ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Learning and Memory Enhancing Effects of BacoLive ® (An Enriched Composition of Bacopa monnieri Extract) in Scopolamine Induced Memory Impaired Mice
R&D Department of Phytochemistry, Olive Life sciences Pvt Ltd, Nelamangala, Karnataka, India
- *Corresponding Author:
- Sudhakar A
R&D Department of Phytochemistry
Olive life sciences Pvt Ltd, No. 5805/05
NH-4, Nelamangala - 562123, Bangalore, Karnataka, India
Tel: + 9607156596
E-mail: sudhakar@olivelifesciences.com
Received Date: June 28, 2021; Accepted Date: July 16, 2021; Published Date: July 20, 2021
Citation: Sudhakar A, Shantakumar J, Prasad CP, Joseph MV (2021) Learning and Memory Enhancing Effects of Bacolive® (An Enriched Composition of Bacopa monnieri Extract) in Scopolamine Induced Memory Impaired Mice. Am J Phytomed Clin Ther Vol.9 No.7:32.
Abstract
Objective: The objective of this study was to evaluate the learning and memory enhancement activity of BacoLive® (a composition enriched with bacosides from Bacopa monnieri) in various experimental models i.e., passive shock avoidance test, Y- maze test and novel object recognition test.
Methodology: BacoLive® was administered for 7 days at the dose of 100, and 200 mg/kg to mice in passive shock avoidance test, Y-Maze test and in novel object recognition test. Scopolamine (0.5 mg/kg) was used to induce memory (amnesia) loss and piracetam (200 mg/kg) served as reference standard. After 7 days, cognitive and memory scales were measured as per standard test protocols.
Results: In the passive shock test for memory, BacoLive ® reduced the latency to reach the shock free zone compared to amnesia induced group. In the Y-maze test, the number of arm entries failed to memorize the new space was decreased with BacoLive ® group compared to amnesia induced group. In the new object recognition test, BacoLIve ® improved the percentage preference for new object recognition compared to amnesia induced group.
Conclusion: Based on the present study and findings, BacoLive® demonstrated learning and memory enhancing effects and reversed memory loss effects induced by scopolamine in mice, which suggest its beneficial role in conditions associated with memory and learning dysfunctions. Study indicates that bacopa extract justifies its traditional use in Ayurveda for memory related complications associated with neuro degeneration and stress.
Keywords
BacoLive®; Bacopa monnieri; Bacosides; Memory; Cognition; Neuro degeneration; Stress; Scopolamine; Passive shock avoidance test; Y- Maze test; Novel object recognition test
Introduction
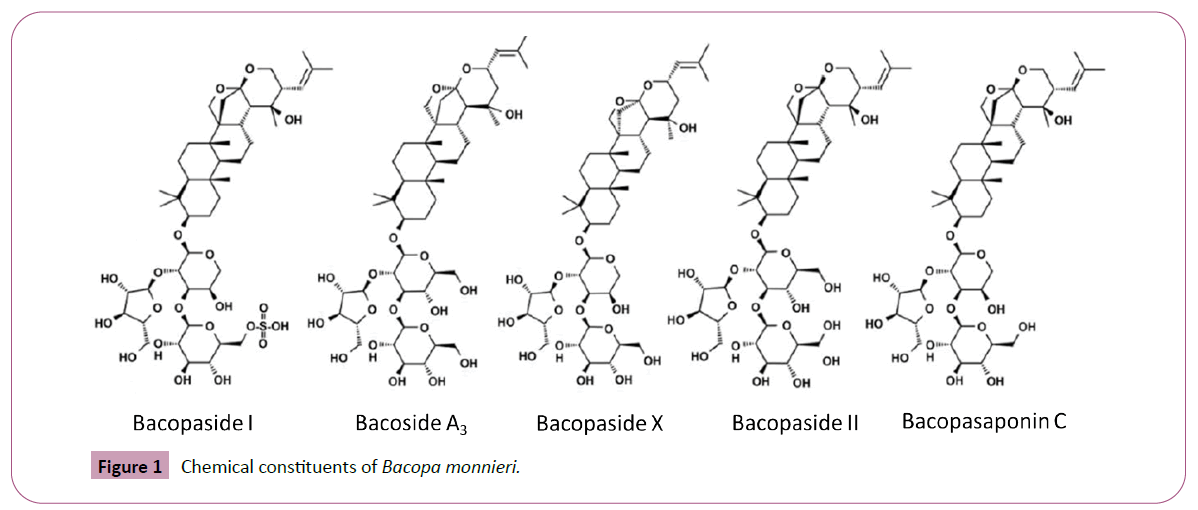
Cognition is a term referring to the mental processes involved in gaining knowledge and comprehension, including thinking, knowing, remembering, judging, and problem solving. These are higher-level functions of the brain and encompass language, imagination, perception, and planning [1]. Cognitive impairment is one of the major health problems in normal aged life as well as in some neurological disease conditions like Alzheimer’s, stroke and Parkinson [2]. The short term or working memory in humans and animals can be disrupted by anticholinergic drugs, like scopolamine, which blocks muscarinic receptors in brain regions. Scopolamine frequently used as amnesic agent for evaluation of anti-amnesic effect of new drugs. In view of the adverse effects of synthetic drugs [3] there is search for natural agents which are safe, effective and may provide a new source of beneficial neuro psychotropic drugs [4]. There are Several studies showing the neuro protective or cognition-enhancing properties of natural products and their components in in vivo and in vitro experimental models [5,6]. Bacopa monnieri (Known as water hyssop and Brahmi) is a creeping perennial herb with small oblong leaves and native to wetlands of southern and Eastern India. It belongs to Plantaginaceae family. Bacopa monnieri is well known in traditional ayurvedic medicine and used to alleviate variety of disorders including neuronal stress [7]. The entire plant is used medicinally in Ayurveda and was initially described. in texts such as the Charaka Samhita, and Sushruta Samhita as a medhya rasayana class of herb, taken to sharpen intellect and attenuate mental deficits. [8]. Bacosides are the major constituents of B. monnieri and these actives were found to ease the learning and memory in normal rats and found to inhibit the amnesic effects induced by scopolamine, electroshock and immobilization stress [9]. The cognition facilitating activity of B. monnieri extract is attributed to the saponins, Bacosides (Figure 1), which are effective in much lower doses in various studies. In another study, improved anticholinesterase and anti-dementic activities in scopolamine induced amnesic mice are shown by B. monnieri extract [9]. In addition, B. monnieri possesses antioxidant [10,11] anti-stress [12] anti-fatigue [13] memory enhancing, anxiolytic [14] and neuroprotective properties [15]. Hence, the current study was undertaken to explore the anti-amnesic effects of BacoLIve® on scopolamine-induced amnesia in various learning and memory test models.
Materials and Methods
Botanical Identification of B. Monnieri
The identity of this plant was confirmed by taxonomists. The voucher specimen sample of B. Monnieri was deposited in Phytochemistry R&D Dept at Olive Lifesciences Pvt. Ltd.
Preparation of test substance BacoLive®
Study material BacoLive® is prepared from whole herb of Bacopa monnieri (Figure 2a) by using aqueous ethanol extraction process. BacoLive® is standardized to contain at least 50% of Bacosides by HPLC (USP method). Dried herb (Figure 2b) is ground until it becomes a coarse powder, extracted using aqueous ethanol 70%, stirred at 60-65°C for 2-3 h and filtered. Extraction continued for 3 more cycles and combined extracts are then filtered under vacuum and evaporated using a rotary evaporator at 50-60°C to obtain a viscous extract. The extract is then washed to remove the resinous/Chlorophyll impurities. Further the extract is purified selectively for enrichment of Bacosides, filtered and wet cake was dried at 60-70°C under vacuum to become powder. This product (Figure 2c) is further standardized to contain 50% Bacosides by HPLC.
Standardization of BacoLive®
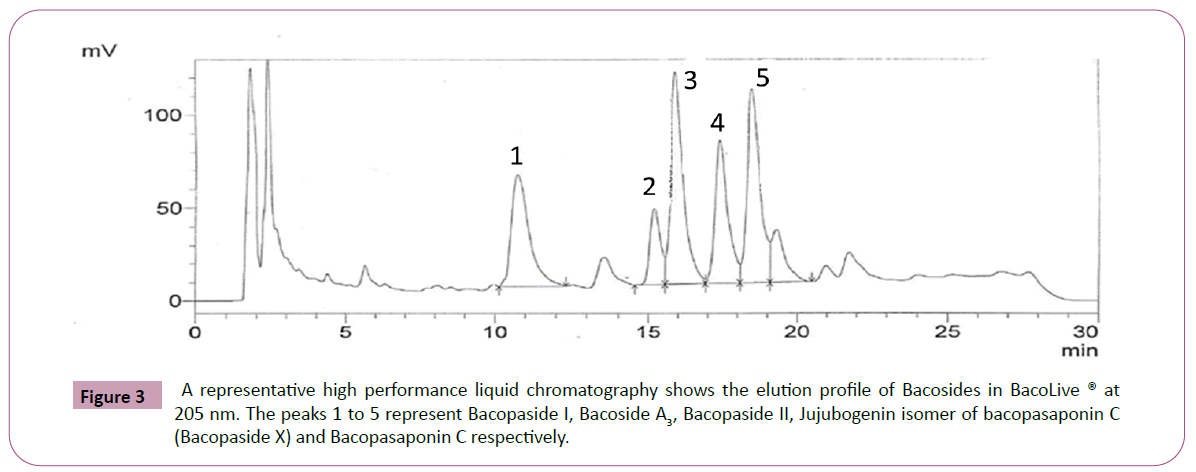
Extraction and purification processes are standardized for BacoLive®. Analytical method (USP) was standardized by HPLC. The triterpene glycosides in Bacopa extract are reported as the sum of Bacopaside I, Bacoside A3, Bacopaside II, Jujubogenin isomer of Bacopasaponin C (Bacopaside X) and Bacopasaponin C and are quantified by using HPLC method. The peaks were separated by a Gradient elution in Shimadzu 2010 HPLC with UV/ PDA detector at 205 nm using a C18, 250 × 4.6 mm, 5 μ Thermo BDS column. Column temperature is set at 27°C. Mobile phase (Acetonitrile: water: Phosphoric acid, 90:10:0.1) and 20 μL of standard and samples are injected with a run time of 30 min. The peaks 1-5 represent the bacosides as shown in the (Figure 3). In addition to these compounds, Bacolive® also consists of other compounds i.e., Bacopasides N1, N2, III, IV, V and Bacopasaponins F and E, which are seen as additional peaks in the chromatogram. Polyaromatic hydrocarbons (PAHs) are tested by GC-MS and ensured that BacoLive® is complying with European regulations. Residual solvents are tested by GC-HS and ensured that BacoLive® complies with European Pharmacopoeia
Chemicals and reagents
Scopolamine was obtained from German Remedies and standard Drug Piracetam capsules obtained from Micro labs, Bangalore. Test Substance, BacoLive® was obtained from Olive life sciences Pvt. Ltd, Bangalore.
Experimental animals
Total 30 Swiss Albino mice of either sex were supplied by an accredited supplier and were divided into 5 groups. Each group containing 6 animals were used in present study. The animals were housed in groups of three in polycarbonate cages with paddy husk bedding. The diet and drinking water were free from any contaminants which might affect the purpose or integrity of the study. The temperature and humidity were set to achieve limits of 18-23°C and 40-70% respectively with 12 hr light and dark cycle.
Grouping and treatment protocol
In passive shock avoidance test, Y- Maze test and Novel object recognition test, mice in 5 groups of six each were used. Groups are divided as follows.
Group I: Control
Group II: Control + Scopolamine (0.5 mg/kg/I.P.) (Memory loss induced group)
Group III: Control + Scopolamine (0.5 mg/kg/I.P.) + Piracetam (200 mg/kg)
Group IV: Control + Scopolamine (0.5 mg/kg/I.P.) + BacoLive® extract (100 mg/kg)
Group V: Control + Scopolamine (0.5 mg/kg/I.P.) + BacoLive® extract (200 mg/kg)
In passive shock avoidance test, Y- Maze test and Novel object recognition test, Group I was considered as control. Group II was injected with scopolamine on 8th day to induce memory loss. Group III, IV & V were treated with Scopolamine Hydro bromide (0.5 mg/kg; I.P) 45 minutes prior to administration of test substance/ standard drug (Piracetam 200 mg/kg; I.P). Drug treatment was given to all groups for 8 days.
Passive shock avoidance test for long-term memory
The passive shock avoidance test was used to examine the longterm memory of animals. Passive avoidance training was done as follows. The mice were put individually on the electric grid and allowed to explore for 1 min. The electric shock (20v) was then applied and latency to reach SFZ recorded three consecutive times as basal readings. Animals that reached the SFZ in 2 min in the first trial were selected for the study. After I hr of the training, each animal was put on the electric grid again and the latency to reach SFZ and the number of mistakes (descents) the animal made in 15 min were recorded as parameters for acquisition and retention respectively [16].
Method: Grouping and treatment followed as per the protocol. On the 8th day, mice were placed individually on the electric grid and allowed to explore the maze for 1 minute. 90 minutes after administration of test/standard substance, the stimulus (20 V) with AC current of 5 mA was applied and latency to reach the shock free zone (SFZ) was recorded. Retention of this learned task (memory) was examined 24h after the last dose (i.e., 9th day). Significant reduction in latency to reach SFZ indicated improvement of memory.
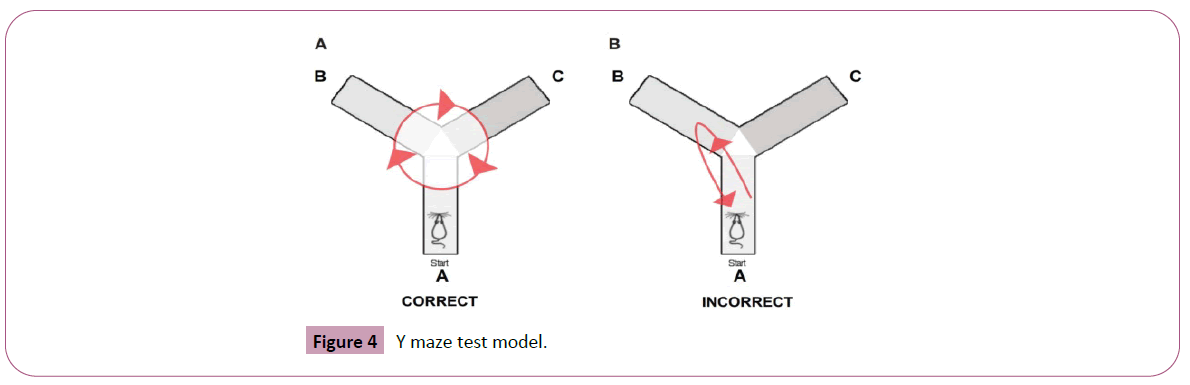
Y-maze test for spatial working memory
The Y-maze test is used to measure spatial working memory through spontaneous alteration of behaviour in mice. The Y-maze is a three-arm maze made of black painted wood with an angle of 120° between each of the two arms (Figure 4). Each arm is 40 cm long, 3 cm wide and 13 cm high. The three identical arms are randomly designed: start arm, in which the animal starts to explore (A); reward arm, with food stimuli (B) and other arm (C). Each rat was initially placed at the end of arm A, allowed to move freely and the sequence and number of arm entries were recorded manually over 8 min period. Rats tend to explore the maze systematically, entering each arm in turn. The ability to alternate required that the rats knew which arm they had already visited. The percentage of triads in which all three arms were represented, i.e., ABC, CAB, or BCA but not BAB, was recorded as an ‘alteration’ to estimate short term memory. Arms were cleaned with water spray between tests to remove odors and residues. The number of arm entries was used as an indicator of loco motor activity [17].
Method: Grouping and treatment followed as per the protocol. On day 8, after 30 minutes of amnesic agent induction, trails were taken on Y-maze and retention was observed on 8th and 9th day. Briefly, each time animal was placed just inside arm B facing away from centre and allowed to move through apparatus for 8 min, while being monitored by tracking system. Trial was terminated at the specified time duration. Each arm entry (defined as all four paws entering arm) was scored and recorded. Animals were returned to home cage and number of fecal pellets was counted in the Y-maze and data was recorded. Y-Maze was cleaned with alcohol between trials of each animal.
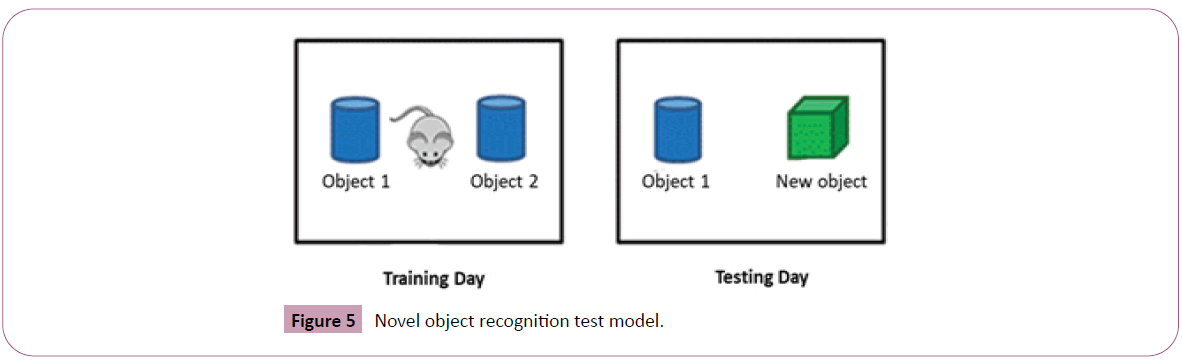
Novel object recognition test
Novel object recognition (NOR) test is one of the best-known tests used for assessing recognition memory in rats and mice. It has become increasingly useful tools for basic and preclinical research investigating the neural basis of memory. The apparatus consists of a Plexiglas box is 40 cm × 40 cm × 40 cm. The colour of Plexiglas is frosted white or transparent. The size of objects is 4 cm × 4 cm × 4 cm approximately. The shape of objects is simple like the building blocks. The objects made by wood, metal or hard plastic are preferred, because those are resistant to biting demolition [18] (Figure 5).
Method: Grouping and treatment followed as per the protocol. The mice were treated as per manipulated environment for decreasing their stress. Mice were first transported to preparing area located near the operative space for 10 min. Then the mice are put into the apparatus for analysis for 10 min to acclimate.
This procedure is carried out for two days. On day 3 (training day), similar to Day 1 and 2, mice were first transported to preparing area located near for the operative space for 10 min. Two identical objects were placed in the boxes for mice to explore for 10 min. On day 4 (Testing Day) all the processes were same as the Day 3, except that one of old objects is randomly replaced with a new different object. The exploration time were counted by the period that mice stay near the new object. If the total of exploration time found less than 20 sec, mouse were likely under the stress and data should be excluded. All groups are stressed and tested for exploring new object. Time spent on exploring new object by each group of animals is noted. Results are interpreted based on % increase in time in exploring the new object by STD & treatment group. This indicates ability of each group animals to explore new object recognition under stressed condition. The preference rates were measured by dividing the total exploration time by the time to explore object. The formula to calculate the object preference is:
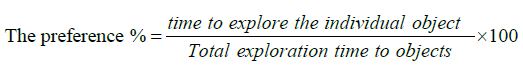
Results
Passive shock avoidance test for recall memory
Scopolamine (0.5 mg/kg, I.P.) significantly increased (p< 0.01) latency to reach Shock Free Zone SFZ as compared to control group indicating impairment of memory. BacoLive® (100 and 200 mg/kg p.o) administered for 8 days reversed the amnesia induced by scopolamine and improved memory as evidenced by dose dependent decrease in latency to reach SFZ when compared to scopolamine group. The group of mice treated with standard non tropic agent Piracetam (200 mg/kg, I.P.) showed reversal of amnesia induced by scopolamine and improved memory which is evident by decrease in latency to reach SFZ (Table 1).
| Group | Treatment | Latency to reach SFZ (Sec) |
|---|---|---|
| Â I | Control | 15.7 |
| II | Control + Scopolamine (0.5 mg/kg/i.p.) (memory loss induced group) | 36.3 |
| III | Control + Scopolamine (0.5 mg/kg/i.p.) + Piracetam (200 mg/kg) | 17.2 |
| IV | Control + Scopolamine (0.5 mg/kg/i.p.) + BacoLive® extract (100 mg/kg) | 31.2 |
| V | Control + Scopolamine (0.5 mg/kg/i.p.) + BacoLive® extract (200 mg/kg) | 24.3 |
Table 1: Effect of BacoLive® on the latency to reach Shock Free Zone in passive shock avoidance test model in Swiss albino mice.
Y- Maze Test for spatial memory
Group II exhibited a higher spontaneous alteration (repeated arm entry to old space), elaborating the amnestic effects of scopolamine, which indicates number of animals failed to memorize the new space are more. For Group III, 1V & V there was a decrease in number of arm entries indicating that amnesic effects are decreased in a dose dependent manner (Table 2).
| Group | Treatment | Number of arm entries |
|---|---|---|
| I | Control | 24 |
| II | Control + Scopolamine (0.5 mg/kg/i.p.) (memory loss induced group) | 31 |
| III | Control + Scopolamine (0.5 mg/kg/i.p.) + Piracetam (200 mg/kg) | 17 |
| IV | Control + Scopolamine (0.5 mg/kg/i.p.) + BacoLive® extract (100 mg/kg) | 27 |
| V | Control + Scopolamine (0.5 mg/kg/i.p.) + BacoLive® extract (200 mg/kg) | 22 |
Table 2: Effect of BacoLive® on reduced number of arm entries compared to amnesia group in Y- Maze test model in Swiss albino mice.
Novel object recognition test
Scopolamine (0.5 mg/kg, I.P.) significantly decreased the percentage of mice exploring the new object, indicating impairment of memory. The mice treated with Groups III, IV & V showed reversal of amnesia induced by scopolamine and improved memory which is evident by increase in tendency to recognize the new object in a dose dependent manner (Table 3).
| Group | Treatment | % Preference for New object recognition |
|---|---|---|
| I | Control | 66.00 |
| II | Control + Scopolamine (0.5 mg/kg/i.p.) (memory loss induced group) | 58.90 |
| III | Control + Scopolamine (0.5 mg/kg/i.p.) + Piracetam (200 mg/kg) | 65.97 |
| IV | Control + Scopolamine (0.5 mg/kg/i.p.) + BacoLive® extract (100 mg/kg) | 62.52 |
| V | Control + Scopolamine (0.5 mg/kg/i.p.) + BacoLive® extract (200 Â mg/kg) | 64.61 |
Table 3: Effect of BacoLive® on percentage preference for new object recognition compared to amnesia group in Swiss albino mice.
Discussion
Brain health can be affected by stress, age-related changes in the brain, injuries such as stroke or traumatic brain injury, mood disorders such as depression, substance use or addiction, diseases such as Alzheimer’s, insomnia and unhealthy lifestyle habits [19]. The process of storing sequence of information in a systematic manner by which one becomes aware of their surroundings, objects and thoughts is known as Cognition [20]. In normal aged life as well as in some neurological disease conditions like Alzheimer’s, Cognitive impairment is one of the major health problems. The degeneration and dysfunction of cortical cholinergic neurons are closely associated with cognitive dysfunction [21]. Memory tests are important indexes of the brain functions for rodent’s behavior assay. Many memory tasks require external forces (e.g., electric shocks) or intrinsic forces (e.g., hunger and thirstiness) to trigger the responses. Under these conditions, rodents are under stress such as pain, tiredness, malnutrition or dehydration which potentially affects the natural neural responses. Effect of agents on memory process and learning can be evaluated in in-vivo studies by using various experimental models like Mazes that are presently available. Spatial long-term memory in experimental animals is measured by Y- maze [22,23].
The long-term memory based on negative reinforcement is examined by Passive avoidance behaviour [24]. Non-spatial memory with the features of episodic memory is measured by Object recognition test [25]. In the present study, the learning and retention in all the models tested in normal mice found to be improved by using BacoLive®. These findings are supported by earlier studies in literature in which the alcoholic extract of B. monnieri and its bacosides constituents have shown similar effects in animal models [26,27]. In addition, BacoLive® also found to protect the animals from scopolamine induced impairment in learning and memory. This is evident from the reports showing scopolamine induced amnesia was inhibited by the extracts of B. monnieri [28-30]. These results indicate that BacoLive® can be used for improving memory. Also, the Protective effect of BacoLive® on scopolamine induced amnesia indicates its usefulness in neurodegenerative diseases.
Conclusion
Based on the present study and findings, BacoLive® demonstrated its effectiveness in learning and memory enhancing effects and reversed cognitive deficits induced by scopolamine in mice, which suggests its beneficial role in conditions associated with memory dysfunctions. Current study is an attempt to revalidate Bacopa monnieri extract (BacoLive®) using a scientific approach, which supports the basis of its use in Ayurveda System for enhancing memory & brain health.
Acknowledgement
We acknowledge Radiant Research Services Pvt Ltd, Bangalore, India for providing the facility for conducting the trials.
References
- APA Dictionary of Psychology (2018) Cognition. American Psychological Association.
- Vasudevan M, Milind P (2007) Memory enhancing activity of Thespesia populnea in rats. Pharm Biol 45: 267–273.
- Gijtenbeek JMM, Van Den Bent MJ, Vecht CJ (1999) Cyclosporine neurotoxicity: A review. J Neuro 246: 339–346.
- Johnson WC, William OW (2002) Warfarin toxicity. J Vasc Surg Cases 35: 413–431.
- Rabiei Z, Hojjati M, Rafieian-Kopaeia M, Alibabaei Z (2013) Effect of Cyperus rotundus tubers ethanolic extract on learning and memory in animal model of Alzheimer. Biomed Aging Pathol 3: 185–191
- Rabiei Z, Rafieian Kopaeia M, Heidarian E, Saghaei E, Mokhtari S (2014) Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus Basalis of Meynert in rat. Neurochem Res 39: 353–360.
- Singh HK, Dhawan BN (1997) Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn (Brahmi). Ind J Pharmacol 29: 359–365.
- Joshi H, Parle M (2006) Brahmi rasayana improves learning and memory in mice. Evidence-Based Complementary and Alternative Medicine 3: 79–85.
- Dhawan BN, Singh HK (1996) Pharmacological studies on Bacopa monnieri an ayurvedic nootropic agent. Eur Neuropsychopharmacol 3: 144–150.
- Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S (2000) Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum and hippocampus. Phytother Res 14: 174–179.
- Bhattacharya SK, Kumar A, Ghosal S (2000) Effect of Bacopa monniera on animal models of Alzheimer’s disease and perturbed central cholinergic markers of cognition in rats. In: Siva Sanka DV (ed) Molecular aspects of Asian medicines. PJD, New York, USA. p. 21–32.
- Chowdhuri DK, Parmar D, Kakkar P, Shukla R, Seth PK, et al. (2002) Anti-stress effects of bacosides of Bacopa monnieri: Modulation of HSP-70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res 16: 639–645.
- Anand T, Phani Kumar G, Pandareesh MD, Swamy MSL, Khanum F, et al. (2012) Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Phytother Res 26: 587–593.
- Ernst E (2006) Herbal remedies for anxiety-A systematic review of controlled clinical trials. Phytomedicine 13: 205–208.
- Dhanasekaran M, Tharakan B, Holcomb LA, Hitt AR, Young KA, et al. (2007) Neuroprotective mechanisms of ayurvedic antidementia botanical Bacopa monniera. Phytother Res 21: 965–969.
- Kulkarni SK, Verma A (1992) Evidence for Nootropic effect of BR-16A (MENTAT®), Herbal Psychotropic preparation in mice. Indian J Physiol Pharmacol 36: 29-34.
- Bhattacharjee A, Shashidhara SC, Saha S (2015) Nootropic activity of Crataeva Nurvala Buch Ham against scopolamine induced cognitive impairment. EXCLI Journal 14: 335-345.
- Huang TN, Hsueh YP (2014) Novel object recognition for studying memory in mice. Bio-Protocol p. 4
- Mintzer J, Donovan KA, Kindy AZ, Lock SL, Chur LR, et al. (2019). Lifestyle Choices and Brain Health. Front Med
- Ashutosh A, Malini S, Bairy KL, Muddanna SR (2002) Effect of Tinospora cordifolia on learning and memory in normal and memory deficit rats . Ind J Pharmacol 34: 339–349.
- Bartus RT, Dean RL, Beer B, Lippa AS (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 21: 408–414.
- Rajesh V, Riju T, Venkatesh S, Babu G (2017) Memory enhancing activity of Lawsonia inermis Linn. Leaves against scopolamine induced memory impairment in Swiss albino mice. Orient Pharm Exp Med 17 : 127-142.
- Sivasangari Rajan KE (2020) Standardized Bacopa monnieri Extract Ameliorates Learning and Memory Impairments through Synaptic Protein, Neurogranin, Pro-and Mature BDNF Signaling, and HPA Axis in Prenatally Stressed Rat Offspring. Antioxidants 9: 1229.
- Reddy DS (1997) Assessment of nootropic and amnestic activity of centrally acting agents. Ind J Pharmacol 29: 208-221.
- Ennaceur A, Delacour J (1988) A new one-trial test for neurobiological studies of memory in rats. Behavioral data Behav. Brain Res 31: 47-59.
- Singh HK, Dhawan BN (1982) Effect of Bacopa monniera Linn (brahmi) extract on avoidance responses in rat. J Ethnopharmacol 5: 205-214.
- Singh HK, Dhawan BN (1997) Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monniera Linn (Brahmi). Ind J Pharmacol 29: 359-365.
- Dhawan BN, Singh HK (1996) Bombay, Abstr. Int Conv Biol Psychiat p. 59
- Kishore K, Singh M (2005) Effect of bacosides, alcoholic extract of Bacopa monniera Linn (brahmi), on experimental amnesia in mice. Indian J Exp Biol 43: 640-645.
- Das A, Shanker G, Nath C, Pal R, Singh S, et al. (2002) A comparative study in rodents of standardized extracts of Bacopa monniera and Ginkgo biloba: Anticholinesterase and cognitive enhancing activities Pharmacol Biochem Behav 73: 893-900.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences