ISSN : 0976 - 8688
Der Pharmacia Sinica
LC-MS/MS Method Development and Validation of Mifepristone and its Metabolites in Human Plasma
Muhammed Rafsal KK* and Sijo Pattam
National College of Pharmacy, Manassery, Kerala, India
Abstract
A rapid, specific and sensitive liquid chromatography-ESI mass spectrometry method was developed and validated for determination mifepristone and its metabolites in human plasma using levonorgestrel as internal standard. The extraction was performed by solid phase extraction procedure using waters Oasis HLB cartridge (1 cc, 30 mg). The column used was Hypurity C18 column (50 × 4.6 mm, 5 µm), the mobile phase was a combination of methanol water containing 0.2% Acetic acid at a ratio of 75:25 in isocratic mode. The flow rate was 0.5 ml/min with an injection volume of 2 µl and the total run time was 4 min. The detection was performed on a triple guard electron spray ionization mass spectrometry by selective reaction monitoring (SRM) mode. The target ions were monitored at [m+H] + m/z 430.3→134, 416.3→120, 446.3→109.1 for Mifepristone, N-Demethyl Mifepristone and Hydroxy Mifepristone respectively. Linearity was established in the range of 51.89 ng/ml to 4059.14 ng/ml, 51.9063 ng/ml to 4059.69 ng/ml, 12.68 ng/ml to 992.36 ng/ml for the determination of Mifepristone, N-Demethyl Mifepristone and Hydroxy Mifepristone respectively in K3EDTA using solid Phase Extraction procedure. The lower limit of quantification was found and reproducible (LLOQ) at 50.95 ng/ml, 54.11 ng/ml and 12.81 ng/ml for Mifepristone, N-Demethyl Mifepristone and Hydroxy Mifepristone respectively.
Keywords
LC-MS/MS method development, Solid phase extraction procedure, Hypurity C18 column
Introduction
Mifepristone (RU38486) is a progesterone receptor antagonist clinically. It is a synthetic drug used as an abortifacient. It is a 19-norsteroid with potent competitive anti-progestational and significant anti-glucocorticoid as well as anti-androgenic activity. RU38486 used to reduce glucocorticoid receptor activation on potential therapy in the metabolic syndromes and depression, used as investigational tool to dissect hypothetical-pituitary axis which regulates glucocorticoid production. The dose used for anti-glucocorticoid therapy is comparatively less compared to gynecological purpose [1]. Previous method of quantitative analysis of RU38486 includes radio immune assay however antibody used has cross reactivity provides more preference for HPLC analysis. Homer et al. developed a quantitative analysis of RU38486 by HPLC triple quadrapole mass spectrometry with a linearity range of 0.5-500 ng (r2=0.997) and a Limit of Detection (LOD) at 50 pg injected on column [2]. Guo et al. developed an HPLC method for the determination of mifepristone in human plasma with a linearity range of 10 ng/ml to 20 μg/ml (r2=0.9991) and an LOD of 6 ng/ml [3] whereas Tang et al. was developed a method for simultaneous determination of mifepristone and monodemethyl mifepristone in human plasma by liquid chromatography-tandem mass spectrometry method using levonorgestrel as internal standard with a linearity range of 5-2000 ng/ml for mifepristone and monodemethyl mifepristone with an LOD of 1.0 ng/ml for both analytes [4]. Whereas Guo et al. developed a HPLC method for simultaneous determination of rivanol and mifepristone in human plasma by solid phase extraction method, linearity was found as 5-100000 ng/ml and an LOD of 3 ng/ml [5]. In this project aimed to develop a highly specific and accurate method for analysis of mifepristone and its metabolites(N-demethy mifepristone and hydroxyl mifepristone) in human plasma by using LC-MS/MS (Tables 1 and 2).
| Mifepristone | N-demethyl Mifepristone | Hydroxy Mifepristone | ||||||
|---|---|---|---|---|---|---|---|---|
| Analyte Area | IS Area | Analyte Area | IS Area | Analyte Area | IS Area | |||
| 1275 | 16611 | 1485 | 16611 | 462 | 16611 | |||
| 1270 | 16406 | 1461 | 16406 | 408 | 16406 | |||
| - | 1376 | 18081 | 1457 | 18081 | 505 | 18081 | ||
| 1305 | 18284 | 1450 | 18284 | 439 | 18284 | |||
| 1382 | 17615 | 1485 | 17615 | 445 | 17615 | |||
| 1186 | 19392 | 1493 | 19392 | 426 | 19392 | |||
| AVERAGE | 1299.00 | 17731.50 | 1471.83 | 17731.50 | 447.50 | 17731.50 | ||
| SD | 73.54 | 1114.51 | 17.94 | 1114.51 | 33.52 | 1114.51 | ||
| % CV | 5.66 | 6.29 | 1.22 | 6.29 | 7.49 | 6.29 | ||
Table 1: Specificity and selectivity test for mifepristone analyte and internal standard (Aqueous)
| Mifepristone | N-demethyl Mifepristone | Hydroxy Mifepristone | ||||
|---|---|---|---|---|---|---|
| Analyte Area | IS Area | Analyte Area | IS Area | Analyte Area | IS Area | |
| 1048 | 14361 | 1177 | 14361 | 336 | 14361 | |
| 1197 | 13799 | 1146 | 13799 | 371 | 13799 | |
| - | 1062 | 14197 | 1314 | 14197 | 375 | 14197 |
| 1139 | 16134 | 1499 | 16134 | 398 | 16134 | |
| 1020 | 14515 | 1285 | 14515 | 388 | 14515 | |
| 1110 | 16288 | 1366 | 16288 | 351 | 16288 | |
| 1226 | 15454 | 1315 | 15454 | 350 | 15454 | |
| 1186 | 15014 | 1237 | 15014 | 349 | 15014 | |
| AVERAGE | 1129.500 | 14970.2500 | 1292.3750 | 14970.2500 | 364.7500 | 14970.2500 |
| SD | 76.000 | 916.11661 | 111.45266 | 916.11661 | 21.61844 | 916.11661 |
| % CV | 6.72 | 6.12 | 8.62 | 6.12 | 5.93 | 6.12 |
Table 2: Specificity and selectivity test for mifepristone analyte and internal standard (Spiked)
Materials and Methods
Mifepristone was purchased from vivan life sciences, N-demethyl mifepristone (RU 42 633) and hydroxyl mifepristone (RU 42 698) was purchased from TLC pharma chem.., Inc. The internal standard levonorgestrel was purchased from Vivan life Sciences. HPLC grade methanol and phosphoric acid purchased from Fisher Scientific, HPLC grade acetic acid purchased from Merck. Plasma was purchased from Laxmi Sai Clinical lab, India and HPLC grade Milli Q water was produced in house by using Milli Q-RO named Millipore. Solid phase extraction cartridge (Oasis HPLC 1 ml 30 mg), the HPLC separation column was HyPURITY c18, 50 × 4.6 μm, 5 μm from thermo scientific, centrifuge of 5810R, micropipettes of variable adjustment from eppendrof, vortex mixer of vibramax 110 from Heidolph, Microbalance of MX5 from Mettler Toledo, Deep freezer of -75°C Deep freezer from Sanyo and Low volume evaporator of Turbovap® (LV) from Caliper life sciences (Tables 3 and 4).
| Standard ID | Std-1 | Std-2 | Std-3 | Std-4 | Std-5 | Std-6 | Std-7 | Std-8 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (µg/ml) | 51.8992 | 103.7984 | 259.496 | 576.6578 | 1441.6444 | 2110.7532 | 3247.3126 | 4059.1408 | ||
| Back Calculated Concentration (µg/ml) | PA-02 | 50.5810 | 112.2690 | 234.4243 | 600.9680 | 1433.4766 | 2177.8631 | 3150.5721 | 4066.9174 | |
| PA-03 | 53.6505 | 94.8508 | 278.3877 | 550.8086 | 1383.7387 | 2007.7490 | 3274.0338 | 4486.2422 | ||
| PA-04 | 53.6027 | 96.8367 | 265.6633 | 543.1534 | 1467.6403 | 2108.0269 | 3153.1109 | 4387.3201 | ||
| AVERAGE | 52.6114 | 101.3188 | 259.4918 | 564.9767 | 1428.2852 | 2097.8797 | 3192.5723 | 4313.4932 | ||
| SD | 1.75856 | 9.53496 | 22.62214 | 31.40354 | 42.19103 | 85.50980 | 70.55918 | 219.19421 | ||
| %CV | 3.34 | 9.41 | 8.72 | 5.56 | 2.95 | 4.08 | 2.21 | 5.08 | ||
| % ACCURACY | 101.37 | 97.61 | 100.00 | 97.97 | 99.07 | 99.39 | 98.31 | 106.27 | ||
Table 3: Back calculated calibration curve concentrations for mifepristone and internal standard
| Standard ID | Std-1 | Std-2 | Std-3 | Std-4 | Std-5 | Std-6 | Std-7 | Std-8 | |
|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (µg/ml) | 51.9063 | 103.8126 | 259.5315 | 576.7367 | 1441.8416 | 2111.0419 | 3247.7568 | 4059.696 | |
| Back Calculated Concentration (µg/ml) | PA-02 | 54.8809 | 93.3984 | 243.9745 | 592.6648 | 1534.5458 | 2088.3308 | 3273.4116 | 4116.1187 |
| PA-03 | 53.8270 | 94.8954 | 270.2119 | 572.2216 | 1366.9640 | 1999.7207 | 3295.9596 | 4487.5563 | |
| PA-04 | 53.4805 | 100.3317 | 258.7929 | 463.9041 | 1552.9200 | 2158.4919 | 3229.4398 | 4497.3802 | |
| AVERAGE | 54.0628 | 96.2085 | 257.6598 | 542.9301 | 1484.8099 | 2082.1811 | 3266.2703 | 4367.0184 | |
| SD | 0.72938 | 3.64843 | 13.15535 | 69.19768 | 102.47022 | 79.56406 | 33.83000 | 217.34106 | |
| %CV | 1.35 | 3.79 | 5.11 | 12.75 | 6.90 | 3.82 | 1.04 | 4.98 | |
| % ACCURACY | 104.15 | 92.68 | 99.28 | 94.14 | 102.98 | 98.63 | 100.57 | 107.57 | |
Table 4: Back calculated calibration curve concentrations for N-demthyl Mifepristone and internal standard
Chromatographic system
The LC-MS/MS system 6460 of Triple Quard from Agilent with an automatic injecting system was used. System management and hardware interface for data management from agilent [6]. The mobile phase was combination of Methanol: Water containing 0.2% acetic acid (75:25 v/v) Column oven temperature was 25°C, Auto sampler temperature was 5°C, flow rate was set at 0.5 ml/min, and injection volume was set at 2 μl with a run time of 4 min.
Preparation of stock solutions and working solutions
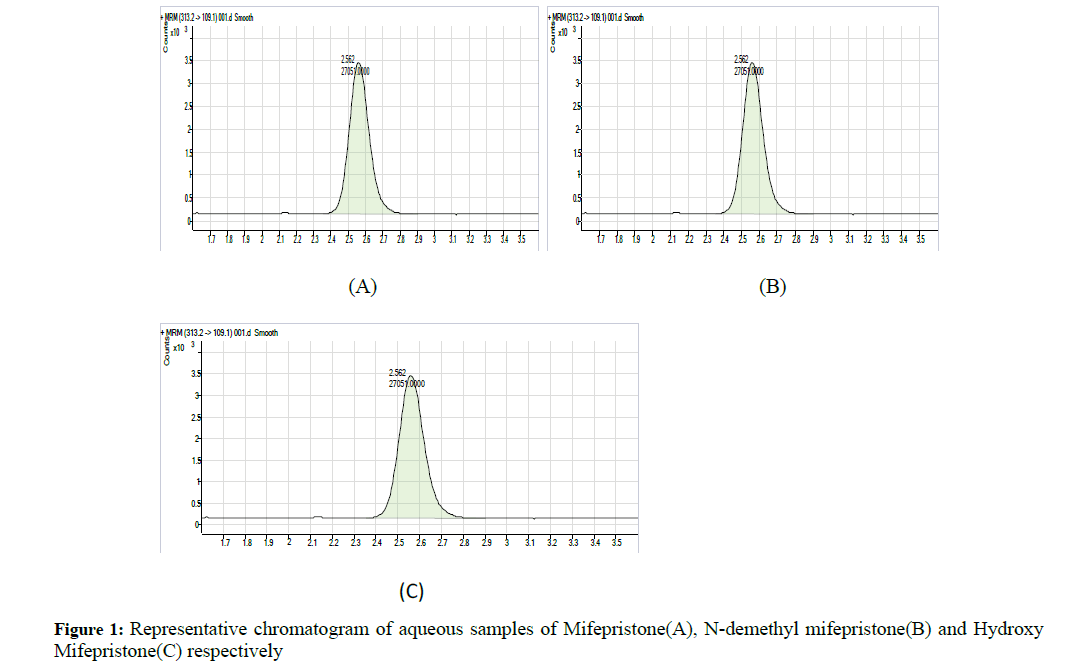
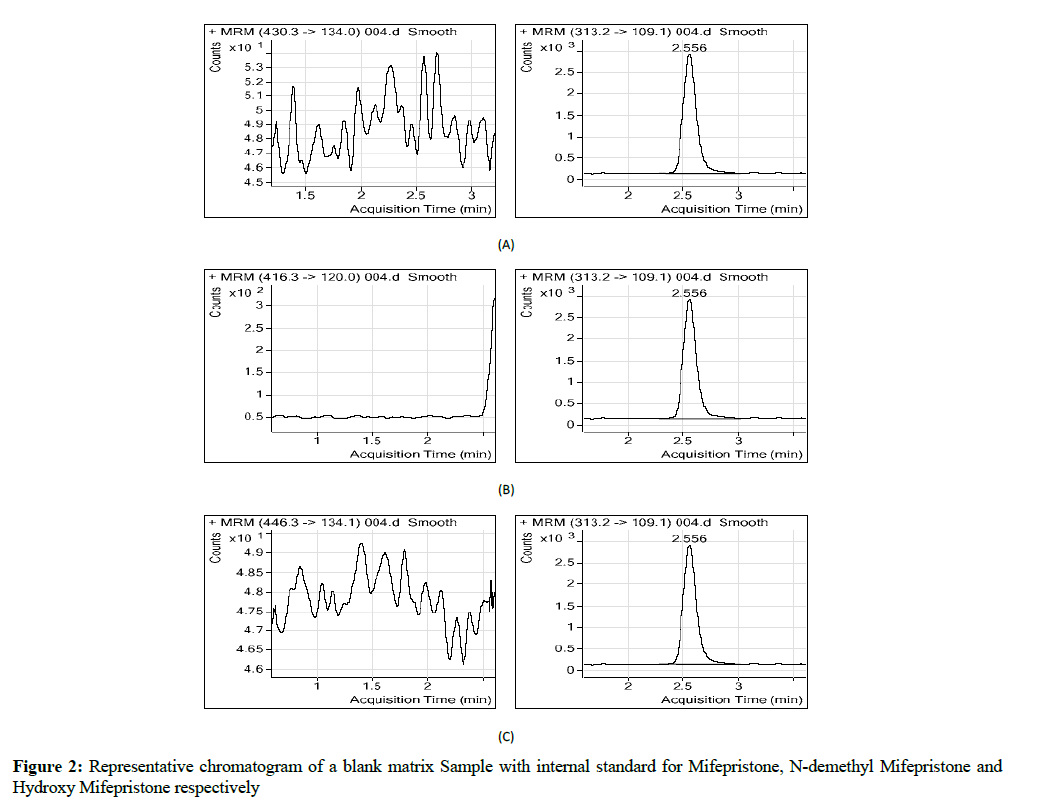
Stock solution of Mifepristone was prepared in diluents combination of methanol: water (90:10 v/v) and stored in clean glass bottle (Figures 1 and 2). They were later stared at 5°C until they were used for the preparation of working solutions by appropriate volume of diluents. Short term stock solution stability was carried out for all analytes (RU 38 486, RU 42 633 and RU 42 698) after storing it at room temperature for 2 hrs against freshly prepared samples (Table 5) [7].
| Standard ID | Std-1 | Std-2 | Std-3 | Std-4 | Std-5 | Std-6 | Std-7 | Std-8 | |
|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (µg/ml) | 12.6881 | 25.3762 | 63.4405 | 140.9789 | 352.4473 | 516.0282 | 793.8896 | 992.362 | |
| Back Calculated Concentration (µg/ml) | PA-02 | 12.8824 | 24.1474 | 64.8572 | 148.5087 | 356.0282 | 518.0672 | 766.9526 | 969.7274 |
| PA-03 | 13.1046 | 23.1908 | 67.2153 | 140.8820 | 340.6015 | 483.3853 | 791.6380 | 1085.8252 | |
| PA-04 | 12.6910 | 25.4607 | 66.4779 | 118.7894 | 374.9019 | 515.7603 | 762.2273 | 1074.3844 | |
| AVERAGE | 12.8926 | 24.2663 | 66.1835 | 136.0601 | 357.1772 | 505.7376 | 773.6060 | 1043.3124 | |
| SD | 0.20703 | 1.13962 | 1.20630 | 15.43530 | 17.17906 | 19.39200 | 15.79392 | 63.98265 | |
| %CV | 1.61 | 4.70 | 1.82 | 11.34 | 4.81 | 3.83 | 2.04 | 6.13 | |
| % ACCURACY | 101.61 | 95.63 | 104.32 | 96.51 | 101.34 | 98.01 | 97.45 | 105.13 | |
Table 5: Back calculated calibration curve concentrations for RU 42 698 and Internal standard
Preparation of calibration standards and QC samples in plasma
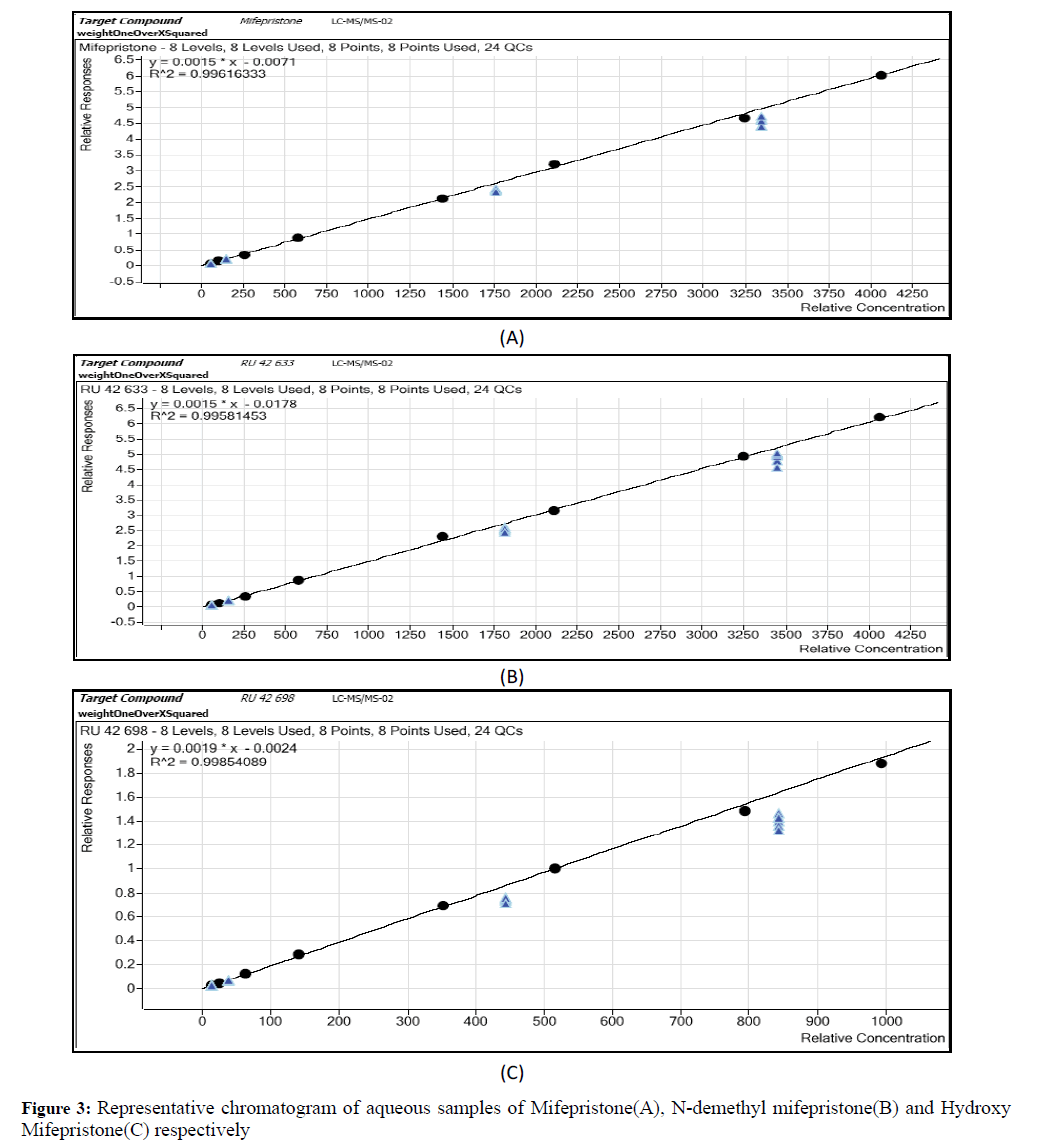
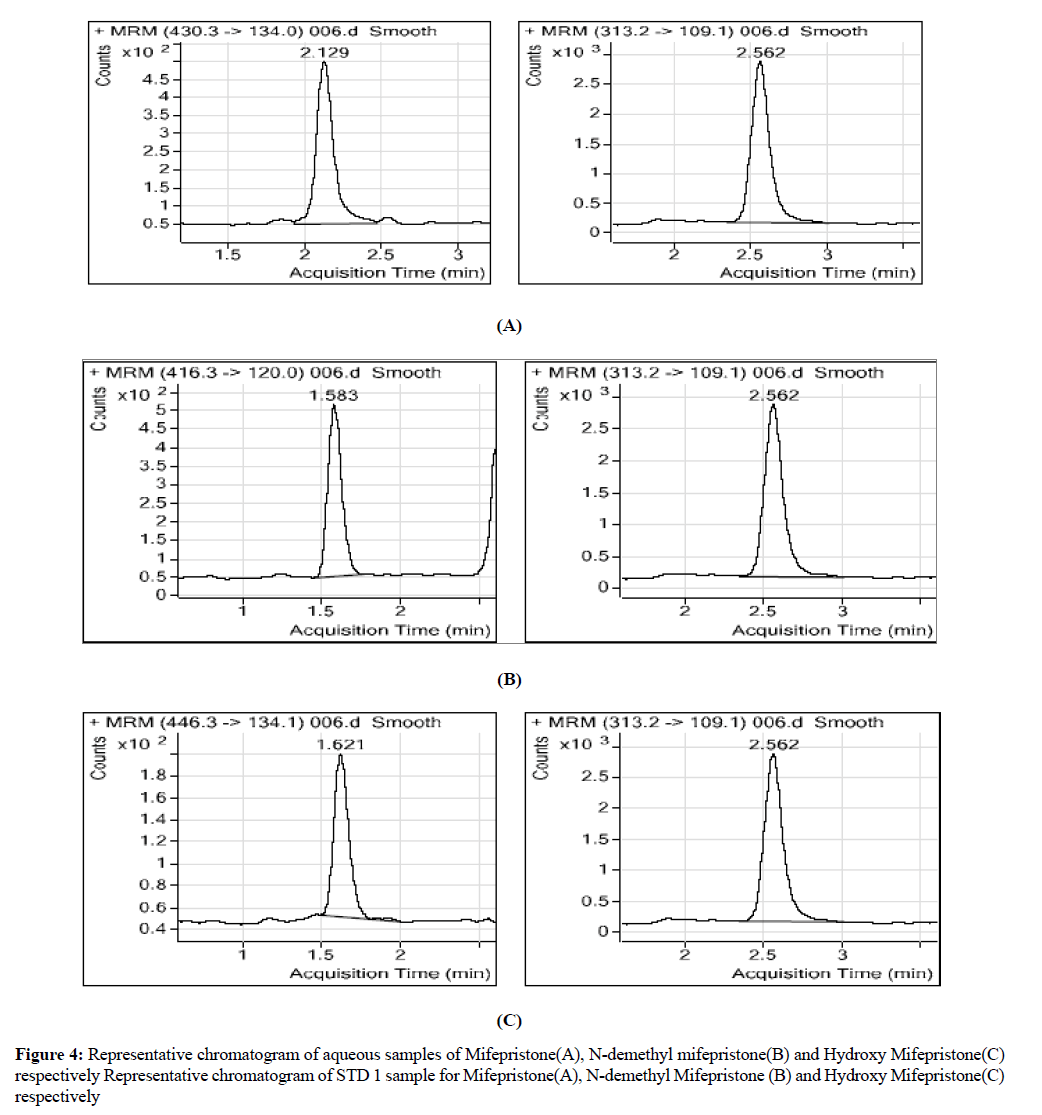
Calibration standards was prepared at a concentration rage of 52.4332-4100.9056 ng/ml for mifepristone, 54.5042- 4262.8800 ng/ml for N-demithyl mifepristone and 13.1661-1029.7455 ng/ml for hydroxy mifepristone by spiking 0.2 ml of each and made to 10 ml by adding diluents (Methanol: Water): 50:50 v/v (Diluents), QC samples was prepared by adding 0.2 ml of each and made to 10 ml by adding diluents (Figures 3 and 4) [8].
Extraction procedure
ll samples, including blank, unknown and standard was extracted using Oasis HLB cartridge of 1 ml, 30 mg before analysis. The optimized extraction procedure was as follows. Set HLB cartridge, condition the cartridge with 1 ml of 100% methanol, equilibrate the cartridge with 1 ml of water (MilliQ water 100%), load the sample in to HLB cartridge (3 mg) 1CC Oasis cartridge, elute with elution solvent (100% methanol), evaporate the eluent to dryness at 40°C and 20 psi nitrogen using low volume evaporator, reconstitute the dried residue with 0.2 ml of mobile phase, transfer the sample into appropriately labeled auto sampler vials and load the samples in to LC-MS/MS system [9].
Results and Discussion
Specificity/Selectivity
A series of chromatograms were shown in Figure 4 of all analytes including the internal standard in auto scale mode, Figure 2 shows the blank chromatograms which indicate the lack of interference, the method were identified as specific for analytes. The retention times are 2.129, 1.577, and 1.615 for mifepristone, N-demethyl mifepristone, hydroxyl mifepristone respectively and 2.562 for internal standard (levonorgestrel). Figure 1 was for mifepristone, N-demethyl mifepristone and hydroxyl mifepristone at concentrations of 52.4334, 54.5042 and 13.1661 ng/ml respectively [10].
Standard curve and linearity of the method
Calibration standards in plasma was prepared for analytes in the ranges of 52.43-4100.90 ng/ml for mifepristone, 54.50- 4262.88 ng/ml for N-demethyl mifepristone and 13.16-1029.74 ng/ml for hydroxyl mifepristone and an equal concentration of levonorgestrel was spiked to all the analytes. From the above standard graph LLOQ, LQC, MQC and HQC were found respectively (53.80, 158.24, 1883.85 and 3588.29 ng/ml for mifepristone), (55.92, 164.49, 1958.26 and 3730.02 ng/ml for N-demethyl mifepristone), (13.58, 39.96, 475.74 and 906.17 ng/ml for hydroxyl mifepristone) [11].
Accuracy and precision
The accuracy and precision was carried out. Inter day accuracy and precision was carried out by four QC as LOQQC, LQC, MQC and HQC respectively by six replicates on each analyte in three days, results are listed in Tables 6-9, intraday precision and accuracy was carried out on the same QC’s listed and was run by gape as six replicates on each analyte. All the readings are within the limit according to the guidelines [12-15].
| Standard ID | Std-1 | S/N Ratio | Standard ID | Std-1 | S/N Ratio | Standard ID | Std-1 | S/N Ratio |
|---|---|---|---|---|---|---|---|---|
| Actual Concentration (µg/ml) | 51.8992 | - | Actual Concentration (µg/ml) | 51.9063 | - | Actual Concentration (µg/ml): | 12.6881 | - |
| PA-02 | 50.581 | 0.78 | PA-02 | 54.8809 | 0.78 | PA-02 | 12.8824 | 0.78 |
| PA-03 | 53.6505 | 1.4 | PA-03 | 53.827 | 1.4 | PA-03 | 13.1046 | 1.4 |
| PA-04 | 53.6027 | 1.41 | PA-04 | 53.4805 | 1.41 | PA-04 | 12.691 | 1.41 |
| Stability | 52.6163 | 10.4 | FTS_IIS | 53.5262 | 10.4 | FTS_IIS | 12.3308 | 10.4 |
| PA-O1_RIR | 44.3003 | 0.66 | PA-O1_RIR | 54.8355 | 0.66 | PA-O1_RIR | 13.0487 | 0.66 |
| Average | 50.9501 | - | Average | 54.11 | - | Average | 12.8115 | - |
| SD | 3.91985 | SD | 0.69603 | SD | 0.31338 | |||
| %CV | 7.69 | %CV | 1.29 | %CV | 2.45 | |||
| % ACCURACY | 98.17 | % ACCURACY | 104.25 | % ACCURACY | 100.97 |
Table 6: Lower Limit of quantification for Mifepristone, N-demethyl mifepristone and Hydroxy Mifepristone respectively
| QC ID | LOQQC | LQC | MQC | HQC | |
|---|---|---|---|---|---|
| Nominal Concentration (µg/ml) | 52.0203 | 153.0010 | 1758.6322 | 3349.7756 | |
| Back Calculated Concentration (µg/ml) |
P & A 01 | 58.79 | 146.69 | 1642.93 | 2978.84 |
| 58.66 | 150.57 | 1582.76 | 3085.04 | ||
| 49.64 | 152.44 | 1641.57 | 2973.25 | ||
| 56.44 | 149.38 | 1627.77 | 3092.29 | ||
| 51.64 | 131.47 | 1623.95 | 3131.48 | ||
| 49.16 | 144.50 | 1629.22 | 3176.94 | ||
Table 7: Inter batch precision and accuracy for Mifepristone
| QC ID | LOQQC | LQC | MQC | HQC | |
|---|---|---|---|---|---|
| Nominal Concentration (µg/ml) | 53.6093 | 157.6745 | 1812.3505 | 3452.096 | |
| Back Calculated Concentration (µg/ml) |
P & A 02 | 59.19 | 160.09 | 1695.09 | 3164.67 |
| 53.10 | 148.54 | 1628.07 | 3304.69 | ||
| 55.40 | 160.39 | 1695.82 | 3028.08 | ||
| 62.61 | 134.20 | 1748.57 | 3283.26 | ||
| 56.96 | 152.17 | 1658.95 | 3268.16 | ||
| 56.88 | 146.05 | 1689.99 | 3329.34 | ||
Table 8: Inter batch Precision and accuracy for N-demethyl Mifepristone
| QC ID | LOQQC | LQC | MQC | HQC | |
|---|---|---|---|---|---|
| Nominal Concentration (µg/ml) | 13.0977 | 38.5228 | 442.7906 | 843.4106 | |
| Back Calculated Concentration (µg/ml) |
P & A 02 | 13.1178 | 39.4054 | 389.8331 | 735.2812 |
| 13.1564 | 39.9848 | 369.5023 | 736.2313 | ||
| 12.9913 | 37.3303 | 388.4016 | 744.1352 | ||
| 12.7259 | 34.1807 | 391.2912 | 719.5374 | ||
| 12.4704 | 34.6297 | 380.4300 | 753.0830 | ||
| 12.6959 | 33.4218 | 391.0660 | 735.2019 | ||
Table 9: Inter batch Precision and accuracy for Hydroxy Mifepristone
Conclusion
A rapid, specific/selective and accurate method by liquid chromatography ESI triple quard mass spectrometry for the analysis of mifepristone and its metabolites (N-demethyl mifepristone and hydroxyl mifepristone) was freshly developed and validated in human plasma [16-20]. The assay used levonorgestrel as internal standard. The method was found linear in the range of 51.89 ng/ml to 4059.14 ng/ml, 51.90 ng/ml, 4059.69 ng/ml, 12.68 ng/ml, 992.36 ng/ml for Mifepristone, N-demethyl Mifepristone, and Hydroxy Mifepristone respectively in Biological Matrix using Solid phase extraction procedure. The LLOQ was found to be 50.95, 54.11 and 12.81 ng/ml for Mifepristone, N-demethyl Mifepristone, and Hydroxy Mifepristone respectively. The run time was fixed at 4.5 with peaks of superior resolution for all the analytes. The result shows that this developed and validated method can be used for routine examination of large number of biological samples [19-22].
Acknowledgment
All the praise and gratitude to the almighty God. The research work was supported by Azidus laboratories Ltd Chennai, would like to have my sincere gratitude to them all. My sincere thanks to Dr. S. Jasemine, Mr. Anand Babu and Mrs. Jisha Mol who helped me a lot to the way through.
References
- Jiang, B., et al., Journal of Chromatography, 2010. 878(1): p. 719-723.
- Natalie, Z., et al., Journal of Chromatography, 2009. 877(1): p. 497-501.
- Cheng, T., Hui-chang, B., Guo-ping, Zhong., Journal of Chromatography, 1993. 48(1): p. 133-149.
- Zhiyong, G., et al., Journal of Chromatography, 2006. 832(1): p. 181-184.
- Zhiyong, G., et al., Journal of Chromatography, 2007. 856(1): p. 12-17.
- https://www.waters.com/webassets/cms/library/docs/720002710en.pdf
- Hsia-lien, L., Haoming, Z., Hollenberg, P., Journal of Pharmacology and Experimental Tharapeutics, 2009. 329(1): p. 26-37.
- Junbo, B., et al., European Journal of Pharmaceutical Sciences, 2010. 39(1): p. 421-427.
- Zhiyong, G., et al., Contraception, 2007. 76(1): p. 228-232.
- Kishore, K., et al., Drug Metabolism & Disposition, 2002. 30(9): p. 985-990.
- Lui, C., et al., Acta Pharmacologica Sinica, 2002. 23(1): p. 117-182.
- https://www.crcpress.com/Liquid-Chromatography-Mass-Spectrometry-Third-Edition/Niessen/p/book/9780824740825
- https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf
- https://www.jpmedpub.com/JPadmin/TableContents/978-81-8448-085-6/TOC/TOC.pdf
- Satoskar, R., et al., Journal of Pharmacology and Pharmacotherapeutics, 2013. 15(2): p. 923.
- Bramankar, D., Jairwal, S., Journal of Pharmacology and Pharmacotherapeutics, 2007. 1(1): p. 283-303.
- Ahuja, S., et al., Hand Book of Pharmaceutical Analysis, 2009. 6(1): p. 500-564.
- Foye, W., Principle of Medicinal Chemistry, 1989. 3(1): p. 79-113.
- Beckett, A., Stenlake, J., Practical Pharmaceutical Chemistry, 2002. 4(2): p. 477.
- https://www.dcne.ugto.mx/Contenido/MaterialDidactico/amezquita/Analitica4/Silverstein%20-%20Spectrometric%20Identification%20of%20Organic%20Compounds%207th%20ed.pdf
- Kalsi, P., Spectroscopy of Organic Compounds, 2004. 6(2): p. 415-513.
- Skoog, A., et al., Fundamental of Analytical Chemistry, 2011. 8(1): p. 980-981.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences