ISSN : 2348-9502
American Journal of Ethnomedicine
Isolation of Lichen Forming Fungus of Everniastrum cirrhatum and Evaluate its Antagonistic and Antimicrobial Activity
1Centre of Excellence for Sanitary and Phytosanitary (SPS), Department of Plant Pathology, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut-250110, India
2Department of Botany, Guru Ghasidas Vishwavidyalaya, Bilaspur- 495009 (CG), India
3Department of Microbiology, Meerut Institute of engineering and Technology, Meerut-250005, India
4Department of Agriculture Biotechnology, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut-250110, India
Abstract
Mycobiont is a lichen forming fungi (LFF) symbiotically associated with phycobiont (algae) of lichens. Here LFF was isolated from Everniastrum cirrhatum lichen and checked its antagonist and antimicrobial properties against pathogens with minimum inhibitory concentration (MIC). The isolated LFF was inhibited the growth of several plant pathogens viz, Fusarium moniliforme, F. oxysporum and F. udum and Human pathogenic fungi viz, Epidermophyton floccosum, Microsporum gypseum and Trichophyton rubrum as well as pathogenic bacteria viz, Streptococcus mutant, Staphylococcus aureus, Salmonella typhimurium and Salmonella typhi. The antagonistic activity of LFF was found most effective against Fusarium udum and caused 55.55% inhibition of mycelial growth and antimicrobial substances from LFF were also compared with natural thallus extract, maximum activity was observed by acetone extracts of LFF against Microsporum gypseum (21mm) and its MIC was found to be 0.78125×10-7 μl against Microsporum gypseum and Candida albicans. Therefore acetone extracts of LFF found most effective inhibitor rather than other extracts. This is the 1st attempt to evaluate antagonistic and antimicrobial properties of LFF (Everniastrum cirrhatum) against pathogenic fungi and bacteria.
Keywords
Lichen forming fungus, Everniastrum cirrhatum, Antagonists, Antimicrobials.
INTRODUCTION
Lichens are the small plants like organisms having a symbiotic association between fungus as mycobiont and algae as phycobiont [1]. The thallus of Lichen does not show any resemblance to either partner. Lichens are well known for having unusual secondary metabolites which have medicinal properties and been used in medicinal purposes from very long times. Some lichens such as Cladonia islandica, Cladonia speres and Lobaria pulmonaria were found to be effective in the pulmonary tuberculosis treatment [1].
Some secondary metabolites which not produced by higher plants or even in free living fungi are secreted by the mycobiont partner of Lichens Nobuo Hamada [2-3]. This is the main reason that’s why the isolation and extraction of mycobiont which is also known as Lichen Forming Fungus (LFF) for secondary metabolites is necessary.
Antimicrobial secondary metabolites produced by some LFF have manifold more active biological properties like antibiotic, antiviral, allergenic, antitumor, plant growth inhibitory, ecological roles enzyme inhibitory and many more [4-5].
The antibacterial and antifungal activities of lichens have studied by a number of investigators. The antibiotic property of lichen was firstly studied by Burkholder [6]. The antifungal and antibacterial activity of many lichens and their extracts was evaluated against different group of microorganisms like fungi, grampositive and gram- negative bacteria [7-9].
In this context the purpose of the present study was to standardize the methodology for isolation of Lichen Forming Fungus (LFF), study the antagonistic activity of this fungus and to screen their antimicrobial potential by solvent extracts of natural thallus and LFF of lichen Everniastrum cirrhatum.
MATERIALS AND METHODS
Collection and identification
The lichen was collected from Nainital (Utrakhand, India), identified by Dr. D. K Upreti, lichen laboratory, NBRI, Lucknow [10]. Then this lichen was dried at room temperature for 72 hours and preserved it in -200C for further investigation [10].
Some microorganisms which produced diseases viz. Streptococcus mutans (MTCC 890), Staphylococcus aureus (MTCC 7443), Salmonella typhi (MTCC 3216), Salmonella typhimurium (MTCC 3224), Trichophyton rubrum (MTCC 7859), Fusarium oxysporum (MTCC 6569) Microsporum gypseum (MTCC 6041), Epidermophyton floccosum (MTCC 7880), Candida albicans (MTCC 227), and Fusarium moniliforme (MTCC 6576), Fusarium udum (MTCC 4290) were obtained from Microbial Type Culture Collection (MTCC), IMTECH, Chandigarh. Colonies of bacterial cultures were preserved in NA (Nutrient agar) and fungi cultures were preserved in PDA (Potato dextrose agar) media at 4°C for further studies.
Isolation of lichen forming fungus (LFF)
The separation and isolation of LFF give a better opportunity to gather the morphological and biochemical study of the mycobiont components of lichen. For isolation of LFF many types of media like Potato Dextrose Agar (PDA), 2% malt agar (MA), Sabordaud Dextrose Agar (SDA) and Malt Yeast Extract Agar (MYEA) were used [11].
Lichen’s apothecia were surface sterilized with 0.01% HgCl2, removed a drop of HgCl2 from the surface of apothecia was triple time washed by distilled water. After it small pieces of apothecia were inoculated on a different medium. All inoculated plates were incubated at 24+ 2°C for 48 hrs. Plates were daily checked for contamination and immediate transfer the non- contaminated colony on to fresh culture plate [11].
Antagonistic effect of LFF
The antagonistic effect of LFF of lichen Everniastrum cirrhatum was evaluated against different pathogenic fungi viz., Fusarium moniliforme, F. oxysporum F. udum, Trichophyton rubrum, Microsporum gypseum and Epidermophyton floccosum by dual culture technique [12] in triple replications. 5 mm diameter discs of LFF were placed on one side of poured PDA plates aseptically while, the discs of fungus pathogen were placed on opposite sides of LFF in the same Petri plates here control were also maintained with both LFF as well as pathogen separately. These Petri plates were incubated at 20 + 2°C for 12 days. After each 24 hours, the mechanism of interaction was observed and the data were recorded as per cent inhibition by the formula given here:
Percent growth inhibition over control= dc – dt /dc × 100.
where,
dc = colony diameter in control
dt = colony diameter in treatment
Preparation of extracts
4 g air dried natural thallus (NT) of lichen powder and same amount of air dried LFF was dipped in 8 ml of each solvent (i.e. ethanol and acetone) separately for 48 hours at room temperature [10]. By using filter paper (Whatman No.1) all extracts were filtered and under reduced pressure they evaporated till dryness. The obtained residues, 8 ml of Dimethly sulfoxide (DMSO) were added in each filtrate for final concentration [10]. The obtained solutions were kept at 4°C for further study [10].
Antimicrobial screening of LFF and NT of lichen extracts against pathogens
For antimicrobial screening discdiffusion method was used [12,13]. A sterile disc of 6 mm (Hi Media) was impregnated with 20μl of the extracts and placed in a Luria Bertani agar plate which is inoculated with the pathogen. All plates were incubated at 30+ 2° C for 24 hours and with DMSO only the control was also maintained.
MIC of acetone (LFF) extract of lichen against pathogens
Minimum Inhibitory Concentration (MIC) is the lowest concentration which inhibited the growth of microorganisms and judged by the lack of turbidity in a tube. The MIC of acetone extract of LFF was checked against pathogens by microtiter plate assay used by Sarker et. al., 2007 [14].
RESULTS AND DISSCUSION
Isolation of LFF
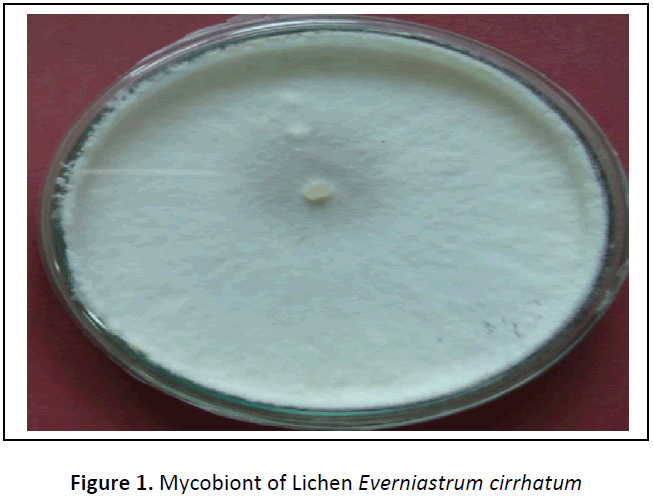
From different media tried, Malt, Yeast Extract Agar medium was supported best for the isolation and good growth of LFF. The pure culture of LFF, colony texture was white smooth with wavy margin (figure 1). Similar results were found by Dharmadhikari et al., 2010 but they found MGYP medium best for the growth of the mycobiont of lichen Parmelinella simplicior [11]. For the analysis of secondary metabolites and their mass production, liquid media were found to be more convenient so that LFF can easily separate from liquid medium as compare to solid medium. The growth rate of the cultured LFF in the laboratory could be improved to harvest large quantities of these novel secondary metabolites. Thus, to identify the chemical constituents of the extracts of LFF more study is necessary. In addition, the data may also suggest that the extracts of LFF could be used as an easily accessible source of secondary metabolites for the antimicrobial properties in the pharmaceutical purpose.
Antagonistic effect of LFF
The antagonist effect of LFF of lichen Everniastrum cirrhatum was evaluated against different pathogenic fungi viz., Fusarium oxysporum, F. moniliforme, F. udum, Trichophyton rubrum, Microsporum gypseum and Epidermophyton floccosum were estimated on the basis of the percentage of inhibition of pathogenic strains, the results were presented in table 1.
Table 1: Antagonistic effect of lichen against different pathogenic fungi
| S. No | Name of pathogen | Percent of inhibition after 14 days |
|---|---|---|
| 1. | Fusarium moniliforme, | 38.88 |
| 2. | Fusarium oxysporum | 36.11 |
| 3. | Fusarium udum | 55.55 |
| 4. | Trichophyton rubrum | 25.27 |
| 5. | Microsporum gypseum | 30.55 |
| 6. | Trichophytan rubrum | 16.66 |
The data showed that the LFF of lichen was found a most effective antagonist against Fusarium udum and caused 55.55% inhibition of mycelial growth and against Trichophytan rubrum showed minimum antagonist i.e. 16.66%.
Antimicrobial screening of extracts of LFF and NT against test pathogens
The antibacterial activity of acetone and ethanol extracts of dried lichen and dried LFF against the different pathogenic microorganisms was estimated on the basis clear zone inhibition of pathogenic strains, the results were presented in table 2.
Table 2: Inhibition of pathogenic bacteria and fungi by natural extract and LFF
| S. No. | Extracts | Zone of inhibition against pathogens (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sm | Sa | St | Sty | Tr | Mg | Ef | Ca | ||
| 1. | Acetone (NT) | 12 | 15 | 13 | 15 | 14 | 16 | 15 | 14 |
| 2. | Ethanol (NT) | 09 | 10 | 05 | 13 | 10 | 09 | 12 | 12 |
| 3. | Acetone (LFF) | 17 | 18 | 14 | 19 | 18 | 21 | 17 | 19 |
| 4. | Ethanol (LFF) | 16 | 15 | 15 | 16 | 15 | 13 | 14 | 15 |
| 5. | Aqueous extract (NT) | - | 05 | - | - | - | - | 05 | 06 |
Streptococcus mutant (SM), Staphylococcus aureus (SA), Salmonella typhi (ST), Salmonella typhimurium (Sty), Trichophyton rubrum (Tr), Microsporum gypseum (Mg), Epidermophyton floccosum (Ef), Candida albicans (Ca), Cultured lichen (LFF) and Natural thallus (NT).
The aqueous extract (NT) showed no activity Staphylococcus aureus, Salmonella typhi, Salmonella typhimurium, Trichophyton rubrum and Microsporum gypseum. The minimum activity was recorded against Streptococcus mutant (05mm), maximum activity was found against Candida albicans (06mm). The acetone extract of natural thallus showed minimum activity against Streptococcus mutant (12mm), maximum activity was observed against Microsporum gypseum (16mm). The acetone extract of LFF showed minimum activity against Salmonella typhi (14mm), maximum activity was observed against Microsporum gypseum (21mm). The ethanol extract of natural thallus showed minimum activity against Salmonella typhi (05mm), maximum activity was observed against Salmonella typhimurium (13mm). The ethanol extract of LFF showed minimum activity against Microsporum gypseum (13mm), maximum activity was observed against Streptococcus mutant and Salmonella typhimurium (16mm). The differences of antibacterial activity between lichen extracts were dependent upon the solvent used for extraction. Rankovic et. al., 2007 found that the extracts of lichens Lasallia pustulata, Umbilicaria crustulosa, Parmelia sulcata and extracts of Umbilicaria cylindrical, with solvents used were acetone, methanol and aqueous have active antimicrobial properties against bacteria15. Rowe et. al., 1989 were also reported that many different extracts of lichens viz. Evernia prunastri, Pseudovernia furfuracea and Alectoria capillaries were active against gram-positive bacteria and Candida albicans [16]. All these studies indicated that the lichens inhibited the growth of mostly gram-positive bacteria. However, this may be due to the biochemical variations between grampositive and gram-negative bacteria. If so, it is great to note that Everniastrum cirrhatum inhibited the growth of both gram positive and gram negative bacteria as well as the growth of fungi also.
MIC of acetone (LFF) extract against pathogens
The MIC is the lowest concentration at which the bacterial growth is inhibited by the lichen extract and could be detected by pink well showing reduction of resazurin in the dilution series. The results obtained after microtiter plate assay are shown in table 3.
Table 3: MIC of acetone extract (LFF) against pathogens
| S. No. | Pathogenic culture strains | Minimal inhibitory concentration (µl/ml) |
|---|---|---|
| 1. | Streptococcus mutant | 6.25 × 10-4 |
| 2. | Salmonella typhimurium | 3.125 × 10-5 |
| 3. | Salmonella typhi | 12.5 × 10-3 |
| 4. | Staphycoccus aureus | 3.125 × 10-5 |
| 5. | Trichophyton rubrum | 3.125 × 10-5 |
| 6. | Microsporum gypseum | 0.78125 × 10-7 |
| 7. | Epidermophyton floccosum | 6.25 × 10-4 |
| 8. | Candida albicans | 0.78125 × 10-7 |
The MIC of acetone (LFF) extract showed 0.78125×10-7 μl against Microsporum gypseum and Candida albicans, while 3.125 × 10-5 μl against Staphycoccus aureus, Salmonella typhimurium and Trichophyton rubrum. The MIC of Streptococcus mutant and Epidermophyton floccosum was found to be 6.25 × 10-4 μl and the MIC of Salmonella typhi was found to be 12.5 × 10-3 μl where as Santiago et. al., 2010 were shown the Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of the crude extracts of lichen Ramalina dendriscoides (Rd), against S. aureus, which gave MIC/MBC of 156 μg/ml and 2500 μg/ml, respectively [17].
CONCLUSIONS
Malt, Yeast Extract Agar medium was the best medium for the isolation of mycobiont, antagonistic activity of mycobiont was observed maximum against Fusarium udum (55.55%) while, out of the extracts, acetone extract of LFF (Everniastrum cirrhatum) showed maximum antimicrobial activity against Microsporum gypseum (21mm), Salmonella typhimurium (19mm) and Candida albicans (19mm).
ACKNOWLEDGMENT
Thanks are due to Head, Department of Microbiology, Chaudhary Charan Singh University, Meerut for providing the facilities. Special thanks to the Centre of Excellence for Sanitary and Phytosanitary, SVPUA&T, Meerut for giving me a Senior Research Fellowship (SRF).
REFERENCES
- Hamada N, Ueno. Depsides from an isolated mycobiont. Agricullture and Biological chemistry. 1987, 51:1705-1706.
- Vartia KO. Antibiotics in lichens. The lichens Academic Press Inc., New York: Ahmadjian, V., Hale, M.E. (Eds.).1973, 547-561.
- Hamada N, Miyagawa H. Secondary metabolites from isolated mycobionts cultured under osmotic conditions. Lichenologist. 1995, 27:201-205.
- Huneck. The Significance of lichens and their metabolites. Naturwissenschaften. 1999, 86: 559-576.
- Aslan A, Aptroot A, Yazıcı K. New Lichens for Turkey. Mycotaxon. 2002; 84: 277-280.
- Burkholder PR, Evans AW, McVeigh I, Thornton HK. Antibiotic Activity of Lichens Proceedings on National Academic Science, USA, 1944, 30(9): 250-255.
- Tolpysheva T, Yu. Effects of lichen extracts of fungi II. Effects of joint preparation obtained from Cladina stellaris and C. rangiferins on growing soil fungi, J. Mycol. Phytopathalogy, 1984, 18(5): 384-388.
- Turk AO, Yilmaz M, Kivanc M, Turk H. Antimicrobial activity of extracts of the lichen Cetraria aculeata and its protolichesterinic acid constituent, Verlag der Zeitschrift fur Naturforschung (C). 2003, 58: 850-854.
- Yilmaz, M, Tay T, Kivanc M, Turk H, Turk AO. The Antimicrobial activity of extracts of the Lichen Hypogimnia tubulosa and its 3- Hydroxyphysodic Acid Constituent. Verlag der Zeitschrift fur Naturforschung [C]. 2005, 60(1-2): 35-38.
- Javeria S, Shahi SK, Shahi MP, Uperti DK. Parmotrema nilgherrense: potential antimicrobial activity against drug resistant pathogens. Microbial Resource Technology. 2013, 2(1): 36-40.
- Dharmadhikari M, Jite PK, Chettiar S. Antimicrobial activity of extracts of the lichen Parmelinella simplicior and its isolated mycobiont. Asian J. Exp. Biol. Sci. Spl. 2010, 54-58.
- Morton DJ, Strouvle WH. Antagonistic Stimulatory effect of soil microorganism upon Sclerotium rolfsii. Phytopath, 1955, 45, 417-420.
- Collins CH, Lyne PM. Microbiological Methods. 3rd ed. Baltimore7 University Park Press; 1970.
- Sarker SD, Nahar L, Kumarasamy. Microtiter Plate –Based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in vitro antibacterial screening of phytchemical. Methods. 2007, 42(4): 321-324.
- Rankovic B, Misjic M, Sukdolak S. Evaluation of Antimicrobial activity of the Lichens Lasallia pustulata, Parmelia sulcata, Umbilicaria crustulosa and Umbilicaria cylindrica J. Microbiol, 2007, 76: 723-727.
- Rowe JG, Saenz MT, Garcia. Contribution a a’l e’ tudedel’ activite antibactérienne de queques lichens du sudde l’Espagne. Pharmaceutique francais. 1989, 47: 89-94.
- Santiago1 KAA, Borricano1 JNC, Canal1 JN, Marcelo1 DMA, Perez1 MCP, Cruz1 TEE. Antibacterial activities of fruticose lichens collected from selected sites in Luzon Island, Philippines Philippine Science Letters. 2010, 3(2): 18-29.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences