Laparoscopic Fenestration of Hepatic Cysts Called "Simple Cysts of the Liver": Assessment of Evolution and Complications
1Department of General Surgery, Tizi Ouzou Teaching Hospital, Algeria
2Department of General Surgery, Ain Taya Hospital, Algiers, Algeria
- *Corresponding Author:
- Bendjaballah A
Department of General Surgery
Ain Taya Hospital, Algiers, Algeria
Tel: 213551764640
E-mail: ali_bendjaballah@yahoo.fr
Received Date: February 21, 2019; Accepted Date: May 13, 2019; Published Date: May 20, 2019
Citation: Habarek M, Bendjaballah A (2019) Laparoscopic Fenestration of Hepatic Cysts Called "Simple Cysts of the Liver": Assessment of Evolution and Complications. Gen Surg Rep. Vol.3 No.1:9
Abstract
Hepatic Cysts (HC) known as "simple cysts", are benign, frequent and most often asymptomatic. They are present in about 2.5% to 4.7% of the population. They are divided into congenital and acquired. Congenital cystic lesions include polycystic liver disease, simple cysts, duct related and ciliated hepatic foregut cysts. Acquired cystic lesions are divided into infectious represented by hydatid cyst, the amoebic abscess, and the pyogenic abscess. The non-infectious represent the most frequently reported differential diagnoses which are cystadenoma, cystadenocarcinoma. The aim of this retrospective study is to report and evaluate the results of laparoscopic fenestration of this kind of hepatic cysts.
Keywords
Laparoscopic approach; Fenestration; Simple cyst; Liver cyst; Congenital; Acquired cysts
Introduction
Liver Cysts (LC) are benign, frequent, single or multiple lesions and most often asymptomatic [1]. They are present in 2.5% to 4.7% of the population [2]. The term hepatic cyst generally refers to solitary nonparasitic cysts of the liver also known as simple cysts. Nevertheless more than a few other cystic lesions must be distinguished from true simple cysts. The classification of hepatic cysts includes congenital and acquired types. Among the congenital, must be distinguished the simple or sporadic forms and Polycystic Liver Disease (PCLD). It should be noted that the congenital forms can be associated with renal cysts. The acquired forms include cysts as an evolution of organized traumatic hematomas, parasitic cysts (hydatid cysts), pyogenic cysts (abscess cysts) neoplastic cysts (Cystoadenoma, Cystoadenocarcinoma), and metastatic cysts [3]. These conditions can usually be distinguished on the basis of the patient's symptoms, clinical history, and the radiographic appearance of the lesion. Ductal cysts, choledochal cysts, and Caroli disease are differentiated from hepatic cysts by involvement of the bile ducts and they are not studied in this work. The treatment of simple hepatic cysts presents some relevant controversies [4].
Patients and Methods
This is a retrospective study of 12 cases of liver cysts. These patients were collected in the General Surgery Department of Tizi Ouzou University Hospital, Algeria over a period of 08 years from January 2010 to December 2017 divided between 10 women and 2 men. The average age was 57.5 years (range 31-74 years). The site of the liver cyst the associated complications were determined using imaging techniques. Therapeutic methods and short- and long-term results were noted. The surgical indication and the choice of the type of surgical treatment, with possible laparoscopic approach, on the basis of the immediate results and the possibility of relapse of the disease, are also under discussion (Tables 1 and 2).
Table 1 Clinical finding in patients with liver cysts [5].
| Patient case number | Symptoms |
|---|---|
| 1 | Epigastric pain, gallbladder lithiasis |
| 2 | Epigastric pain, gallbladder lithiasis |
| 3 | Abdominal pain |
| 4 | Abdominal and right shoulder pain |
| 5 | Abdominal pain with nauseas and vomiting |
| 6 | Right shoulder pain |
| 7 | Epigastric pain with vomiting |
| 8 | Right shoulder pain |
| 9 | Epigastric pain |
| 10 | Abdominal pain with nauseas and vomiting |
| 11 | Abdominal and right shoulder pain |
| 12 | Abdominal pain |
Table 2 Gigot’s classification for polycystic liver disease [5].
| Type | Description |
|---|---|
| I | Presence of less than 10 large hepatic cysts measuring more than 10 cm in maximum diameter |
| II | Diffuse involvement of liver parenchyma by multiple cysts with remaining large areas of non-cystic liver parenchyma |
| III | Presence of diffuse involvement of liver parenchyma by small and medium-sized liver cysts with only a few areas of normal liver parenchyma |
Results
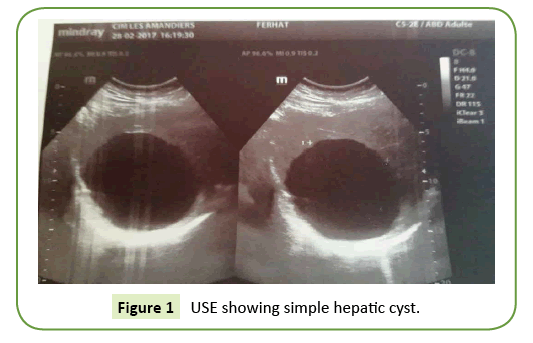
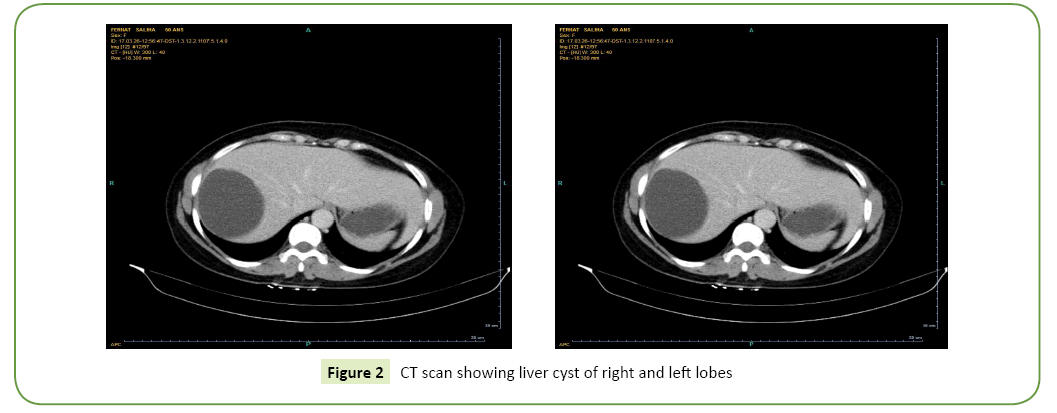
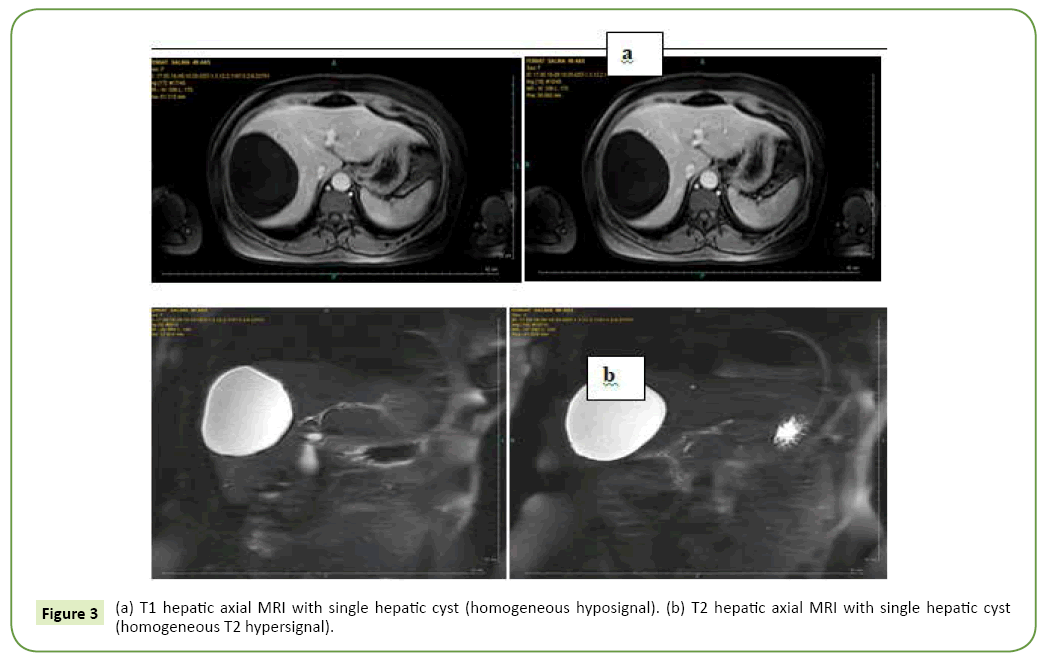
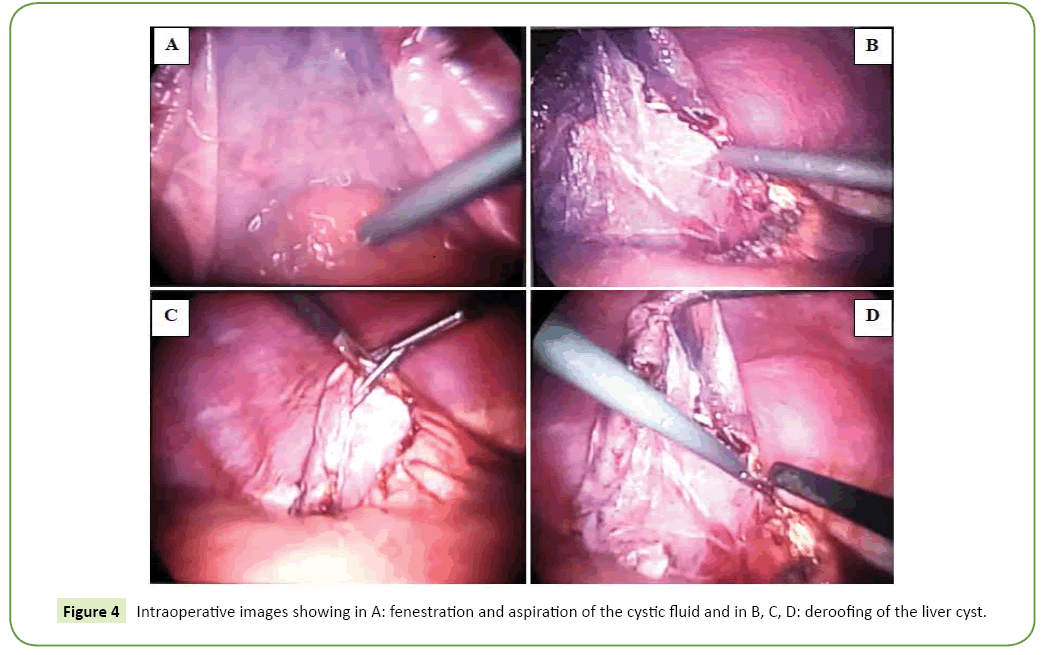
All our patients were symptomatic. The isolated or associated clinical signs leading to the discovery of the hepatic cyst were exclusively type of abdominal pain in the right hypochondrium in nine patients and digestive nausea and vomiting in three patients. The average period of onset of clinical symptomatology compared to the finding of the liver cyst is 07 years with extremes of 06 to 15 years. Abdominal Ultrasound (USE) was performed in all our patients (Figure 1). This examination revealed a single liver cyst in ten cases and multiple with two or more cysts in two cases. In addition, gallbladder stones are associated with the hepatic cyst in two cases. Abdominal computed tomography CT scan (Figure 2) accomplished in all patients also, confirmed the diagnosis of liver cyst in 9 cases and suspected cystadenoma or hydatid cyst in 3 cases. For 3 patients, Magnetic Resonance Imaging (MRI) showed extensive hepatic cystic formation (Figure 3). The location of hepatic cysts was at segment III in three cases (with 8, 10 and 15 cm in diameter, respectively), at segment VI in two cases (with 18 and 20 cm in diameter, respectively), at the segment VII in two cases (with 14 and 10.5 cm in diameter), at segment IV in one case (12 cm in diameter), at segment V in one case (14 cm in diameter) and at segment II (8 cm in diameter). The other two patients with multiple cysts had a variable diameter between 6 and 20 cm interesting two to four segments. All patients furthered from a hydatid cyst serology. It was negative in all cases. Tumor markers (CA19-9 levels and ACE levels) also were negative in all cases. All our patients were treated by laparoscopic fenestration. The fenestration began with a careful puncture of the cyst, then all the fluid was aspirated and the prominent dome was resected (Figure 4). The operative piece was extracted in a bag and sent to histopathological examination. Abdominal drainage was performed in 9 patients. There were no conversions. The postoperative period was passed smoothly for all our patients. We did not notice any recurrence. The histological study result was benign in all cases (Table 3).
Table 3 Direct results with the laparoscopic approach.
| Parameter | Hepatic cyst |
|---|---|
| Mortality | No |
| Conversion | No |
| Mean operative time (min) | 52 (range 35–90) |
| Hospital stay (days) | 7 (range 4–21) |
| Ascites | No |
| Disappearance of the symptomatology | Complete |
| Histology of the removed wall | Simple cyst |
Discussion
First, it is useful to define the general clinical characteristics of this pathology, to institute an appropriate and adequate therapeutic program. In fact, there are three fundamental points:
1. This is a benign pathology,
2. Often asymptomatic or with few signs and symptoms (vague abdominal pain, mild dyspnea, etc).
3. This is accompanied by normal hepatic function tests.
Then apart from the open or laparoscopic approach (as the interventions can readily be overlapped), the surgical indication in general must be recognized on the basis of the appearance of the symptoms. Two elements of debate are in evidence: the anatomical-clinical characteristics of the pathology and the surgical indication. These are related to the presence of hepatic cysts i.e., the presence of another disease such as cholelithiasis [3]. The simple cystic liver usually affects women (sex ratio=3/2), its prevalence is 1% to 2% in adults [5-7] and increases with age (until the age of 40) [8]. It contains a serous fluid. Histopathological examination describes LC as an abnormal cavity separated from the healthy hepatic parenchyma by a cuboid or cylindrical epithelial lining that does not communicate with the bile ducts. Usually the described symptoms arise from the effect of the volume of the cyst(s). Furthermore, about 15% [9] of patients are asymptomatic, but the most common problem is the sense of bulk and the mild pain in the upper abdominal quadrants. Even though rare, infection of the cysts (more frequent in polycystosis) and the presence of jaundice from compression of the biliary tree can be found [10]. The increase of pressure in the cysts can lead to inflammatory reaction, even parietal erosion and bleeding (intra-cystic bleeding) or, if the cyst is extra-hepatic it can occur exceptionally an intraperitoneal rupture. It is also possible to observe infections of these cysts either by the hematogenous or by the biliary way. The literature reports no case of malignant degeneration [1] as is the case in our series. Hepatic cysts grow slowly and are a long asymptomatic [9] as in our patients who have never presented particular symptoms for, on average, 7 years duration, when they become symptomatic (32%), the main manifestation (95%) is the pain. Signs of digestive or thoracic compression such as dyspnea may be observed, as well as cholestasis or portal hypertension. The diagnosis can be easy on ultrasound, abdominal CT or MRI by highlighting the rounded or oval appearance of the cyst with a thin non calcified wall, associated with the absence of septa and wall nodules and absence of peripheral enhancement to CT and magnetic resonance imaging and finally homogeneity of the content. Difficulty of differentiation between cystadenomas and cystadenocarcinomas can be observed particularly in cases of intra-cystic bleeding and infection [11,12]. Despite the progress in imaging technology and increasing acknowledgement of biliary Cystadenoma and Cystadenocarcinoma, preoperative diagnosis is uncertain. Cystic neoplasms are estimated to be about 5% of cystic liver lesions and 5% of them are malignant. The overall incidence of malignant hepatic tumors is lower than 0.41% [13,14]. The histogenesis of CA remains unclear but either a congenital origin or a reactive process to some focal injury is supposed.
Most of the Cystadenomas stay quiet and are discovered incidentally or they appear causing tumor compression abdominal symptoms [13]. The rate of malignant transformation is low (5%-10%), nevertheless all suspected Cystadenomas must be and a liver resection with clear margins, open or laparoscopically, protects from the possibility of synchronous presence of Cystadenocarcinomas at the borders of the cyst [15,16]. Veroux et al. [17] reported occasions of incidental finding of biliary Cystadenoma after laparoscopic fenestration of a cystic hepatic lesion. They stated that when a complete laparoscopic enucleation of the cyst may be ensured, a strict clinical, biochemical, and imaging follow-up evaluation should be considered as the definitive treatment. In the period of less invasive surgery, a laparoscopic biopsy of the cyst wall is possible but frozen sections cannot certainly exclude or confirm the diagnosis of Cystadenoma, especially of Cystadenocarcinoma. The current treatment modality is open or laparoscopic liver resection due to the risk of malignancy in all suspected Cystadenomas [14,18]. In hepatic Cystadenocarcinoma there is no place for laparoscopic methods, with formal hepatic resection being the only suggested therapy. The distinction between cystadenomas and cystadenocarcinomas still difficult, principally in cases with infection and intra-cystic hemorrhage. Therefore, this difficulty requires an echo-guided punction of the cyst for a cytological examination in search of tumor cells and an immunological examination by the assay in the cystic fluid of CEA and CA 19-9. In information, the increase in CEA and CA 19-9 in cyst fluid and the increase in serum CA 19-9 are predictive of cystadenomas and cystadenocarcinomas [19,20]. Sometimes the diagnosis is made only after the histological examination of the operative specimen, particularly the cystic wall. Immunolabeling demonstrates epithelial cell expression of anti-CEA and anti-CA 19-9 antibodies. In our series, the histological study of the cystic wall concludes with a benign simple cyst. In addition, the clinical history leads to the conclusion that a congenital cyst is present. On the other hand in our country and in other endemic countries, the serology of hydatidosis is systematically requested. Surgery for simple cyst is indicated when they become symptomatic or compressive or when associated with complications [20-26]. In our series, the surgery was motivated by the pain and the large volume of the cyst. Recent options are wide fenestration by laparoscopy or drainage associated with a sclerosing agent (ethanol, minocycline hydrochloride) [27]. This technic is followed by a high incidence of relapse [28-30]. Drainage alone has also a high rate of recurrence [31]. The treatment by fenestration and deroofing was suggested and largely carried out by the laparoscopic attitude and it was largely accepted as a safe and feasible method. Open deroofing should be reserved for the treatment of inaccessible cysts by laparoscopy. On the full, surgical therapy with fenestration by deroofing of the cystic wall is considered as the therapy of choice for the treatment of simple hepatic cysts because of the low morbidity rates, the high level of effectiveness and the very small relapse index [9,32-35]. The viability and safety of the intervention of fenestration and deroofing of the cysts are well established now, and this procedure can be executed with the same results by laparoscopic and open approaches. The fenestration and deroofing is not very different in the treatment of both the hepatic polycystosis and the simple cysts, so the relapse incidence is unimportant. Surgical indication requires a careful selection of the patients, overlapping with simple cysts, for which the laparoscopic fenestration and deroofing are also safe and efficacious. The laparoscopic fenestration for congenital liver cysts is feasible and effective, with superior shortterm outcomes as compared with the open fenestration, and laparoscopic treatment of uncomplicated liver cysts is considered as a “Gold standard” treatment [36]. Hepatic resection is indicated as a second level choice, after a first deroofing but with relapse of disease and/or complications [25]. In addition, hepatic resection is the preferred treatment for patients with multiple cysts or in PCLD and with a wide hepatic involvement [24], but leaving a sufficient amount of hepatic parenchyma. A further subdivision among the PCLD cases was suggested by Gigot et al., which distinguishes type 1, with large cysts (>10 cm), in limited number (<10); type 2 with multiple and widespread cysts of moderate size, but with largely undamaged parenchyma; type 3 with widespread small cysts, but with small amounts of undamaged residual parenchyma [37]. The therapeutic attitude for polycystosis type 2 (Gigot's type 3) is much more complicated, so the perspective of transplantation is very tangible. In certain, patients with congenital hepatic fibrosis and with extensive substitution of the parenchyma by the cysts (polycystosis type 2), are candidates for hepatic transplantation. Temporarily, hepatic transplantation is mostly indicated for cases of polycystosis with severe hepatic failure and/or in case of relapse after previous hepatic resections. In adding, some of these patients also suffer from polycystic kidney and kidney failure, so combined kidney/ liver transplantation can be the appropriate therapeutic choice for this kind of patients [38-41]. After fenestration, several authors leave a drain in the cystic cavity [22,42], this is the case of 9 of our patients, others fill the residual cavity by the omentum realizing an omentoplasty, especially when the cyst is more than 10 cm long [26]. Recurrences are difficult to assess because the published series have few cases and often mix Liver simple Cyst (LC) and polycystic [43]. Symptomatic relapses are rare, about 5% [44] in patients with liver simple cyst (LC) but the persistence of an asymptomatic LC often less voluminous than before the fenestration, is represented by postoperative imaging in nearly 30% of cases [45]. Finally concerning the injection of alcohol in the LC the modalities are not yet consensual, but some precautions must be taken. It is a painful procedure requiring at least a local anesthesia associated with sedation, or even a general anesthesia. The volume of alcohol (between 20 and 100 mL) depends on the size and the number of cysts to be treated. The duration of contact should be at least 10 minutes with patient mobilization so that the entire surface of the cyst comes into contact with the alcohol. The alcohol content must be at least 95%. Ultrasound monitoring at 6 months and then at one year is recommended. Published results seem comparable to fenestration for symptomatic and asymptomatic recurrence (30%).
Conclusion
The diagnosis of simple hepatic cysts is usually easy facilitated by the use of imaging techniques. Surgical indications arise when they become symptomatic or have complications. The treatment of choice is the fenestration of the cyst in view of the good results as: reduction of postoperative pain, more comfort, early mobilization, shorter hospital stay, considerable esthetic advantage, but above all, the resolution of the symptomatology and the low incidence of distant relapse of disease. Minimally invasive techniques are possible and safe options to the open approaches for treating LC. The most used techniques are percutaneous drainage, laparoscopic fenestration, and laparoscopic hepatectomy. But, the exact method that is favored in each circumstance depends on the type of the cystic lesion, its size and its location in the liver. More studies are needed in order to well determine the exact indications and complications of minimally invasive modalities when they are applied in LC.
References
- Plard L, Guedin P, Le Pennec V, Chiche L (2008) Kystes hépatiques dits kystes biliaires du foie. J Chir 145: 217-225.
- Desmet VJ (2005) Cystic diseases of the liver. From embryology to malformations. Gastroenterol Clin Biol 29: 858-860.
- Neri V, Ambrosi A, Fersini A, Valentino TP (2006) Laparoscopic treatment of biliary hepatic cysts: short- and medium-term results. HPB (Oxford) 8: 306-310.
- Robert EG (2018) Hepatic cyst. John Geibel, Medscape.
- Vardacostas D, Damaskos C, Garmpis N, Antoniou EA, Kontzoglou K, et al. (2018) Minimally invasive management of hepatic cysts: Indications and complications. Eur Rev Med Pharmacol Sci 22: 1387-1396.
- Castaing D, Adam R, Azoulay D (2006) Conditions particulières de la chirurgie des kystes hépatiques. In: Castaing D, Adam R, Azoulay D (eds.). Chirurgie du foie et de l’hypertension portale. Techniques chirurgicales (digestif). Masson, Paris, pp: 178-182.
- Gigot JF, Metairie S, Etienne J (2001) The surgical management of congenital liver cysts. The need for a tailored approach with appropriate patient selection and proper surgical technique. Surg Endosc 15: 357-363.
- Larssen TB, Rorvik J, Hoff SR, Horn A, Rosendahl K (2005) The occurrence of asymptomatic and symptomatic simple hepatic cysts. A prospective, hospital-based study. Clin Radiol 60: 1026-1029.
- Gigot JF, Hubert C, Banice R, Kendrick ML (2004) Laparoscopic management of benign liver disease: where are we? HPB 6: 197-212.
- Parks RW, Garden OJ (2001) In: Hepatobiliary and pancreatic surgery. London: WB Saunders, pp: 75-105.
- Barnes PA, Thomas JL, Bemardino ME (1981) Pitfalls in the diagnosis of hepatic cysts by computed tomography. Radiology 141: 129-133.
- Vuillemin-Bodaghi V, Zins M, Vullierme MP, Denys A, Sibert A (1997) Imagerie des kystes atypiques du foie: étude de 26 lésions opérées. Gastroenterol Clin Biol 21: 394-399.
- Hai S, Hirohashi K, Uenisi T, Yamamoto T, Shuto T (2003) Surgical management of hepatic cyst neoplasm. J Gastoenterol 38: 759-764.
- Vogt DP, Hendersen JM, Chmielewski E (2005) Cystadenoma and cystadenocarcinoma of the liver. A single center experience. J Am Coll Surgeons 200: 727-733.
- Thomas KT, Welch D (2005) Effective treatment of biliary cystadenoma. Ann Surg 241: 769-773.
- Flamengo P, Verroux M, Cillo U, Basso S, Buffone A (2004) Incidental cystadenoma after laparoscopic treatment of hepatic cyst: which strategy?. Surg Laparosc Endosc Percutan Tech 14: 282-284.
- Veroux M, Flamengo P, Cillo U, Tedeschi U, Brolese A, et al. (2005) Cystadenoma and laparoscopic surgery for hepatic cystic disease: A need for laparotomy? Surg Endosc Other Interv Tech 19: 1077-1081.
- Koffron A, Rao S, Ferrario M, Abecassis M (2004) Intrahepatic biliary cystadenoma: role of cyst fluid analysis and surgical management in the laparoscopic era. Surgery 136: 926-936.
- Trésallet C, Jordi-Galais P, Nguyen-Thanh Q, Aubriot-Lorton M, Costedoat-Chalumeau N (2003) Cystadénome hépatique avec taux d’ACE et de CA 19-9 intrakystiques élevés. Gastroenterol Clin Biol 4: 413-415.
- Lee JH, Chen DR, Pang SC, Lai YS (1996) Mucinous biliary cystadenoma with mesenchymal stroma: expressions of CA 19-9 and carcinoembryonic antigen in serum and cystic fluid. J Gastroenterol 31: 732-736.
- Cala Z, Cvitanović B, Perko Z, Velnić D, Rasic Z (2000) Laparoscopic fenestration of non parasitic cysts of the liver and spleen. Endoscopia 3: 21-23.
- Diez J, Decoud J, Gutierrez L, Suhl A, Merello J (1998) Laparoscopic treatment of symptomatic cysts of the liver. Br J Surg 85: 25-27.
- Kitajima Y, Okayama Y, Hirai M, Hayashi K, Imai H, et al. (2003) Intracystic hemorrhage of a simple liver cyst mimicking a biliary cystadenocarcinoma. J Gastroenterol 38: 190-193.
- Martin IJ, McKinley AJ, Currie EJ (1998) Tailoring the management of non parasitic liver cysts. Am J Surg 228: 167-172.
- Tocchi A, Mazzoni G, Costa G, Cassini D, Bettelli E (2002) Symptomatic nonparasitic hepatic cysts. Options for and results of surgical management. Arch Surg 137: 154-158.
- Szabó LS, Takács I, Arkosy P, Sápy P, Szentkereszty Z (2006) Laparoscopic treatment of non parasitic hepatic cysts. Surg Endosc 20: 595-597.
- Dicataldo A, Azzarello G, Lanteri R, Licitra E, Licita A (2005) Traitement par alcoolisation des kystes biliaires. Expérience personnelle sur 13 cases. G Chir 26: 17-20.
- Kairaluoma MI, Leinonen A, Stahlberg M, Paivansalo M, Kiviniemi H (1989) Percutaneous aspiration and alcohol sclerotherapy for symptomatic hepatic cysts. An alternative to surgical intervention. Ann Surg 210: 208-215.
- Montorsi M, Torzilli G, Fumagalli U, Bona S, Rostai R (1994) Percutaneous alcohol sclerotherapy of simple hepatic cysts. Results from a multicentre survey in Italy. HPB Surg 8: 89-94.
- Regev A, Reddy KR, Berho M, Sleeman D, Levi JU (2001) Large cystic lesions of the liver in adults: a 15-year experience in a tertiary center. J Am Coll Surg 193: 36-45.
- Saini S, Mueller PR, Ferrucci JT Jr., Simeone JF, Wittenberg J, et al. (1983) Percutaneous aspiration of hepatic cysts does not provide definitive therapy. Am J Roentgenol 141: 559-560.
- Moorthy K, Mihssin N, Houghton PW (2001) The management of simple hepatic cysts: sclerotherapy or laparoscopic fenestration. Ann R Coll Surg Engl 83: 409-414.
- Zacherl J, Imhof M, Fugger R, Fritsch A (1996) Laparoscopic unroofing of symptomatic congenital liver cysts. Surg Endosc 10: 813-815.
- Kwon AH, Matsui Y, Inui H, Imamura A, Kamiyama Y (2003) Laparoscopic treatment using an argon beam coagulator for nonparasitic liver cysts. Am J Surg 185: 273-277.
- Hansman MF, Ryan JA Jr, Holmes JH 4th, Hogan S, Lee FT, et al. (2001) Management and long-term follow-up of hepatic cysts. Am J Surg 181: 404-410.
- Jian-Guo Q, Hong W, Hui J, Ji-Wei H, Prasoon P (2011) Laparoscopic fenestration vs open fenestration in patients with congenital hepatic cysts: A meta-analysis. World J Gastroenterol 17: 3359-3365.
- Gigot JF, Jadoul P, Que F, Van Beers BE, Etienne J, et al. (1997) Adult polycystic liver disease. Is fenestration the most adequate operation for long-term management? Ann Surg 225: 286-294.
- Swenson K, Seu P, Kinkhabwala M, Maggard M, Martin P, et al. (1998) Liver transplantation for adult polycystic liver disease. Hepatology 28: 412-415.
- Jeyarajah DR, Gonwa TA, Testa G, Abbasoglu O, Goldstein R, et al. (1998) Liver and kidney transplantation for polycystic disease. Transplantation 66: 529-532.
- Mekeel KL, Moss AA, Reddy KS, Douglas DD, Vargas HE, et al. (2008) Living-donor liver transplantation for polycystic liver disease. Transplantation 77: 480-481.
- Gustafsson BI, Friman S, Mjornstedt L, Olausson M, Backman L (2003) Liver transplantation for polycystic liver disease - indications and outcome. Transplant Proc 35: 813-814.
- Fiamingo P, Tedeschi U, Veroux M, Cillo U, Brolese A, et al. (2003) Laparoscopic treatment of simple hepatic cysts and polycystic liver disease. Surg Endosc 17: 623-626.
- Palanivelu C, Rangarajan M, Senthilkumar R, Madankumar MV (2007) Laparoscopic management of symptomatic multiple hepatic cysts: a combination of deroofing and radical excision. JSLS 11: 466-469.
- Garcea G, Pattenden CJ, Stephenson J, Dennison AR, Berry DP (2007) Nine-year single center experience with nonparastic liver cysts: diagnosis and management. Dig Dis Sci 52: 185-191.
- Tan YM, Ooi LL, Soo KC, Mack PO (2002) Does laparoscopic fenestration provide long-term alleviation for symptomatic cystic disease of the liver?. ANZ J Surg 72: 743-745.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences