ISSN : 0976-8505
Der Chemica Sinica
Liquid-liquid Extraction of Co (II) from Nitrate Solution Using the Mixture of TOPO and TOA
1Veterinary Research Division, Department of Parasitology and Animal Diseases, National Research Centre, 33 El Bohouth St. Dokki, Giza, Egypt
2Department of Chemistry, Centurion University of Technology and Management, Khurda, India
- *Corresponding Author:
- Bhabani Shankar Panda
Department of Chemistry
Centurion University of Technology and Management
Khurda-752050, India
E-mail: bpanda607@gmail.com
Received Date: November 06, 2021; Accepted Date: November 22, 2021; Published Date: November 28, 2021
Citation: Rauta NK, Panda BS, Swain N, Ahemad MA (2021) Liquid-liquid Extraction of Co (II) from Nitrate Solution Using the Mixture of TOPO and TOA. Der Chem Sin Vol.12 No.12:55.
Abstract
A large volume of waste is generated due to rapid growth of mining and metallurgical industries and the dissolution of heavy metals pollute the industrial belt water. Mostly copper, zinc, cadmium, arsenic, lead, nickel, cobalt etc. are responsible for environmental pollution. So, they should be removed before being discharged to surface water. Cobalt is most popular of metals because of its vast area of application. Cobalt is used in electroplating because of its appearance, hardness and resistance to oxidation. Cobalt is essential to the metabolism of all animals. But if present in excess amounts it may cause several toxic effects. In hydrometallurgical processes, liquid-liquid extraction is a well-established technique for the removal and separation of various metal ions after leaching and able to produce pure metal solutions which are used for electrowinning processes. In fact, the techniques can be applied for treatment of both concentrated and dilute solutions. In the present investigation attempts have been made to extract Co (II) from nitrate solution using mixture solutions of TOPO (tri-n-octyl phosphine oxide) and TOA (tri-n-octyl amine) in toluene. The effects of nitric acid concentration, equilibration time, concentration of aqueous phase and presence of salt have been studied. The extraction equilibrium was achieved in 15 minutes. The percentage of extraction of Co (II) decreased with increase in cobalt concentration in aqueous solution. The percentage of extraction of cobalt was found to be insignificant in the presence of salt. The extraction of cobalt using mixture of extractants, TOPO and TOA changed from antagonistic to synergistic with the increase in concentration of acid.
Keywords
Extraction; Tri-n-octylamine (TOA); Tri-n-octyl phosphine oxide (TOPO); Cobalt (II); Potassium nitrate
Introduction
A great deal of experimental research has been carried out on solvent extraction. In hydrometallurgical process solvent extraction is a well-established technique for removal and separation of various metal ions after leaching and able to produce pure metal solutions which are used for electro winning purposes. The techniques of liquid-liquid extraction have been applied to the extraction of heavy metals. In hydrometallurgical processes Solvent extraction is a great strategy for the separation and eradication of different metal particles in the wake of filtering and ready to deliver unadulterated metal arrangements which are utilized for electrowinning purposes.
Environmental pollution due to different types of metallic pollutants is of great concern because metals are nonbiodegradable [1,2]. Metals have widespread industrial applications in paints, pigments, nonferrous metal industries, fertilizers, wood pulp and photogenic industries [3,4]. The increasing use of metals affects the flora and fauna directly or indirectly on a daily basis because of their vast area of application. Though these metals play an important role in human metabolism, if present in excess amounts these may cause several toxic effects [5,6]. Excess concentration of cobalt may cause genetic and metabolic disorders. Industrial wastes therefore must be treated with a proper technique before discharge. Various techniques are available for the treatment of metal containing hazardous wastes such as co-precipitation, adsorption, electrochemical deposition, ion exchange, solid phase extraction, liquid-liquid extraction, etc. Among these methods liquid-liquid extraction is a cost-effective technique for the treatment of metal containing waste due to its high reproducibility and high sample capacity even in trace amounts of heavy metals. Liquid-liquid extraction is a very popular technique due to its simplicity, rapid method development and reasonable selectivity. With the proper choice of extracting agents this technique can achieve group separation or selective separation of trace elements with high efficiency. The measure of metals contained in risky squanders created overall is huge. The disposal of such residues in landfills results in the loss of valuable metals, energy and money. Therefore, recycling is essential to assure sustainability.
The extraction and stripping characteristic of CYANEX 301 paired extractant frameworks were researched for the recuperation of cobalt and nickel from sulfate arrangements was concentrated by Bourget C, et al. [7]. The selectivity properties of these frameworks against calcium, manganese and magnesium were likewise examined. Based on criteria such as extraction stripping characteristics (efficiency and rates) and selectivity against calcium, manganese and magnesium, screening experiments were performed to select the most appropriate binary extractant systems. From this screening work, the CYANEX 301/amine systems all demonstrated that cobalt and nickel could be selectively extracted from calcium, magnesium, and manganese. A large synergistic effect on the stripping kinetics and efficiencies of cobalt and nickel has been also observed when any amine extractant was added to CYANEX 301. Complete extraction of cobalt and nickel could be accomplished in 2-3 phases while as yet keeping a high selectivity against calcium, manganese and magnesium. Complete stripping of cobalt could also be achieved in 2-3 stages while complete nickel stripping was shown difficult.
The synergistic extraction of nickel and cobalt with a mixture of di (2-ethylhexyl) phosphoric acid and 5-dodecylsalicylaldoxime was studied by Zhang P, et al. [8]. The synergistic extraction of nickel and Cobalt from acidic aqueous solution has been observed with a mixture of di (2-ethylhexyl) phosphoric acid and 5-dodecylsalicylaldoxime. The rates of extraction and stripping were observed to be quick, in any event, for nickel, and depriving of nickel-loaded organic phase was promptly accomplished by contact with 0.50M mineral corrosive (acids). The partition of nickel and cobalt is conceivable dependent on contrasts in stripping practices of the two metals.
Solvent extraction of titanium by tributylphosphate, trioctylphosphine oxide (TOPO) and decanol from chloride media was studied by Allal KM, et al. [9]. Solvent extraction of titanium from both hydrochloric acid and calcium chloride solutions has been investigated. Polarographic analysis showed that the dissolved titanium is present as Ti(IV) in chloride solutions. Two extractants, tributylphosphate (TBP) and decanol, were used separately and mixed. The effects of the reaction time, the concentration of HCI and CaC12 in the aqueous phase, and the extractant concentration in the organic phase were studied. It is shown that the kinetics of the extraction process is very fast, since equilibrium is reached after 3 min. In addition, the extraction of Ti(IV) increases with the total chloride concentration in the aqueous phase, as well as with the extractant concentration in the organic phase. Extraction of about 95% of titanium by 1 mol/l TBP from hydrochloric acid media was possible, as well as the extraction of 99% of titanium by 0.5 mol/l TOPO from a 6 mol/l solution of total chloride.
The synergistic extraction and separation of Co (II)/Ni(II) by solvent extraction technique using TIOA/TOPO as carriers was studied by Okatan A, et al. [10]. The powerful boundaries on the extraction and the depriving of the cobalt were explored, and ideal synergistic extraction and stripping conditions were distinguished. The cobalt extraction from aqueous Co/Ni solutions in various molar concentrations was examined in the optimum conditions to determine the synergism between TIOA and TOPO.
In the present work, Tri-n-octylphosphine-oxide (TOPO) an organic complexing agent has been used as an analytical reagent for the extraction and separation of many metal ions. Trioctylamine (TOA), a well-known chemical organic compound in the group of aliphatic amines and tertiary amines has also been used along with TOPO for the extraction of cobalt. The present work introduces the liquid–liquid extraction of Cobalt from nitrate solution. To investigate the effect of solvent on the extraction of metal ions, toluene is used as the diluent.
Liquid-liquid extraction is an inter phase transport process in which two immiscible liquids are kept in contact and the distribution of a chemical substance between two phases is studied. The distribution of a substance depends on its solubility’s and the phase volume ratio. This technique is employed in analytical separation method. It has been used to separate metals of similar nature in solution and to purify solution for subsequent recovery of pure products. It ensures simpler and cleaner separation of materials at both macro and tracer level concentrations. The term liquid-liquid extraction is also used for solvent extraction. In the biphasic liquid system, one is organic phase which contains the extracting agent and sometimes along with diluents, and the other one is aqueous phase containing the metal ion. Diluent is used to lower the viscosity of the extractant and improve its extracting ability. Sometimes in industrial applications a component called modifier is added to the organic phase to prevent third phase formation. Liquidliquid extraction is extensively used in pharmaceutical industries, organic synthesis, bio-molecular processes, petrochemical industries, etc. New liquid-liquid extraction processes have been developed to separate and then concentrate long-lived actinide isotopes and fission products from nuclear waste solutions in atomic energy industry. For quantitative recovery of nuclear materials, continuous and multistage extraction system is used. The first report on liquid-liquid extraction of uranyl nitrate with diethyl ether as solvent was published in 1842 by Peligot EM [11]. The principles, applications and scope of liquid-liquid extraction techniques have been reviewed by several workers [12-21].
The use of trioctylphosphine oxide for solvent extraction recovery and purification of transplutonium elements was reported by Kosyakov V, et al. [22]. Extraction of trivalent curium, berkelium, californium, einsteinium, and europium with trioctylphosphine oxide (TOPO) from nitric acid solution just as extraction of curium and cerium from lactic acid solution containing DTPA relying upon aluminium nitrate fixation have been contemplated. The conveyance of cobalt, nickel, chromium, iron, aluminium, titanium, zirconium, and niobium has been contemplated and coefficients of berkelium decontamination from the components examined have still up in the air under the states of extractionchromatographic recuperation of berkelium. The impact of gauged measures of something very similar pollutants on the yield of berkelium in its extraction-chromatographic partition has been examined.
The dissolvable extraction of nickel and cobalt with synergistic system comprising of carboxylic acid and aliphatic hydroxyoxime was accounted for by Chu CY [23]. Metal extraction what's more, stripping kinetics with Versatic 10 acid and LIX 63 and modifier TBP are trying to create synergistic SX framework for the partition, filtration of nickel and cobalt from leach solutions. The blend of LIX 63 with Versatic 10 acid came about in huge synergistic movements for nickel, cobalt, copper, zinc and manganese and adversarial shift for calcium and manganese. The extraction and stripping kinetics of cobalt, copper, zinc and manganese were quick and the extraction and stripping kinetics of nickel were slow with the Versatic 10 acid/LIX 63 synergistic framework. The nickel stripping kinetics expanded with the expansion of TBP expansion. Within 2 min, the stripping productivity of nickel expanded from 18% with no TBP to 91% with 0.5 M TBP addition. The addition of TBP to the Versatic 10 acid/LIX 63 framework additionally further developed the nickel extraction kinetics. It is proposed that in the Versatic 10 acid/LIX 63 synergistic system, LIX 63 plays the role of an extractant and Versatic 10 acid a synergist for nickel while LIX 63 plays the role of a synergist and Versatic 10 acid an extractant for cobalt.
Synergistic extraction of copper (II) from sulfate medium with capric acid and tri-n-octylphosphine oxide in chloroform was studied by Ajdel F and Barkat D [24]. The synergistic solvent extraction of copper (II) from 0.33 mol/dm3 Na2SO4 aqueous solutions with capric acid (HL) in the absence and presence of tri-n-octylphosphine oxide (TOPO) in chloroform at 25°C has been studied. The extracted species when capric acid was utilized alone is CuL2(HL)2. Within the presence of TOPO, the complex is CuL2(HL)2(TOPO). The TOPO–HL collaboration firmly impacts the synergistic extraction proficiency. The extraction constants were determined.
Solvent extraction of uranium and lanthanides from phosphoric acid utilizing a synergistic DOPPA–TOPO blend was reported by Krea M and Khalaf H [25]. The majority of the phosphate rocks utilized financially contain little amounts of metals like uranium, lanthanides and yttrium. During the phosphate handling, by responding the phosphate rocks with sulphuric acid, ca. 30% of lanthanide and yttrium and over 80% of uranium, present at initially in the rocks, end up in phosphoric acid. In this work the general extraction of uranium and lanthanides from phosphoric acid has Synergistic extraction been considered. The influence of various factors such H3PO4, (SO4)2−, Fe (III), DOPPA and TOPO concentration on the degree of extraction has been established. Kinetic study shows that the initial extraction rates of uranium and lanthanides are of the same order of magnitude.
The uranium (VI) extraction mechanism of D2EHPA-TOPO from a wet process phosphoric acid was studied by Girgin S, et al. [26]. This paper reports the aftereffects of an examination concerning the solvent extraction of uranium from specialized grade phosphoric acid utilizing industrial accessible extractants as D2EHPA and TOPO diluted in specialized grade kerosene. Fundamental tests showed that, the impact of various boundaries for example, uranium oxidation stage, temperature and the molar proportion of D2EHPA/TOPO on the uranium recuperation was inacceptable concurrence with those of past examinations. In any case, a nitty gritty examination concerning the impact of phosphoric acid concentration, organic concentration what's more, acid stage proportions recommended that the component of D2EHPA/TOPO synergism was fairly perplexing and it introduced an alternate character contingent upon the acid concentration.
The synergistic liquid-liquid extraction and transport of Zn (II) and Cu (II) across polymer consideration layers with a combination of TOPO and Aliquat 336 was reported by Pospiech B [27]. Removal of zinc (II) and copper (II) particles from aqueous solution by synergistic extraction and transport through polymer consideration films (PIMs) has been examined. A combination of trioctylphosphine oxide (TOPO) and trioctymethylammonium chloride (Aliquat 336) was utilized as a specific extractant also as a particle transporter in polymer layers. The impacts of hydrochloric acid concentration in the watery stage and extractants fixation in the organic phase on the division cycle of zinc (II) and copper (II) particles have been contemplated. Zn (II) particles were effectively isolated from Cu (II) particles in dissolvable extraction process utilizing 0.025 M TOPO and 0.06 M Aliquat 336 in kerosene. Polymer consideration layers (PIMs) containing a combination of TOPO and Aliquat 336 as the particle transporter have been arranged and the facilitated with transport of Zn (II) and Cu (II) particles has been contemplated. The impact of layer membrane structure on the transport kinetic of Zn (II) and Cu (II) has been assessed. Zn (II) ions were preferably transported from the aqueous solutions containing Cu (II) and above 87% of Zn (II) ions were effectively recovered from the 0.5 M HCl solution as the source phase through PIM into 0.5 M H2SO4 as the stripping phase.
The solvent extraction of Zirconium and Hafnium from hydrochloric acid solutions using acidic organophosphorus extractants and their mixtures with TOPO was studied by Wang Yun Ling LY, et al. [28]. Solvent extraction of Zr and Hf from hydrochloric acid solution was performed by using acidic organophosphorus extractants and their mixtures with TOPO. In the HCl concentration range from 1 to 4 mol/dm3, solvation reaction was responsible for the extraction of Zr and Hf by single acidic organophosphorus extractants, such as D2EHPA, PC88A and Cyanex272. Although the extraction percentage of both metals by single and mixture of extractants increased with the increase of HCl concentration, the dependence of separation factor on the HCl concentration showed opposite behavior in both extractant systems. Synergistic extraction of Zr and Hf was acquired by the mixtures. Single Cyanex272 and a combination of Cyanex272 and TOPO were observed to be the most proficient in isolating the two metals.
The dissolvable extraction of copper (II) by synergistic combination of TOPO and Lauric acid was concentrated by Oukebdane K, et al. [29]. The solvent extraction of copper (II) by combinations containing tri-n-octyl phosphine oxide (TOPO) and lauric corrosive (LA) was considered. The effects of the concentration of the extracting agent in the organic phase and KCl in the water phase were investigated. The comparison between the results of extraction tests obtained with each separate extracting agent and those obtained with their mixtures, showed a synergistic effect. Yield of 100% in a single stage was attained in the cases of mixture. The ratio TOPO/LA in the complex in cyclohexane solution was determinated as 1:1. The complex formed in the organic phase may be (CuCl)2TOPO (LA-H)2. The coordination bonds of the Cucomplex were determined by infrared spectroscopy.
The solvent extraction of trivalent lanthanides with hexafluroacetone (HFAA) and tri-n-octylphosphineoxide (TOPO) was studied by Murthy KSR, et al. [30]. Trivalent lanthanides can be synergistically removed from aqueous solution as blended ligand compound with HFAA and TOPO with the general formula, Ln (HFAA)3. 2TOPO. The ideal condition for extraction like equilibration time, pH, impact of solvent, metal ion concentration and reagent concentration were set up.
The extractants used in present work are tri-n-octyl phosphine oxide (TOPO) and tri-n-octyl amine (TOA). Tri-n-octylphosphineoxide (TOPO) is an organophosphorous compound with the formula OP(C8H17)3. It is an air-stable white coloured solid at room temperature. It is an organic complexing agent and has been used as an analytical reagent for the extraction and separation of many metal ions. Tri-n-octyl amine (TOA) is an amine-based extractant. It is a liquid anion exchanger.
In the present investigation this extractant has been effectively used for the extraction of Co (II) from nitrate solution. The other extractant used along with TOA for study of extraction of cobalt is tri-n-octyl phosphine oxide (TOPO) which is a solvating solvent. Extraction of Co (II) was carried out by using mixture of extractants, TOA and TOPO.
Materials and Methods
The stock solution of Co (II) (0.01 M) was prepared by dissolving the required amount of cobalt nitrate, Co(NO3)2 in double distilled water. Samples of TOPO supplied by heavy metals, Talcher and TOA supplied by Sigma-Aldrich were used without further purification. Toluene was used as diluent. The stock solutions of TOPO (0.05 M) and TOA (0.05 M) were prepared by weighing the desired amount of extractants and diluting them in toluene. Working solutions of both aqueous (0.0005 M) and organic mixture solution (0.0025 M TOPO +0.0025 M of TOA) were prepared by diluting both the aqueous and organic stocks as per the requirement. All other reagents used were of analytical grade.
Extraction tests were done in a separating funnel by shaking equivalent aliquots (10 ml) of aqueous phase and organic phase with the assistance of a mechanical shaker for a time of 15 min, aside from time variety. After the disengagement of phase, the aqueous phase was analyzed to decide the metal concentration by utilizing shading creating reagents (10 mL KSCN, 2 mL N/2 HCl, 25 mL CH3)2CO) at 585 nm utilizing UV-noticeable spectrophotometer (SYSTRONICS 105). The concentration of metal ion in the organic phase was determined by the distinction of grouping of metal ion in the aqueous phase prior and then afterward extraction. Every one of the trials was done at 250°C temperature.
Results and Discussion
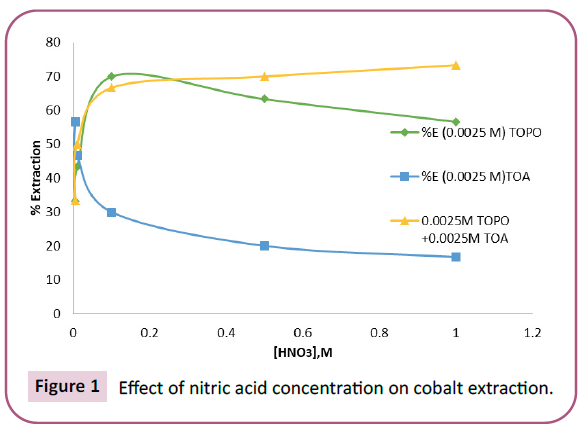
The extraction of Co (II) from 0.0005 M cobalt nitrate solution with 0.0025 M TOPO, 0.0025 M TOA and with mixture of 0.0025 M TOPO and 0.0025 M TOA were studied by varying the concentration of nitric acid from 0.005 M – 1 M. The percentage extraction of Co (II) using 0.0025 M TOPO increased from 33.33% at acid concentration 0.005 M to 70% at acid concentration 0.1 M and then decreased to 56.66% at acid concentration 1 M. The percentage extraction of Co (II) using 0.0025 M TOA decreased from 56.66% at acid concentration 0.005 M to 16.66% at acid concentration 1 M (Figure 1). The percentage extraction of Co (II) using mixture of 0.0025 M TOPO and 0.0025 M TOA increased from 33.33% at acid concentration 0.005 M to 73.34% at acid concentration 1 M.
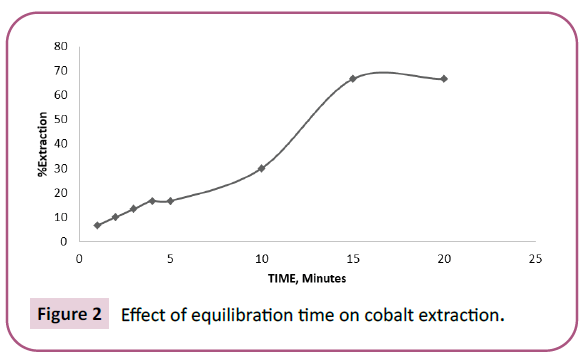
The effect of equilibration time on the extraction of Co (II) from the solution of 0.0005 M Co (NO3)2 and 0.1 M HNO3 using mixture of 0.0025 M TOPO and 0.0025 M TOA in toluene was studied. Experiments were carried out at room temperature to study the effect of equilibration time on the extraction of cobalt from 0.0005 M Co (II) solution in the range 1-20 minutes with mixture of 0.0025 M TOPO and 0.0025 M TOA in toluene. The percentage extraction of cobalt increased from 6.66% in 1 minute to 66.66% in 15 minutes and further increase in time has no effect on percentage extraction of cobalt. Hence in all the experiments 15 minutes of shaking time was maintained in Figure 2.
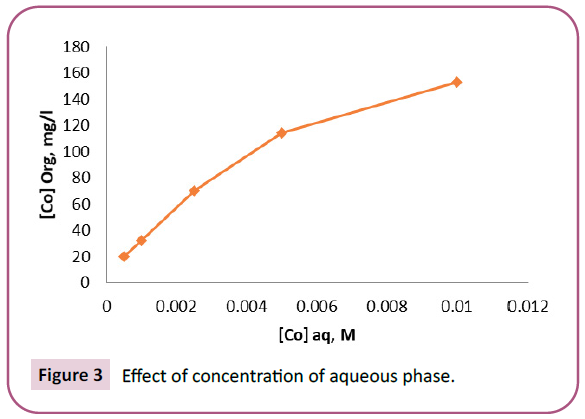
Different concentrations of Co (II) in the range 0.0005 M-0.01 M were used to study the effect of extraction of Co (II) using 0.1 M HNO3 and mixture solution of 0.0025 M TOPO and 0.0025 M TOA in toluene. The percentage extraction of Co (II) decreased with increase in cobalt concentration in the aqueous solution but the loaded cobalt in the organic phase increased with increase in cobalt concentration. For example, when concentration of Co (II) was 0.0005M (30ppm), concentration of cobalt, [Co] loaded in organic phase was 20 ppm and percentage extraction is 66.67% and when it was 0.01 M (590 ppm), [Co] loaded in organic phase was 153 ppm and percentage extraction is 25.93% as presented in Figure 3.
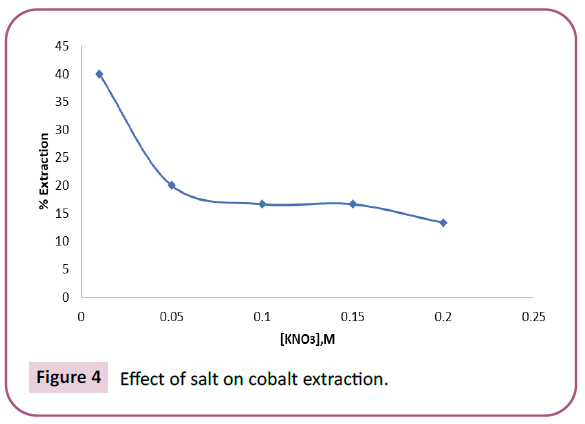
The different concentrations of KNO3 ranging from 0.01 M–0.2 M were used to know the involvement of nitrate ions in the extraction of cobalt from the solution of 0.0005 M Co (II) and 0.1 M HNO3 using mixture solution of 0.0025 M TOPO and 0.0025 M TOA in toluene. It was found that the extraction of metal decreased with increase in concentration of nitrate ions, found to be maximum at a concentration of 0.01 M and then decreased with further increase in concentration. A plot of % of Extraction vs. Concentration of KNO3 is shown in Figure 4.
To study the synergistic/ antagonistic effect on cobalt extraction, experiments were carried out with different concentrations of nitric acid using mixture solution of 0.0025 M TOPO and 0.0025 M TOA. It was found that ΔD i.e., Dmix- (DTOPO+DTOA) values were negative at lower concentration of acid, for example, when concentration of acid was 0.005 M, ΔD was -2.8 and then became positive with increase in concentration of acid i.e., 1.24 at acid concentration 1 M. The results are reported in Table 1. Antagonistic effect was first observed and then synergistic effect was observed. Antagonistic effect was observed at lower concentration of acid and from 0.1 M concentration of nitric acid synergistic effect was observed.
| Sl. No. | [HNO3], M | DTOPO | DTOA | Dmix | ΔD |
|---|---|---|---|---|---|
| 1 | 0.005 | 2 | 1.30 | 0.5 | -2.8 |
| 2 | 0.01 | 0.76 | 0.87 | 1 | -0.63 |
| 3 | 0.1 | 0.42 | 0.42 | 2 | 1.52 |
| 4 | 0.5 | 1.72 | 0.25 | 2.34 | 0.36 |
| 5 | 1.0 | 1.30 | 0.2 | 2.75 | 1.24 |
Table 1: Synergistic/Antagonistic effect on cobalt extraction. Aq: 10 mL 0.0005 M Co (II) and Org: 10 ml mixture solution of 0.0025 M TOPO and 0.0025 M TOA in toluene.
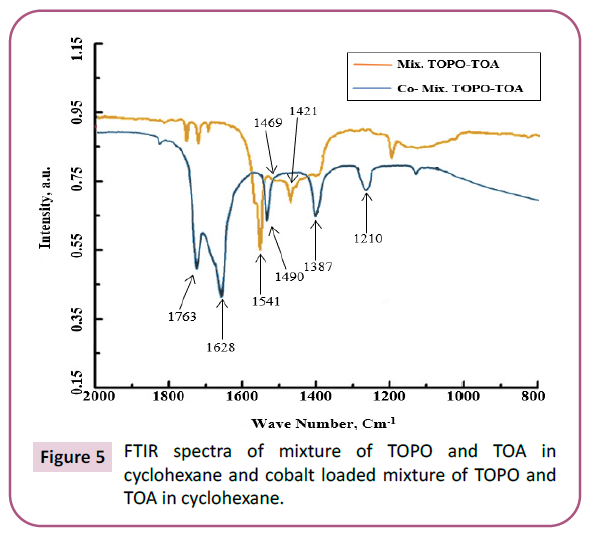
In order to observe the mechanism of extraction, FTIR spectra of mixture of trioctylphosphine oxide and trioctylamine (i.e. mix. TOPO-TOA) before and after extraction were noted. The spectra of the mixture extractant and loaded extractant are noted and as shown in the Figure 5. From the spectrum, it was noticed that a peak obtaining at 1541 cm-1 in case of mixture TOPO-TOA shifted to 1490 cm-1 for indicating N-CH2 symmetric-stretching vibration. The peak at 1490 cm-1 gave a more intense as well as a broad peak after loading. Also, a peak corresponding to CH3 bending vibration of mixture TOPO-TOA shifted from 1421 cm-1 to 1387 cm-1 loaded mixture TOPO-TOA. Another characteristic peak was obtained at 1210 cm-1 forming C-N stretching vibration.
Conclusion
The extraction of Co (II) from aqueous solution was carried out at room temperature using mixture of TOPO and TOA in toluene. It was observed that equilibration time was achieved in 15 minutes. Hence in all the experiments 15 minutes of equilibration time was maintained to ensure complete equilibration. The percentage extraction of Co (II) using 0.0025 M TOPO increased from 33.33% at acid concentration 0.005 M to 70% at acid concentration 0.1 M and then decreased to 56.66% at acid concentration of 1 M. The percentage extraction of Co (II) using 0.0025 M TOA was 56.66% at acid concentration 0.005 M and decreased to 16.66% at 1 M. The percentage extraction of Co (II) using mixture of 0.0025 M TOPO and 0.0025 M TOA increased from 33.33% at acid concentration 0.005 M to 73.34% at acid concentration 1 M. The percentage extraction of Co (II) using mixture of 0.0025 M TOPO and 0.0025 M TOA decreased from 66.67% to 25.93% with increase in concentration of the aqueous solution from 0.0005 M to 0.01 M. The percentage of extraction using mixture of TOPO and TOA was found to be insignificant in presence of the salt, KNO3. Antagonistic effect was observed at lower concentration of acid and from 0.1 M concentration of nitric acid synergistic effect was observed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors are very much thankful to Odisha University of Agriculture and Technology, Odisha and Centurion University of Technology and Management, Odisha for their heartly support to carry out the experiments.
References
- Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review. J Environ Manage 92:407-418.
- Wang J, Chen C (2014) Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour Technol 160:129-141.
- Hua M, Zhang S, Pan B, Zhang W, Lv L, et al. (2012) Heavy metal removal from water/wastewater by nano sized metal oxides: A review. J Hazard Mater 211:317-331.
- Sharma YC, Srivastava V, Singh VK, Kaul SN, Weng CH (2009) Nano- adsorbents for the removal of metallic pollutants from water and wastewater. Environ Technol 30:583-609.
- Ahmed SA (2011) Batch and fixed-bed column techniques for removal of Cu(II) and Fe(III) using carbohydrate natural polymer modified complexing agents. Carbohydro Polym 83:1470-1478.
- Malki M, Toril EG, Sanz JL, Gomez F, Rodriguez N, et al. (2006) Importance of the cycle in bio hydrometallurgy. Hydrometallurgy 83:223-228.
- Bourget C, Jakovljevic B, Nucciarone D (2004) CYANEX 301 binary extractants in cobalt/nickel recovery from acidic sulphate solutions. Hydrometallurgy 75:25-36.
- Zhang P, Yokoyama T, Suzuki TM, Inoue K (2001) The synergistic extraction of nickel and cobalt with a mixture of di (2-ethylhexyl) phosphoric acid and 5-dodecylsalicylaldoxime. Hydrometallurgy 61:223-227.
- Allal KM, Hauchard D, Stambouli M, Pareau D, Durand G (1997) Solvent extraction of titanium by tributylphosphate, trioctylphosphine oxide and decanol from chloride media. Hydrometallurgy 45:113-128.
- Okatan A, Eyüpoğlu V, Kumbasar RA, Turgut Hİ (2016) Synergistic extraction and separation of Co (II)/Ni (II) by solvent extraction technique using TIOA/TOPO as carriers. AIP Conf Proc 1726: 02011
- Peligot EM (1842) Recherches sur l’uranium. Ann Chim Phys 5: 47.
- Morrison GH, Freiser H (1957) Solvent extraction in analytical chemistry. J Am Pharm Assoc.
- Marcus Y, Kertes S (1969) Ion Exchange and solvent extraction of metals complexes. Wiley-Interscience, New York, London.
- De AK, Khopkar SM, Chalmers RA (1970) Solvent extraction of metals. Van Nostrand-Reinhold co, New York, London.
- Sekine T, Hasegawa H (1977) Solvent extraction chemistry, fundamentals and applications. Marcel Dekker, New York.
- Ritcey GM, Ashbrook AW (1984) Solvent extraction: Principles and applications to process metallurgy. Elsevier, Amsterdam.
- Rydberg J, Musikas C, Choppin GR (1992) Principles and practices of solvent extraction. Marcel Dekker, New York.
- Sahu SK (2000) Liquid-liquid extraction of trivalent lanthanides and some early actinides.
- Rice NM (1978) Recent developments and potential uses for carboxylic acid extractants- A review. Hydrometallurgy 3:111-133.
- Shmidt VS (1987) Some problems of the development of the physicochemical principles of modern extraction technology. Russ Chem Rev 56:792.
- Nernst W, Physik Z. Chem (1891), 8,110.
- Kosyakov V, Yerin E, Vitutnev V (1980) The use of trioctylphosphine oxide for solvent extraction recovery and purification of transplutonium elements. J Radioanal Nucl Chem 56:83-92
- Cheng CY (2006) Solvent extraction of nickel and cobalt with synergistic systems consisting of carboxylic acid and aliphatic hydroxyoxime. Hydrometallurgy 84: 109-17.
- Adjel F, Barkat D (2011) Synergistic extraction of copper (II) from sulfate medium with capric acid and tri-n-octylphosphine oxide in chloroform. J Coord Chem 64: 574-582.
- Krea M, Khalaf H (2000) Liquid-liquid extraction of uranium and lanthanides from phosphoric acid using a synergistic DOPPA-TOPO mixture. Hydrometallurgy 58: 215-225.
- Girgin S, Acarkan N, Sirkeci AA (2002) The uranium (VI) extraction mechanism of D2EHPA-TOPO from a wet process phosphoric acid. J Radioanal Nucl Chem 251: 263-271.
- Pospiech B (2014) Synergistic solvent extraction and transport of Zn (II) and Cu (II) across polymer inclusion membranes with a mixture of TOPO and Aliquat 336. Sep Sci Technol 49:1706-1712.
- Wang LY, Lee HY, Lee MS (2013) Solvent extraction of zirconium and hafnium from hydrochloric acid solutions using acidic organophosphorus extractants and their mixtures with TOPO. Mater Trans 54: 1460-1466.
- Oukebdane K, Didi MA, Azzouza A, Villeminb D (2004) Solvent extraction of copper (II) by synergistic mixtures of trioctylphosphine oxide and lauric acid. Sci. Study Res: Chem Chem Eng (1-2): 59-64.
- Murthy KSR, Krupadam RJ, Anjaneyulu Y (1997) The solvent extraction of trivalent lanthanides with hexafluroacetone (HFAA) and tri-n-octylphosphineoxide (TOPO). Proc Indian Acad Sci 110: 83-88.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences