Management of difficult colorectal polyps Review of the Literature
Department of General Surgery, Braga’s Hospital, Portugal
- *Corresponding Author:
- Charlène Viana
Resident of General Surgery
Department of General surgery
Braga’s Hospital, Portugal
Tel: +351 253 027 000
E-mail: vianacharlene@gmail.com
Received Date: January 31, 2018 Accepted Date: March 23, 2018 Published Date: March 30, 2018
Citation: Viana C*, Pinto JP, Goulart A, Nogueira F, Leão P, et al. (2018) Management of Difficult Colorectal Polyps: Literature Review. J Med Oncol. Vol.1 No.2:6
Abstract
Colorectal cancer remains one of the worldwide leading causes of death. Fortunately, screening programs led to an earlier diagnosis and a subsequent approximately 50% decrease in colorectal cancer mortality.
It’s known that the majority of malignant polyps arise from adenomatous polyps.
Macroscopically, polyps can be classified by many characteristics that generally try to determine de risk to malignancy. Size, location and morphology can be helpful to do an “optical biopsy” as well as Paris and Kudo Classifications, which are powerful signs that help endoscopists to determine the malignancy potential of each lesion.
When resected, polyps have to be completely characterized in terms of: size, grade of dysplasia, margins status, and invasion of submucosa, among others features. Independently of the polypectomy technique applied, the goal is always the same: be as most informative as possible about the resected lesion in order to characterize the polyp in a low or a high risk group. Despite that, sometimes there are polyps of difficult decision and in this case, management can be controversial. This reinforces the need of multidisciplinary approach in order to decide always the best for each patient.
We present a literature´s review about management of difficult colorectal polyps, introducing polyp classifications, features of potential malignancy, endoscopic techniques for polypectomy and management of malignant polyps.
Keywords
Colorectal cancer; Narrow band imaging; Multiband imaging; Endoscopic mucosal resection
Introduction
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in Europe and a leading cause of death in Europe and worldwide [1]. It remains a challenging clinical entity to diagnose and treat. Fortunately, with advances in screening programs and early diagnosis, CRC in initial stages is becoming more and more frequent [2]. Observational studies of populations screened by colonoscopy shows a reduction in CRC mortality of approximately 50% [3]. Despite the huge importance of screening, 60-80% of polyps detected are diminutive (≤ 5 mm) and those almost never are malignant which undermines the benefit of removal diminutive polyps [3,4]. So, the decision to do a polypectomy should be tailored approached.
More than 95% of CRC arise from adenomatous polyps [5]. Those different phases between normal epithelium, adenoma, dysplasia and carcinoma may occur for a long time, indolently, and by accumulation of multiple genetic mutations, some inherited and others acquired [2]. The adenomatous polyps characterization must inform us about adenoma subtype, grade of dysplasia and, if polypectomy is intact, an assessment of size and margin status with respect to involvement by dysplasia [6]. Larger adenomas can be difficult to remove so, new endoscopic technologies are being applied [2,4].
We present a literature review concerning the management of difficult colorectal polyps: classification types, endoscopic resection techniques, definition of high risk patient’s pathology and management of malignant polyps, among others.
Colorectal polyps
A colorectal polyp is an abnormal protrusion of the mucosa into the bowel lumen. It is known that the majority of CRC arises from precursor adenomatous polyps [2]. The prevalence of large bowel adenomas increases with age and can be symptomatic or not, found during screening colonoscopy. The majority of removed polyps are small and the risk of malignant transformation is known to increase with many factors, like size [7].
Thereabout 80-90% of adenomas are <1 cm and can be submitted to complete resection with conventional endoscopic techniques. Larger polyps can be more difficult to remove and require more advanced endoscopic expertise that will be discussed later in this review. The final is goal is always to permit a total excision in order to acquire more comprehensive histological examination [2,7].
A malignant polyp implies the presence of cancer cells invading through the muscularis mucosa into the underlying submucosa T1 cancer according to TNM classification (Table 1) [8]. They usually have a benign endoscopic appearance but the presence of invasive adenocarcinoma cells on final histology creates many difficulties in final orientation and often controversial decisions [2,7]. Studies show that malignant polyps, therefore early carcinoma, are found in 0.75% to 5.6% of large bowel polyps removed in all colonoscopies [7].
| TNM Clinical Classification | |
|---|---|
| T-Primary tumour | |
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ: invasion of lamina propria |
| T1 | Tumor invades submucosa |
| T2 | Tumor invades muscularis propria |
| T3 | Tumor invades subserosa or into non-peritonealised pericolic or perirectal tissues |
| T4 | Tumor directly invades other organs or structures b, c, d and/or perforates visceral peritoneum |
| T4a | Tumor directly invades visceral peritoneum |
| T4b | Tumor directly invades other organs or structures |
| N-Regional lymph nodes | |
| NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in 1-3 regional lymph nodes |
| N1a | Metastasis in 1 regional lymph node |
| N1b | Metastasis in 2-3 regional lymph nodes |
| N1c | Tumor deposit(s), i.e., Satellites, in the subserosa or in non-peritonealised pericoliv or perirecctal soft tissue without regional lymph node metastasis |
| N2 | Metastasis in 4 0r more regional lymph nodes |
| N2a | Metastasis in 4-6 regional lymph nodes |
| N2b | Metastasis in 7 0r more regional lymph nodes |
| M-Distant metastasis | |
| M0 | No distant metastass |
| M1 | distant metastasis |
| M1a | Metastasis confined to one organ (liver, lung, ovary, non-regional lymph node(s)) without peritoneal metastsis |
| M1b | Metastasis in more than one organ |
| M1c | Metastasis to the peritoneum with or without other organ involvement |
aTis includes cancer cells confined within the mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the
submucosa
binvades through to visceral peritoneum to involve the surface
cdirect invasion in T4b includes invasion of other organs or segments of the colorectum by way of the serosa, as confirmed on microscopic
examination or for tumors in a retroperitoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond
the muscularis propria
dTumor that id adherent to other organs or structures, macroscopically, is classified cT4b. However, if no tumor is present in the adhesion
microscopically, the classification should be pT1-3, depending on the anatomical depth of wall invasion
eTumor deposits (satellites) are discrete macroscopic or microscopic nodules of cancer in the pericolorectal adipose tissue’s lymph drainage area of
a primary carcinoma that are discontinuous from the primary and withput histological evidence of residual lymph node or identifiable vascular or
neural structures. If a vessel wall is identifiable on H&F, elastic or other strains, it should be classified as perineural invasion (pn1). The presence of
tumor deposits does not change the primary tumor T category, but changes the node status (N) to pN1c if all regional lymph nodes are negative or
pathological Examination
H&E: Hematoxillin and Eosin; U icc: The Union for International Cancer Control; TNM: Tumor, Node, Metastasis
Reprinted from Uno and Munakata [16], with permission from John Wiley & Sons, Inc.
Table 1: TNM classification for CCR8.
Colorectal polyps can be classified by many endoscopic characteristics that generally try to determine de risk of malignancy.
Endoscopic appearance – “Optical Biopsy”
Endoscopically, there is no possibility of achieving a definitive diagnosis in terms of benign or malign pathology. However, there are many characteristics like size, location and morphology that can support “optical biopsy” for potential malignancy.
Size is one of the most important risk factors for malignancy. Studies shows that polyps ≤ 5 mm have negligible risk of malignancy while those with >35 mm show ~75% of risk to be malign [7].
In terms of location, is known that right colonic polyps have major risk to malignancy. Increasingly detailed location is required for the exact determination of the site of polyp [7].
About morphology, polyps can be pedunculated or sessile. Sessile are, obviously, more difficult to remove and more specific techniques could be needed [2].
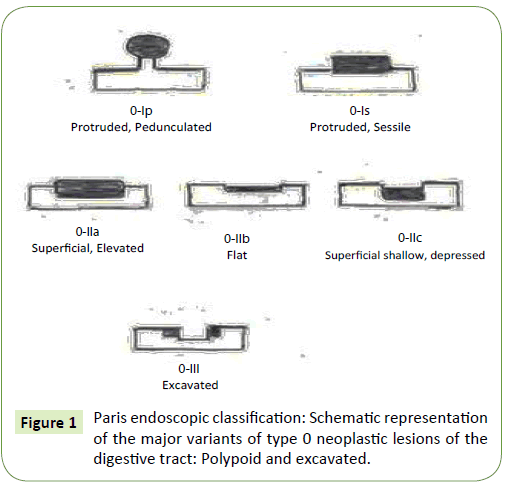
Macroscopically, polyps also have more risk to be malignant when they have irregular contour or ulceration or when the consistency is hard. Paris (Diagram 1 and Figure 1) and Kudo Classifications (Figure 2) allow us to predict the polyp histology [9-11]. Despite the classifications utility, they are still poorly standardized globally, so that can lead to marked interobserver variability [2].
Paris classification [10] (Diagram 1 and Figure 1)
It was published in 2002 after international consensus and collaborative meeting, differentiate polyps in “Polypoid/ Protruded” – Type I; and “Non Polypoid” – Type II, that can be: Superficial Elevated, Flat and Depressed. Sometimes, Type III lesions are also described as “Excavated” lesions (ulcers). “Protrude” Polyps can be classified in pedunculated (p) and sessile (s) while “Non Polypoid” lesions are divided on “a”, “b” or “c” lesions. In the case of “a” lesions, polyps are flat and elevated; in “b” polyps, those are completely flat; while “c” polyps are superficially depressed. Often, those polyps have mixed forms and not an isolated type of lesion [10]. Recognition of depression is critical in colorectal lesions because this is many times associated with invasive cancer even in small lesions. True depressed lesions are rare but dangerous because they grow quickly and become advanced tumors at early stages. So, Paris Classification is important because is both descriptive and predictive and is used by endoscopists to predict malignancy in early disease stage [7].
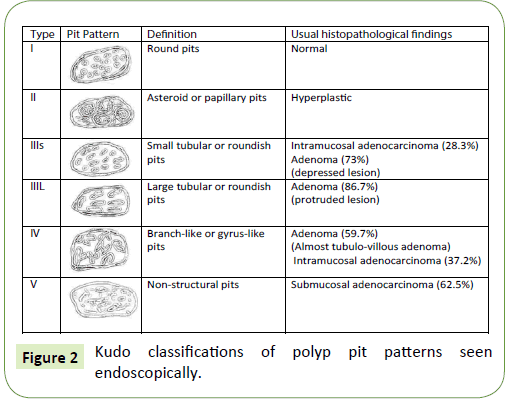
The Kudo classification [11] (Figure 2)
T detailed classification of pit patterns to predict histology in five types (“I-V”) [11,12]. Types “I” and “II” are non-neoplastic, with normal or hyperplastic mucosa; Types “IIIS” (small), “IIIL” (large) an “IV” are most frequently benign; and Type “V” have high risk for malignancy because the invasion of submucosa. It can be divided into “Vi” and “Vn” if it is irregular or nonstructural, respectively. Despite the ability to identify pit patterns requires training and practice, when possible, enables endoscopists to predict malignant invasion and determine appropriate therapy [7,11].
Endoscopic study and the decision to perform a polypectomy according the size
As previously mentioned, colonoscopy and endoscopic resection of malignant polyps play a fundamental role in screening of CRC and early detection of precancerous polyps, with reduction in CRC mortality of approximately 50% [3]. Actually, colonoscopy is safe and allows identification, diagnosis and, in many cases, definitive treatment of benign and malign polyps [7].
Diminutive polyps (<5 mm) are very common (~60-70% of patients screened) and the great majority of this are non-neoplastic. Highrisk histological features were found in only 1.7% of diminutive polyps and can be, usually, identified by trained endoscopists through morphologic patterns like Paris Classification or Kudo Classifications [4,10-12]. When polyps are diminutive or difficult to identificated, techniques to improve polyp’s detection can be improved to minimize missing precancerous/malignant polyps [7]. Despite colonoscopy with polyp resection is an optimal screening method for CRC diagnosis, it had important costs and some risks so, once the majority of diminutive polyps grow up slowly with very small risk to malignant transformation, management of diminutive polyps is acceptable to be done in three ways: endoscopic resection and histopathological analysis; endoscopic resection and discard the sample; or even, nonresection of diminutive polyps (the last two could be considered when done by trained endoscopists and when morphologic patterns are unsuspicious) [7,13].
Mid-size polyps (5-20 mm) can be found during colonoscopy or through other imaging modalities. In all cases, those polyps have relevant risk for malignant transformation so they should be timely resected. Studies revealed advanced pathology in 7% of cases of polyps between 6-9 mm and in 31% of polyps with ≥ 10 mm [12]. Complete resection of those polyps is fundamental to avoid CRC development once lesions with higher risk to malignant transformation are more easily incompletely resected (e.g. larger size, serrated histology) [3,4].
Larger polyps (≥ 20 mm) can have high rate of dysplasia or invasive cancer (~7-68%) [14]. Adenocarcinoma can invade submucosa of polyp in ~7% of polyps with ≥ 20 mm therefore, large polyps must always be totally resected because the high probability of malignant transformation [4,15].
Despite most malignant polyps are larger and easily to see, many polyps can be missed because they are small or because they are not pedunculated but flat and depressed or because they hide behind colon folds or flexures. Good bowel preparation is critical not to miss lesions and also, many techniques to improve polyp detection can be done. “Dye spray chromoendoscopy” using indigocarmine contrast is a technique to enhance mucosal features; other technological techniques like “Optical and processor-based technologies”, for instance, Narrow Band Imaging (NBI), multiband imaging (MBI marked as FICE: Flexible Spectral Imaging Color Enhancement) and i-scan (Pentax) can also be used; and “Cap colonoscopy”, an endoscope with disposable hood that expose hidden mucosa is another technique used to decrease the possibility to miss important lesions. It is generally accepted that, in average risk patients, techniques like NBI (providing detailed observation of microcapillary architecture) and FICE (detecting surface patterns) improve capacity to detect precancerous lesions however, having trained endoscopists is critical for the clinical value of these techniques [3,7].
Another important sign favoring malignant change is the “Nonlifting Sign”. This is made injecting saline or other fluid below polyp. If the lesion don’t lift relative to surrounding mucosa means that there is submucosal invasion and so, endoscopic resection would not be sufficient to clear the lesion. Non-lifting sign seems to be Sm3 lesions by Kikuchi Classification while early cancers that lift are, usually, Sm1 or Sm2 [7,16]. Kikuchi Classification will be explained in detail later on this paper.
Polypectomy: Techniques, Complications and the Importance of Tattooing
Polyps should be resected in best way possible by endoscopists, to give maximum histological information to pathologist. Posteriorly, pathologists have to process and observe it optimally and totally, respectively. Actually, there are many endoscopic techniques to make polypectomy. The goal is, whenever possible, excision in one piece and in one session [7].
Cold Snare Polypectomy is feasible and safe and is, actually, the technique usually applied in pedunculated lesions because that allows good histopathological evaluation with better resection margin than, for example, polypectomy done by monopolar energy [3]. Many times, when histology is informative and margin is favourable, snare polypectomy can be curative. So, cold snare resection is, normally, the preferred method for pedunculated polyps with 5-10 mm [3] and the margin actually accepted that is associated with lowest rate of local recurrence and residual disease is ≥ 2 mm [2,17].
For non-pedunculated polyps, endoscopic mucosal resection (EMR) is, usually, the preferred method [3]. EMR is done lifting the mucosa up by injecting a fluid in submucosal plane, allowing resection of sessile, flat and depressed lesions in bloc. After lifted, lesion can be snared and excised, because of deepening the vertical excision plane [18]. After polypectomy by EMR, tattooing location is critical to accurately identify the previous location of the polyp in a future procedure. Most non-pedunculated polyps, sessile or flat, with more than 20 mm and no other unfavorable features, can require resection in more than one piece, called “piecemeal EMR”. Despite that it not comply the resection enbloc usually required, that can allow adequate local control in unfit person for surgery however, histopathology is difficult to interpret in this case and prognosis cannot be established so, this is not the preferred modality in suspicious lesions [7].
Other endoscopic technique available for resection of large lesions is endoscopic submucosal dissection (ESD). ESD can be used alternatively to EMD to resect en-bloc non pedunculated precancerous/cancerous polyps larger than 20 mm [3]. In this case, submucosal lift is done followed by mucosal incision and submucosal dissection. This technique is complex and should be done only by experts that, when trained, can make resections in one piece in addition to re-excise areas of previous recurrence [7]. Studies described curative rates >80% after ESD of precancerous/ cancerous lesions however, complication rates are high, even when done by experts (~4.9% of perforation and 1.5% of bleeding) [19]. Despite the learning curve is long, that’s necessary, because of the great results associated to this technique [7].
Another emerging strategy for the management of colorectal polyps is underwater endoscopic mucosal resection (UEMR). Studies revealed that UEMR is safe, effective and well tolerated technique for resection of polyps larger than 10 mm, alternatively to EMR. Authors described advantages over EMR because of the water infusion instead of air or carbon dioxide insufflation, not requiring submucosal lifting and so, allowing more complete resection margins, eliminating risks associated with submucosal injection and reducing mucosal injury [20]. Despite UEMR safety and efficacy being demonstrated, data on recurrence rates are limited. An Italian group reported recurrence rates between 0-12% for polyps ≥ 10 mm however, no data exists about resection en-bloc or need of submucosal lift so, more studies are needed to define the efficiency, tolerability an recurrence rates post-UEMR [20,21].
About complications risk after polypectomy, those are variable consonant technique, size of polyps and expertise of endoscopists. Serious complications after colonoscopy are rare and unplanned admissions or episodes of unplanned care after colonoscopy are 0.14%. British Society of Gastroenterology showed perforation and bleeding rate of 0.04% and 0.26%, respectively [22]. According to techniques, different complication rates are described. EMR is associated with risk of perforation of 1% and bleeding risk of 3-10%. ESD, due to its complexity, complication rates are higher, in order to 8% [3]. Obviously that, independently of the procedure of choice, the goal of endoscopy and polypectomy is to acquire best resection and histological information with lowest complication rates.
At the time of polypectomy, tattooing the place of resect polyp is critical because of surveillance of previous endoscopic excision site and because of the possibility of need of surgery. Usually, India Ink or Indocianin Green is injected into submucosa, distally to lesion. If lesion is in caecum or in low rectum, tattooing can be dismissed however, if lesion is between caecum and low rectum, tattooing is fundamental to signalize place needing surveillance or segment needing surgery resection, ensuring resection of the correct segment of colon/rectum. Tattoo is fundamental, mainly in laparoscopic surgery when lesions cannot be palpable and, in the majority of cases, doesn’t affect serosa and so, are not macroscopically identificated [7].
Malignant polyps: Pathology
The NHS Bowel Cancer Screening Program recommends the use of two categories of dysplasia: low-grade and high-grade dysplasia. Like already commented, more than 95% of colorectal cancers arise from adenomatous polyps. Adenomas are benign neoplasms, corresponds of 75% of colorectal polyps resected and all shows some grade of dysplasia (low or high). Architecturally, the majority is tubular (~50%) with lower frequency for tubulovillous (15-24%) and villous (1-6%). Purely villous adenomas show high rates of malignancy (10-18%) while tubulovillous and tubular adenomas show lower risk to convert to malignant polyp (6-8% and 2-3%, respectively) [7].
High grade dysplasia is described on 5-14% of cases. Those shows complex glandular irregularity, cribriform architecture and prominent cellular atypia [7,23,24]. The last revised “highgrade dysplasia” definition includes focal infiltration of cells with carcinoma into lamina propria so, the previous lesions called carcinoma in situ or intramucosal carcinoma. Now, those categories are discouraged because of overtreatment in non-invasive lesions [24]. Still about size, studies reinforce the importance of size evidencing that large polyp size correlates positively with villous morphology and high-grade-dysplasia [7].
Previously defined like hyperplastic or metaplastic polyps, serrated polyps were considered innocuous. Nowadays, they are known having distinct genetic characteristics and different architecture, with high malignant potential because of their adenocarcinoma sequence faster than APC mutation. They can be classified into sessile serrated adenomas, traditional serrated adenomas, hyperplastic polyps and mixed hyperplastic polyps. Serrated polyps seem to be responsible for disproportionate number of CCR which are interval cancers and occur despite recommended screening [7,25,26].
Pathological prognostic factors: Depth of invasion, margins of resection, histology, lymphovascular invasion, tumor budding
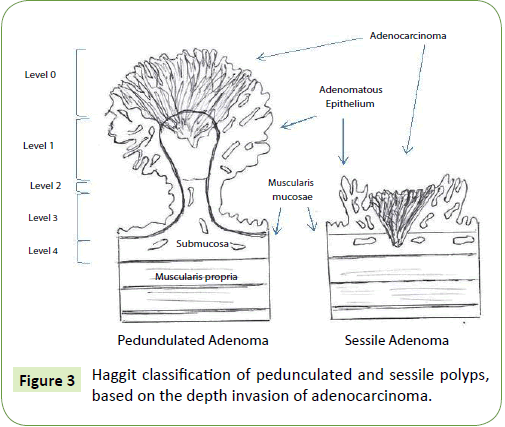
Polyps can be classified, histologically, by several factors. The most important seems to be the depth of invasion. Haggit et al. [27] create, in 1985, a classification system for pedunculated polyps based on the depth invasion of adenocarcinoma (Figure 3). Polyps can be classified into level 0 to 4 of invasion if adenocarcinoma is limited to the mucosa but not penetrate through the muscularis mucosae (level 0), invades the muscularis mucosae into the submucosa just to the head of the polyp (level 1), into the level of the neck (level 2), into the stalk (level 3) and into the submucosa below the stalk, above the muscularis mucosa (level 4) [2,27]. Invasive cancer arising sessile adenoma is, by definition, a level 4 lesion [7,27]. Haggit et al. [16] showed that, when depth of invasion was less than Haggit 4 the risk for local recurrence or locorregional metastasis was very low so, when there are no other negative prognostic features, pedunculated lesions seems to be appropriated for endoscopic resection. Level 4 invasion is associated with statistically adverse prognostic factors [27]. In patients with pedunculated polyps, lymph node metastasis was present in ~6% of cases while when polyps was invaded into level 4, ~27% had lymphatic involvement [28].
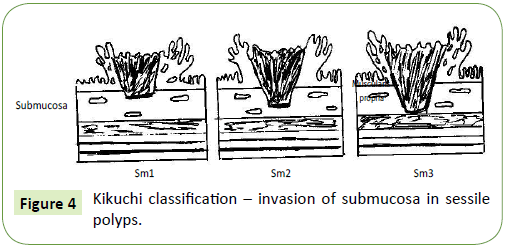
After Haggit, Kikuchi et al. [29] improved classification of malignant sessile polyps (level 4 in Haggit classification), characterizing submucosal invasion dividing submucosa into thirds: Sm1, Sm2 and Sm3 (Figure 4). Tumors involving only the uppermost third of submucosa are subdivided based on extend of horizontal spread of tumor. The Kikuchi classification is defined by [28,29]:
• Sm1: Tumor invasion of the upper third of the submucosa
• SM1a: Less than a quarter of the width of the tumor invading the submucosa
• SM1b: Between a quarter and a half of the width of the tumor invading the submucosa
• SM1c: More than a half of the width of the tumor invading the submucosa
• Sm2: Tumor invasion of the middle third of the submucosa
• Sm3: Tumor invasion of the lower third of the sub mucosa
Kikuchi et al. [29] show different risks of lymph node invasion between 0% in Sm1 to 14.4% in Sm3 polyps however, this classification can be compromised if the resection not include a significant portion of submucosa or some of muscularis mucosa to define the border of sub mucosa which shows the importance of those procedures be done by specialized endoscopists in those subjects. Lesions Sm1 and Sm2, when no other risk features are associated (e.g. lymph vascular invasion, poor differentiation, resection margin <2 mm), can be treated endoscopic ally. In case of pedunculated polyps Haggit et al.’s [27] level 4, sessile polyps Sm1 or Sm2 with other unfavourable risk factors or sessile polyps Sm3, surgical resection should be considered. Once again, the analysis to determine the treatment should be the combination of several factors to achieve the best management.
About margins of resection, this is also important feature in terms of luminal recurrence. When resection margin is involved or when it is <1 mm, the relapse rate is 21-33%. Many authors consider that the ideal resection margin is ≥ 2 mm [2]. If no other adverse features are known, margins >1 mm can be accepted with lymph nodes metastasis risk, residual tumor or recurrence <2% [7].
Histologically, carcinomas can be classified by grades: well, moderately or poorly differentiated. Well-differentiated supposes having well-formed glands with >95% glandular differentiation. The majority of carcinomas are moderately differentiated. Poorly differentiated malignant polyps are unusual findings (~4-7% of cases) and are usually associated with other adverse features. When diagnosed, poorly differentiated malignant polyps should be resected surgically because of greater risk of residual disease (greater incidence of metastasis and worst prognosis [7]. Carcinomas associated with cribriform pattern (gland within gland or/and back-to-back arrangement with stroma in between) seems to be associated with increased risk of lymph node metastasis [30].
Determination of lymph vascular invasion in malignant polyp can be difficult and can be associated with elevated interobserver variation. Lymphatic invasion can be difficult to differentiate from artefacts associated to fixation methods. Vascular markers like CD31 or CD34 can be used to differentiate lymphatic to vascular vessels however; their routine use is not recommended [4]. Lymph vascular invasion seems to be an independent risk factor for lymph node involvement [31]. Nevertheless, parameters have to be analyzed together once lymph vascular invasion without other adverse features is unusual [7].
About tumor budding, it is defined as isolated/small clusters (<5) of malignant cells at the advancing edge of the tumour or five or more buds/20 power fields. Tumor budding seems to be also associated with lymph node metastasis and other adverse outcomes [7].
Combination of Risk Factors and Management
Many factors have to be analyzed to determine polyps/patients and to be part of “low” or “high” risk features. These factors should be analyzed in group since they have a cumulative risk for residual disease after polypectomy that will determine the need to apply another treatment [2,7].
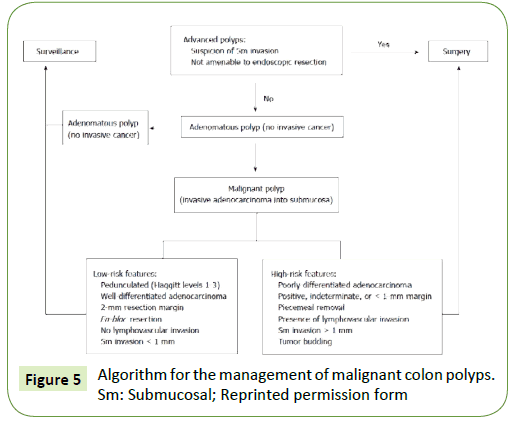
Aaron et al. [2] created an algorithm that guide the management of malignant colon polyps divided into polyps with “low-risk features” and “high-risk features” (Figure 5).
Figure 5: Algorithm for the management of malignant colon polyps. Sm: Submucosal; Reprinted permission form
Polyps are classified into “low risk” when no adverse features are present and, “high risk” when, at least, one adverse factor is present. Studies revealed the importance of each factors attributing more relevance to excision margins (>1 mm) and depth of invasion of the tumor. The degree of cancer differentiation and presence of lymph vascular invasion are also important prognostic factors however, they are usually seen in more deeply invasive malignant polyps [7]. Low risk polyps seems to have less probability of adverse outcomes whereas polyps with at least one high risk feature can have a risk of residual disease close to 50% (depending of number of adverse risk features present). There are studies that also differentiated an intermediate group with intermediate risk that are a group with difficult decision and associated with a greater challenge deciding what to do after the resection. In this cases, when the decision to undergo surgery or follow up strategy is unclear, informing “patient choice” is fundamental, after giving all the information about risks and benefits inherent in each of the options. Many studies show that, many times, when surgery is improved, histologic piece reveals no evidence of residual disease [7]. This reinforces, once again, the need of multidisciplinary teams to decide the best treatment for each case individually.
Surveillance
When surgery is improved following endoscopic polypectomy, the purpose is to remove the risk of progression of any residual disease about excision of the draining lymph nodes. So an adequate lymphadenectomy is required since it will have prognostic significance [7].
When surgery is not performed and surveillance after polypectomy is decided, no established standard for surveillance is established [2].
Non-endoscopic surveillance, contrast-enhanced Computed Tomography (CT) continues being the gold standard for distant disease. Contrast-enhanced liver Magnetic Resonance (MR) and positron emission tomography can be used in specific circumstances. About depth of invasion of colorectal lesion, CT, MR or endoluminal ultrasound can be performed however, none of them appear to be perfect on T staging, especially on T1 cancers [32,33]. The three-dimensional ultrasound actually seems to increase the accuracy detecting T, with accuracy about 25-98% for T1 tumours [34]. For detection the N, the same imaging modalities can be required. MR seems to be accurate detecting lymph nodes however, it is important to define when lymph node is positive because in T1 lesions, lymph nodes can be smaller than in more advanced tumours, making them much harder to identify [35].
About endoscopic surveillance, there is no established protocol of surveillance however; the initial follow-up suggested by British Society of Gastroenterologists/American College Physicians Guidelines is colonoscopy every three months after the procedure. If there is no doubt about totally resection, repeat examination is indicated six month later. This schema three to six months follow-up has to be performed unless twice a year. After that, annually colonoscopy should be done [2,7]. Obviously that, in all cases, combination of factors must to be taken into account to include patients in high or low risk for CRC.
Conclusion
The management of colorectal polyps is often controversial and remains a challenge that requires a multidisciplinary approach.
Polypectomy techniques can be the more conventional or more advanced, since the resected lesion allows the pathologist to provide the most complete information to include a patient in a low or high risk to cancer.
In case of high risk features, surgery should be performed. If polypectomy is chosen, an adequate surveillance must be ensured. In this last option it is crucial not forget tattooing the excision site in order to be able to do an appropriate follow up after polypectomy.
Acknowledgement
The authors would like to thank to Joshua Bleier, MD, FACS, FASCRS, Associate Professor of Surgery, Chief of Colorectal Surgery, and Pennsylvania Hospital for the kindness in providing to us the “Algorithm for the management of malignant colon polyps”.
References
- van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, et al. (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27: 1386-1422.
- Aarons CB, Shanmugan S, Bleier JI (2014) Management of malignant colon polyps: Current status and controversies. World J Gastroenterol 20: 16178-16183.
- von Renteln D, Bouin M, Barkun AN (2017) Current standards and new developments of colorectal polyp management and resection techniques. Expert Rev Gastroenterol Hepatol 11: 835-842.
- Bujanda L, Cosme A, Gil I, Arenas-Mirave JI (2010) Malignant colorectal polyps. World J Gastroenterol 16: 3103-3111.
- Loughrey MB, Shepherd NA (2017) Problematic colorectal polyps. Surg Pathol Clin 10: 947-960.
- Williams JG, Pullan RD, Hill J, Horgan PG, Salmo E, et al. (2013) Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2: 1-38.
- Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, et al. (2017) Rectal cancer: ESMO Clinical practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28: iv22-iv40.
- Williams CB, Whiteway JE, Jass JR (1987) Practical aspects of endoscopic management of malignant polyps. Endoscopy 19: 31–37.
- Participants in the Paris Workshop (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach and colon. Gastrointest Endosc 58: S3-43.
- Kudo S (1993) Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 25: 455-461.
- Lieberman D, Moravec M, Holub J, Michaels L, Eisen G (2008) Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology 135: 1100-1105.
- Gellad ZF, Voils CI, Lin L, Provenzale D (2013) Clinical practice variation in the management of diminutive colorectal polyps: Results of a national survey of gastroenterologists. Am J Gastroenterol 108: 873-878.
- Ahlawat SK, Gupta N, Benjamin SB, Al-Kawas FH (2011) Large colorectal polyps: Endoscopic management and rate of malignancy: Does size matter? J Clin Gastroenterol 45: 347-354.
- Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, et al. (2011) Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 140: 1909-1918.
- Uno Y, Munakata A (1994) The non-lifting sign of invasive colon cancer. Gastrointest Endosc 40: 485-489.
- Cooper HS, Deppisch LM, Gourley WK, Kahn EI, Lev R, et al. (1995) Endoscopically removed malignant colorectal polyps: Clinicopathologic correlations. Gastroenterology 108: 1657-1665.
- Repici A, Pellicano R, Strangio G, Danese S, Fagoonee S, et al. (2009) Endoscopic mucosal resection for early colorectal neoplasia: Pathologic basis, procedures and outcomes. Dis Colon Rectum 52: 1502-1515.
- Saito Y, Matsuda T, Fujii T (2010) Endoscopic submucosal dissection of non-polypoid colorectal neoplasms. Gastrointest Endosc Clin N Am 20: 515-524.
- Siau K, Ishaq S, Cadoni S, Kuwai T, Yusuf A, et al. (2017) Feasibility and outcomes of underwater endoscopic mucosal resection for ≥ 10 mm colorectal polyps. Surg Endosc, pp: 1-8.
- Curcio G, Granata A, Ligresti D, Tarantino I, Barresi L, et al. (2015) Underwater colorectal EMR: Remodeling endoscopic mucosal resection. Gastrointest Endosc 81: 1238-1242.
- Logan RF, Patnick J, Nickerson C, Coleman L, Rutter MD, et al. (2012) Outcomes of the bowel cancer screening programme (BCSP) in England after the first 1 million tests. Gut 61: 1439-1446.
- Hunter J, Harmston C, Hughes P, Wong L (2011) What is the nature of polyps detected by the NHS bowel cancer screening pilots? Colorectal Dis 13: 538-541.
- Foss F, West K, McGregor A (2011) Pathology of polyps detected in the bowel cancer screening programme. Diagn Histopathol 17: 495-504.
- O'Connell BM, Crockett SD (2017) The clinical impact of serrated colorectal polyps. Clin Epidemiol 9: 113-125.
- East JE, Atkin WS, Bateman AC, Clark SK, Dolwani S, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 66: 1181-1196.
- Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD (1985) Prognostic factors in colorectal carcinomas arising in adenomas: Implications for lesions removed by endoscopic polypectomy. Gastroenterology 89: 328-336.
- Nivatvongs S, Rojanasakul A, Reiman HM, Dozois RR, Wolff BG, et al. (1991) The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum 34: 323-328.
- Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, et al. (1995) Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 38: 1286-1295.
- Egashira Y, Yoshida T, Hirata I, Hamamoto N, Akutagawa H, et al. (2004) Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol 17: 503-511.
- Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, et al. (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39: 534-543.
- Mathur P, Smith JJ, Ramsey C, Owen M, Thorpe A, et al. (2003) Comparison of CT and MRI in the pre-operative staging of rectal adenocarcinoma and prediction of circumferential resection margin involvement by MRI. Colorectal Dis 5: 396-401.
- Videhult P, Smedh K, Lundin P, Kraaz W (2007) Magnetic resonance imaging for preoperative staging of rectal cancer in clinical practice: High accuracy in predicting circumferential margin with clinical benefit. Colorectal Dis 9: 412-419.
- Doornebosch PG, Tollenaar RA, De Graaf EJ (2009) Is the increasing role of transanal endoscopic microsurgery in curation for T1 rectal cancer justified? A systematic review. Acta Oncol 48: 343-353.
- Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, et al. (2003) Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 227: 371-377.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences