 11.5% (2.3 billion dollars in 2016), and Europe's is 10.9% (1.5 billion dollars in 2016). Until August 1, 2013, three stem cell therapies has acquired a sale permit for domestic use in Korea: Hearticellgram-AMI by FCB-Phamicell (the world's first stem cell therapy product), Cartistem by Medipost (the world's first allogenous stem cell therapy product) and Cupistem by Anterogen. Besides, 24 medicines are undergoing clinical trial till January 1, 2013.
US$3 billion state taxpayer-funded institute for stem cell research, the California Institute for Regenerative Medicine. It hopes to provide $300 million a year. However, in June 6, 2006, there were delays in the implementation of the California program and it is believed that the delays will continue for the significant future. On July 21, 2006, Governor Arnold Schwarzenegger (R-Calif.) authorized $150 million in loans to the Institute in an attempt to jump start the process of funding research.
Semma Therapeutics, a company developing a cell therapy for Type 1 diabetes, announced today that it has closed a $44 million Series A consisting of equity financing and strategic funding. The Series A financing is led by MPM Capital, with Fidelity Biosciences, ARCH Venture Partners, and Medtronic participating. Alongside the equity financing, Semma entered into an undisclosed agreement with Novartis Pharmaceuticals.
NIH provided US$ 258 Million in funding for three SF universities and US$ 41 Million to SF start-up firms through Small Business Innovation Research grant scheme.
Market Value:
-
2nd Annual Congress on Cellular Therapies, Cancer, Stem Cells and Bio Medical Engineering July
Vienna, Austria
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
