ISSN : 0976-8505
Der Chemica Sinica
Isolation and Identification of Nicotiflorin from the Leaves of Costus spectabilis (Fenzl) K. Schum
1Département Sciences et Techniques, Institut Universitaire de Formation Professionnelle (IUFP), Université de Ségou, Mali
2Faculté des Sciences et Techniques, Département de chimie, Université des Sciences, des Techniques et des Technologies de Bamako, Mali
3Faculté des Sciences et Techniques, Département de Biologie, Mali
4Faculté d’Agronomie et de Médecine Animale (FAMA), Université de Ségou, Mali
5Département de Chimie Moléculaire et SERCO, Université Grenoble Alpes, Mali
Abstract
Costus spectabilis is a rhizomatous geophyte used by traditional medicine to treat internal and external wounds, coughs, inflammation, arthritis, rheumatism, fever, maternal and neonatal infections. It is also recommended for its laxative, purgative, diuretic and ichthyotoxic properties. A phytochemical study of the leaves of Costus spectabilis, revealed the presence of flavonoids, alkaloids, sterols and triterpenes, coumarins, reducing compounds, oses and holosides. The combination of chromatographic (CC and TLC), 1D NMR spectral (1H, 13C), 2D (COZY and HMQC) and spectrometric (ESI-MS positive mode) techniques allowed the isolation and identification of kaempferol 3- O- (6- O- (α-L-rhamnosyl) β-D-glucoside or nicotiflorin of the methanol extract of the leaves of Costus spectabilis.
Keywords
Costus spectabilis, phytochemistry, nicotiflorin, flavonoid, NMR, MS.
Introduction
Costus spectabilis is a rhizomatous geophyte native to much of tropical Africa [1]. In Mali, traditional medicine uses its leaves to treat internal and external wounds. Its leaves are also recommended against inflammation, arthritis, rheumatism, maternal and neonatal infections [2,3]. They are also used to prepare the laxative, the purgative, the diuretic [2] and are chewed and swallowed to give up fevers [4]. Its tuberous roots are used for their ichthyotoxic properties [5]. These pharmacological properties, whose interest is obvious, the new perspectives for the scientific valorization of Costus spectabilis. A study conducted in the United States at the University of Illinois (Chicago) in 2001 shows that the substances were medicated on the market, 122 transferred from plants. Of these naturally occurring molecules, 80% were used at the same time [6]. This work concerns phytochemical screening, isolation and identification of a bioactive compound, a nicotiflorin methanol extract from the leaves of Costus spectabilis. As such, it is a promising field of research, a title of great champion of open applications, as well as the valorization of medicinal plants, the obtaining of new bioactive molecules [7].
Materials and methods
Plant material
The plant material consists of the leaves of Costus spectabilis, harvested in Siby (Bamako) in october 2015. After drying in the shade, the samples were ground to obtain 800 g of fine powder. Botanical identification was carried out at DMT-MALI and confirmed by the nomenclature of the Angiosperms Phylogeny Group classification (APG III, 2009).
Apparatus
columns; silica (230-400 Mesh); analytical and preparative TLC plates (GF 254); Kofler's bench; Bruker Avance III 500 MHz spectrometer for 1H and 125 MHz for 13C; Bruker quadrupole spectrometer under electrospray and in positive mode.
Phytochemical Screening
We characterized the different chemical groups (sterols and polyterpenes, flavonoids, catechin and gallic tannins, alkaloids, coumarins, saponosides, cardiotonic glycosides, quinone compounds, cyanogenic compounds, reducing compounds, oses and holosides) by referring to the techniques described in works of N'Guessan et al. [8], Muanda [9] and Yahya [10].
Extraction, Isolation and Identification
Extraction and isolation
The powder (500 g) of the leaves of Costus spectabilis is extracted with cyclohexane to remove the pigments, then using methanol until exhaustion at room temperature. The resulting extract was evaporated to dryness and weighed. The separation of the methanol extract (JNKE5A2, 5 g) carried out by column chromatography on silica gel (230-400 Mesh), eluting with DCM / MeOH (8: 1) gave three fractions JNKE5A2 (1), 2 and 3). The column chromatographic fraction (JNKE5A2-2) (1.8 g) eluted with AcOEt / MeOH / Water (10: 1: 1) gave in turn three fractions JNKE5A2-3 (1, 2, 3 and 4). ). The compound JNKE5A2-3-1-2 (42 mg) is finally obtained from the JNKE5A2-3-1 pool (556 mg) selected for purification by preparative MMC in the AcOEt / MeOH / Water system (10: 1: 1).
Identification of the compound (JNKE5A2-3-1-2)
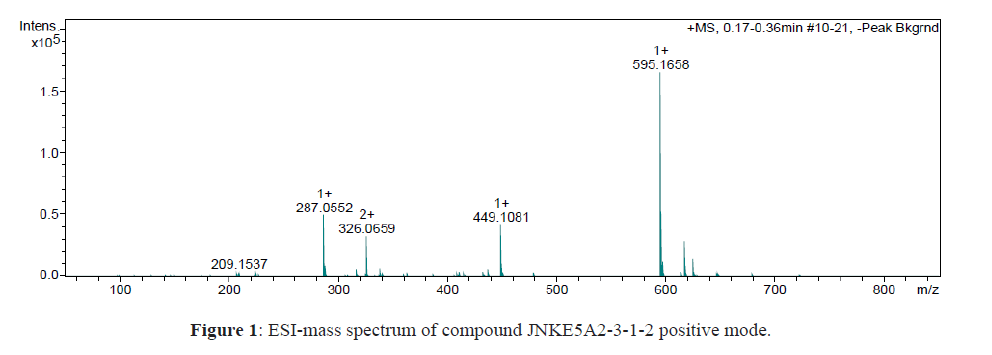
Yellow crystals soluble in MeOH, Rf = 3.6 (AcOEt / MeOH / Water: 10: 1: 1), mp 200°C. The SM / ESI spectrum shows a quasi-molecular ion [M-H] + at m / z 595 suggesting a molecular weight of 594u indicating the molecular formula C27H30O15. Fragmentation in ESI + of [M + Na] + reveals the ions at m / z 449 and at m / z 287 indicating the successive loss of a deoxyhexose (-146 u) then of a hexose (-146-162 u). In addition, the most abundant fragment at m / z 287 assumes the presence of a kaempferol genome (Figure 1) [11,12].
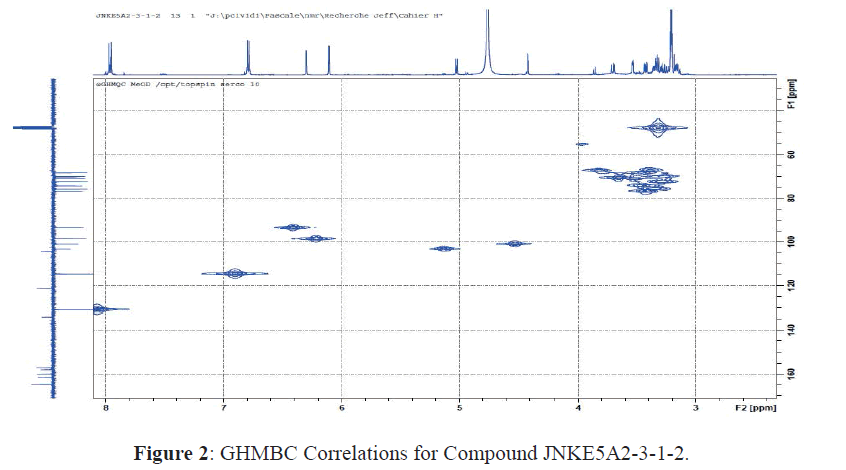
The 13C-NMR spectrum (Table 1) confirms the characteristic signals of the kaempferol genome: signal at δC 179.37 ppm of the carbonyl group, nine quaternary carbons (eight between 130 and 170 ppm and one at δC 105.56 ppm) and five aromatic CH (Table 1) [11-14]. The GHMBC spectrum indicates that these carbons correlate respectively with the anomeric protons at δH 4.53 and 5.00 ppm. These elements confirm the presence of two hexoses [11-14]. The 13 C-NMR shifts in the sugar region are characteristic, on the one hand, of a glucopyranose (C-2 '', C-3 '', C-4 '', C-5 '' and C-6 at δ C 75.76, 78.14, 71.43, 72.08 and 68.55 ppm) in the β-configuration (H-1''J = 7.4 Hz) and on the other hand, rhamnopyranose (C-2 '' ', C-3' '', C-4 '' ', C-5' '' and C-6 '' 'respectively at δC 71.43; 72.28; 73.89; 69,72 and 17,91) in α (H-1 '' 'J = 1.3 Hz) configuration (Figure 2) [11-14].
| n° | δC | δH (J en HZ) | HSQC | HMBC |
| 2 | 159,36 | - | - | C-2', C-6' |
| 3 | 135,50 | - | - | C-1’’ |
| 4 | 179,37 | - | - | - |
| 5 | 162,98 | - | - | - |
| 6 | 100,06 | 6,23 d (2,1) | H-6/C-6 | - |
| 7 | 166, 35 | - | - | C-6 |
| 8 | 94,98 | 6,43 d (2,1) | H-8/C-8 | - |
| 9 | 158,57 | - | - | C-8 |
| 10 | 105,56 | - | - | C-8, C-6 |
| 1’ | 122,74 | - | - | C-3', C-5' |
| 2’ | 132,37 | 8,10 d (8,9) | H-2’/C-2’ | C-6' |
| 3’ | 116,13 | 6,93 d (8,9) | H-3’/C-3’ | - |
| 4’ | 161,50 | - | - | C-2', C-6', C-3', C-5' |
| 5’ | 116,13 | 6,93 d (8,9) | H-5’/C-5’ | - |
| 6’ | 132,37 | 8,10 d (8,9) | H-6’/C-6’ | C-2' |
| 3-O-C(6)-glucoside | ||||
| 1’’ | 104,63 | 5,00 d (7,4) | H-1’’/C-1’’ | C-2’’ |
| 2’’ | 75,76 | 3,41-3,49 m | H-2’’/C-2’’ | C-3’’ |
| 3’’ | 78,14 | 3,41-3,49 m | H-3’’/C-3’’ | C-4’’, C-2’’ |
| 4’’ | 72,08 | 3,28-3,33 m | H-4’’/C-4’’ | C-6’’, C-3’’, C-5’’ |
| 5’’ | 77,20 | 3,41-3,49 m | H-5’’/C-5’’ | C-4’’, C-6’’ |
| 6’’a | 68,55 | 3,83 d (9,6) | Ha-6’’/C-6’’ | - |
| 6’’b | 3,41-3,49 m | Hb-6’’/C-6’’ | ||
| 6’’-O-rhamnosyl | ||||
| 1’’’ | 102,42 | 4,53 d (1,3) | H-1’’’/C-1’’’ | C-6’’ |
| 2’’’ | 71,43 | 3,67 dd (3,4 ; 1,6) | H-2’’’/C-2’’’ | C-4’’’ |
| 3’’’ | 72,28 | 3,56 dd (9,5 ; 3,5) | H-3’’’/C-3’’’ | C-2’’’, C-5’’’ |
| 4’’’ | 73,89 | 3,29-3,36 m | H-4’’’/C-4’’’ | C-6’’’, C-5’’’, C-3’’’ |
| 5’’’ | 69,72 | 3,46-3,50 m | H-5’’’/C-5’’’ | C-4’’’, C-4’’’, C-6’’’ |
| 6’’’ | 17,91 | 1,16 d (6,2) | H-6’’’/C-6’’’ | C-4’’’ |
Table 1: 1-H (500 MHz), 13-C (125 MHz) chemical shifts in CD3OD and GHSQC and GHMBC.
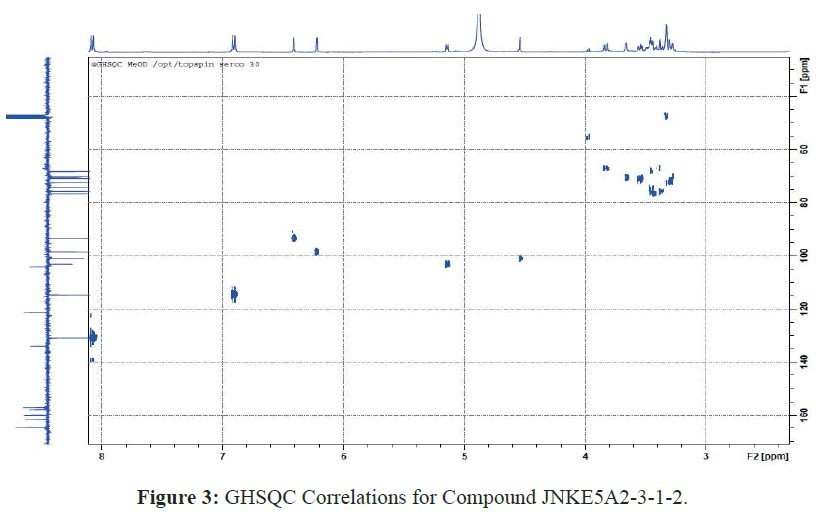
Moreover, the Heteronuclear Single-Quantum Correlation (HSQC) observed between the protons at δH 4.53 ppm (H-1 '' ') and the carbon located at δ 68.55 ppm (C-6' ') made it possible to show that the two sugars are bound in 1-6 and identify the diglycoside as rutinose (rhamnopyranosyl- (α1-6) -glucopyranoside) [11-14]. Rutinosis is attached to the genome Kaempferol at position 3 as evidenced by the HSQC correlation between C-3 carbon and the H-1 'anomeric proton (Figure 3) [11-14].
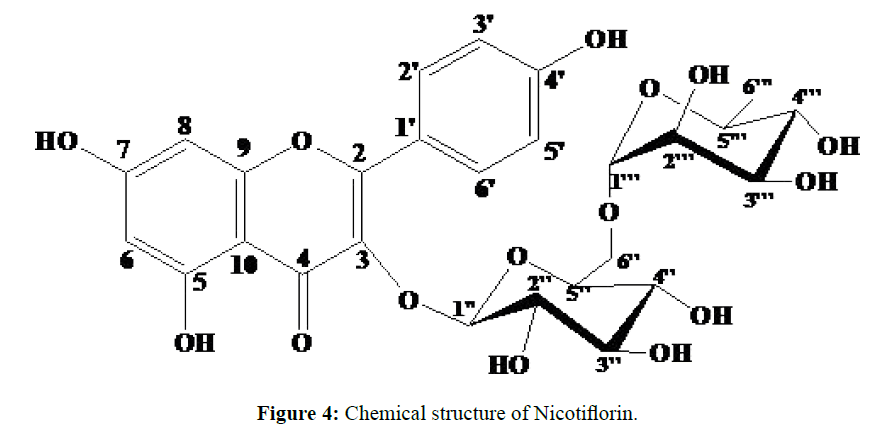
These data allowed us to identify JNKE5A-2-3-1-2 as kaempferol 3-O- (6-O- (α-L-rhamnosyl) β-D-glucoside known as nicotiflorin.
Results and Discussion
Phytochemical screening of the leaves of Costus spectabilis revealed the presence of flavonoids, coumarins, alkaloids, sterols and triterpenes, reducing compounds, oses and holosides. The methanolic extract showed no moderate selectivity between cancer and non-cancer cells [7]. These results suggest toxicity and should caution the traditional use of Costus spectabilis, despite its traditional pharmacological potential [7]. Flavonoids are compounds known for their antioxidant properties, they are therefore at the origin of physiological effects beneficial for the human organism and deserve the growing interest that the research brings to them [15,16]. Nicotiflorin (Figure 4) is a flavonoid diglycosyl type isolated from many plants including Astragalus verrucosus and Astragalus cruciatus [17], Heteropappus altaicus and H. Biennis [18], Solidago canadensis [19], Ficaria verna [20], Clitoria ternatea [21], Staphylea bumalda [22], Trigonotis peduncularis [23], Acalypha indica [24], Carthamus tinctorius [25], Caragana Bungei [26], Solanum campaniform [27], Osyris wightiana [28], Ampelopsis heterophylla [29], Amaranth [30] and Aspergillus awamori [31]. Although it is not an original structure, we note for the first time, the isolation of this compound from the leaves of Costus spectabilis.
Various studies have shown the pharmacological effects of nicotiflorin, such as inhibitors on hACAT1 (human AcylCoA: cholesterol transferase 1) [23], protective against memory dysfunction and oxidative stress in model rats with multiple infarct dementia [25], decreased blood pressure and heart rate [32], anti-glycation [33], hepatoprotective effect on CCl4-induced hepatic injury [34,35], anti-inflammatory, anti-nociceptive [36], inhibitors of α-glucosidase, with an activity more than 8 times higher than that of the reference antidiabetic drug, acarbose [37], antioxidant [37,43], antihypertensive, anti-anaphylactic [38] and neuroprotective [39,40]. Other studies have shown that it inhibits adipogenesis [41] and protects against ischemic brain damage [42,43].
Conclusion
We have found the presence of flavonoids, coumarins, alkaloids, sterols and triterpenes, reducing compounds, oses and holosides in the leaves of Costus spectabilis. This work also isolated nicotiflorin for the first time from Costus spectabilis. This molecule could be used to study and explain the medicinal properties of Costus spectabilis. For greater efficiency, we envision many perspectives including expanding the panel of activities by determining the acute oral toxicity, haemolysis, and cytotoxicity of the aqueous extract to assess the safety of use of Costus spectabilis, and continue the chemical investigations of other extracts of Costus spectabilis.
Acknowledgment
We thank the PADES project of the University of Ségou, through its steering committee for material assistance. We say a big thank you to Pascale Cividino of the DCM-SERCO of Grenoble Alpes University for the spectral analyzes and the SCAC of the French Embassy in Mali for the grant of a scientific stay grant.
References
- Costus (2014) Pacific bulb society.
- Nyananyo BL (2006) Plants From The Niger Delta. IJPAS 3: 21-25.
- Traoré CO (2014) Essai de monographie des soins infantiles traditionnels dans la commune II du district de Bamako: Cas de Medina-Coura. Thèse de doctorat, Faculté de Médecine et d’Odontostomatologie. P: 94.
- Kokwaro JO (1976) The Medicinal Plants of East Africa (Kampala: East Africa Literature Bureau). P: 244.
- Kimpouni V, Apani E, Motom M (2011) Plantes ichtyotoxiques et particularisme des usages au Congo (Brazzaville). Int J Biol Chem Sci 5: 979-990.
- Fabricant DS, Farnsworth NR (2001) The Value of Plants Used in Traditional Medicine for Drug Discovery. Environ Health Perspect 109: 69-75.
- Tagne RS, Telefo BP, Nyemb JN, Yemele DM, Njina SN, et al. (2014) Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac J Trop Dis 7: S442-S447.
- N’Guessan K, Kadjam B, Zirihi GN, Traoré D, Akéassi L (2009) Screening phytochimique de quelques plantes médicinales ivoiriennes utilisées en pays Krobou (Agboville, Côte-d’Ivoire). Sciences & Nature 6: 1-15.
- Muanda FN (2010) Identification de polyphenols, évaluation de leur activité antioxydante et étude de leurs propriétés biologiques. Thèse de Doctorat, Université Paul Verlaine-Metz.
- Al-Yahya MA (1986) Phytochemical studies of the plants used in traditional medicine of Saudi Arabia. Fitoterapia 57: 179-182.
- Ternai B, Markham KR (1976) Carbon-13 NMR studies of flavonoids-i flavones and flavonols. Tetrahedron 32: 565-569.
- Andersen OM, Markham KR (2005) Flavonoids chemistry, biochemistry and applications CRC press, 1st Edition.
- Markham KR (1982) Techniques of Flavonoid Identification; Academic Press: London, UK.
- Markham KR, Ternai B (1976) 13C NMR of flavonoids-II flavonoids other than flavone and flavonolaglycones. Tetrahedron 32: 2607-2612.
- Di-Carlo G, Mascolo N, Izzo AA, Capasso F (1999) Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sciences 65: 337-353.
- Ghedira K (2005) Les flavonoïdes: structure, propriétés biologiques, rôle prophylactique et emplois en thérapeutique. Phytothérapie 4: 162-169.
- Neslihan TKÖ, zgür Ç, Semra H, Fatih Karabey, SI (2018) Identification and production of phenolic nicotiflorin in astragalus chrysochlorus callus. Farmacia 66: 558-562.
- Bader G, Tuja D, Wray V, Hiller K (1993) Flavonol glycosides from Heteropappus altaicus and H. biennis. Planta Med 59: 284-285.
- Apati P, Szentmihalyi K, Balazs A, Baumann D (2002) HPLC analysis of the flavonoids in pharmaceutical preparations from Canadian goldenrod (Solidago canadensis). Chromatographia 56: S65-S68.
- Tomczyk M, Gudej J, Sochacki M (2002) Flavonoids from Ficaria verna huds. Zeitschrift für Naturforschung C 57: 440-444.
- Kazuma K, Noda N, Suzuki M (2003) Flavonol glycosides from the petals of Clitoria ternatea. Phytochem 62: 229-237.
- Sohn SJ, Kwon YS, Kim SS, Chun WJ (2004) Chemical constituents of the leaves of Staphylea bumalda. Nat Prod Sci 10: 173-176.
- Yang HJ, Song MC, Bang MH, Lee JH (2005) Development of biologically active compounds from edible plant sources-XII Flavonol glycosides from Trigonotis peduncularis benth and its hACAT1 inhibitory activity. J Korean Soc Appl Biol Chem 48: 98-102.
- Nahrstedt A, Hungeling M, Petereit F (2006) Flavonoids from Acalypha indica. Fitoterapia 77: 484-486.
- Huang JL, Fu ST, Jiang YY, Cao YB (2007) Protective effects of nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmac Biochem Behavior 86: 741-748.
- Olennikov DN, Tankhaeva LM, Partilkhaev VV, Rokhin AV (2012) Chemical constituents of Caragana bungei shoots. Rev bras farmacogn 22: 490-496.
- Torres MC, das-Chagas LPF, Braz-Filho R, Silveira ER (2011) Antiophidic solanidane steroidal alkaloids from Solanum campaniforme. J Nat Products 74: 2168-2173.
- Lal-Shyaula S, Abbas G, Siddiqui H, Sattar SA (2012) Synthesis and antiglycation activity of kaempferol3-O-rutinoside (nicotiflorin). Med Chem 8: 415-420.
- Chen P, Wu D, Pan Y (2013) Separation and purification of antioxidants from Ampelopsis heterophylla by countercurrent chromatography. J Sep Sci 36: 3660-3666.
- Kraujalis P, Venskutonis PR, Kraujaliene V, Pukalskas A (2013) Antioxidant properties and preliminary evaluation of phytochemical composition of different anatomical parts of amaranth. Plant Foods Hum Nutr 68: 322-328.
- Lin S, Zhu QQ, Wen LR, Yang B (2014) Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous-organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem 145: 220-227.
- Ahmad M, Hassan Gilani A, Aftab KH, Ahmad VU (1993) Effects of Kaempfrol 3-O-Rutinoside on Rat Blood Pressure. Phytother Res 7: 314-6.
- Sajan LS, Ghulam A, Hina S, Samina ASM, Iqbal C, et al. (2012) Synthesis and antiglycation activity of Kaempferol-3-O-rutinoside (Nicotiflorin). Med Chem 8: 415-420.
- Jun Z, Shilei Z, Shuping Y, Tao L, Fang X, et al. (2017) Hepatoprotective effects of nicotiflorin from nymphaea candida against concanavalin a-induced and d-galactosamine-induced liver injury in mice. Int J Mol Sci 18: 587.
- Wang Y, Tang CY, Zhang H (2015) Hepatoprotective effects of kaempferol 3-O-Rutinoside and Kaempferol 3-O-glucoside from Carthamus tinctorius L on CCl4-induced oxidative liver injury in mice. J Food Drug Anal 23: 310-317.
- Gamal-Eldeen AM, Kawashty SA, Ibrahim LF, Shabana MM, El-Negoumy SI (2004) Evaluation of antioxidant, antiinflammatory and antinociceptive properties of aerial parts of vicia sativa and its flavonoids. J Nat Remed 4: 81-96.
- Habtemariam S (2011) A-glucosidase inhibitory activity of kaempferol-3-O-rutinoside. Nat Prod Commun 6: 201-203
- Ishiguro K, Ohira Y, Oku H (2002) Preventive effects of impatiens balsamina on the hen egg-white lysozyme (HEL)-induced decrease in blood flow. Biol Pharm Bull 25: 505-508.
- Nakayama M, Aihara M, Chen YN, Araie M (2011) Neuroprotective effects of flavonoids on hypoxia glutamate and oxidative stress-induced retinal ganglion cell death. Mol Vision 17: 1784-1793.
- Li RP, Guo ML, Zhang G, Xu XF (2006) Neuroprotection of nicotiflorin in permanent focal cerebral ischemia and in neuronal cultures. Biol & Pharmac Bull 29: 1868-1872.
- Jang YS, Wang ZQ, Lee JM, Lee JY (2016) Screening of Korean natural products for anti-adipogenesis properties and isolation of kaempferol-3-O-rutinoside as a potent anti-adipogenetic compound from Solidago virgaurea. Molecules 21: 1-11.
- Li R, Guob M, Zhang G, Xua X, Li Q (2006) Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J Ethnopharmacol 107: 143-50.
- Abreu PM, Braham H, Ben JH, Mighri Z (2007) Antioxidant compounds from Ebenus pinnata. Fitoterapia 78: 32-34.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences