Investigations on the Potential of Marine Copepods (Acartia tonsa) as Starter Feeds for African Catfish (Clarias gariepinus) Larvae
Edun OM1*, Edun OM1 and Ukwe OIK2
1African Regional Aquaculture Center of Nigerian Institute for Oceanography and Marine Research, P.M.B.5122, Port Harcourt, Rivers State, Nigeria
2Department of Fisheries, Faculty of Agriculture, Rivers State University of Science and Technology, P.M.B. 5080, Nkpolu-Oroworkwo, Port Harcourt, Rivers State, Nigeria
- *Corresponding Author:
- Edun OM
African Regional Aquaculture
Center of Nigerian Institute for Oceanography and Marine Research
P.M.B.5122, Port Harcourt, Rivers State, Nigeria.
Tel: +86 20 8445 1346
E-mail: isyakoghert@gmail.com
Received date February 10, 2018; Accepted date March 12, 2018; Published date March 19, 2018
Citation: Edun OM, Edun OM, Ukwe OIK (2018) Investigations on the Potential of Marine Copepods (Acartia tonsa) as Starter Feeds for African Catfish (Clarias gariepinus) Larvae. Insights Aquac Cult Biotechnol Vol.2 No.1:2.
Abstract
This study investigated the effects of marine copepods, Acartia tonsa as live feeds on the survival and growth of Clarias gariepinus larvae in the hatchery. A total number of two hundred newly hatched larvae were fed for three weeks. The fish were assessed for survival, growth and nutrient utilization on weekly basis. The results obtained indicated that there was no significant difference (P>0.05) in the physico-chemical parameters of the water in the study period. The values of the water were within the ideal range for aquaculture practice. The microbial analysis of the experimental waters indicated that total heterotrophic bacteria, vibro, total coliform and Salmonela/Shigella were identified. However, total coliform count and Salmonela/Shigella were less than thirty (<30) in all the water samples. Survival rate (%) and growth of the fish significantly (P<0.05) increased as the experimental period increased. Moreover, there was steady reduction of the microbial flora as the fish gets older. The condition factor was less than one in all the treatments at the end of the third week. An indication that Acartia tonsa can serve as a starter feed for C.gariepinus larvae.
Keywords
Live feeds; Fish larvae; Acartia tonsa; Nutrition; Catfish
Introduction
Live feeds are phytoplankton and zooplanktons eaten by fish, they are readily available and mobile in the fish environment so as to be consumed by fish larvae [1]. They are main part of the diet for fish larvae especially marine fish and can provide the nutritional requirements of fish larvae [2]. They are refers to as “living capsules of nutrition”, because they contain essential nutrients such as proteins, lipids, vitamins, carbohydrates, amino acids, minerals and fatty acids. The nutritional content and composition of fresh water zooplanktons determines the growth and survive of fresh water fish larvae and fry [3]. Live feeds are said to be better than artificial diets in feeding of fish larvae. Adewumi [4] noted that poor growth and survival of fish larvae fed artificial diets have been attributed to the tenderness of the digestive track of the fish. Although feeding habits differ among fishes, all fish require protein rich live feed for proper growth, survival and productive breeding. The presence of live feed is very essential in the survival and growth of fish larvae, fry and fingerlings [5].
Acartia tonsa is a type of copepod. Their nutritional content is said to meet up the requirements for growth and survival of fish larvae and fry [2]. They are very important in global fish production and as at 1999, their support for capture fishery was about 92 million tones. When combined with artemia/rotifer, Acartia tonsa enhance survival and growth of fish larvae and similar result of better growth and survival was also reported for exclusive feeding of Acartia tonsa to fish larvae [6]. The size of the Acartia tonsa determines its acceptability by the fish larvae [7]. They have high nutrient levels, their protein and free amino acid concentrations are higher than that of cultured Rotifers and Artemia, with balanced amino acid composition [8].
The C. gariepinus is a preferred fish for aquaculture for so many reasons which include growth rate, good market price, hardiness, easy to breed, etc., and this has made it a subject of investigation. Some of the studies on C. gariepinus include its nutrition and production of its fingerlings [9]. Hatchery production of larvae as against capture fisheries has become a routine operation in modern aquaculture and the highest mortalities during rearing of C. gariepinus have been observed between the larvae and fry stage. The first important stage in the life of the larval is the transition stage, from the endogenous to the exogenous feeding and live food is a necessity for the C. gariepinus at this stage [10]. This study therefore investigates the potential of marine copepods Acatia tonsa as a starter live feeds for C. gariepinus fish.
Materials and Methods
Study area
The project work was carried out in the hatchery unit of the University of Port Harcourt, Choba Campus, Rivers State, Nigeria. Port Harcourt the capital of Rivers State lies between longitude 4° and 6° East at Greenwich median and 7° and 8° of the Equator The climate of the study area is sub-tropical and characterized by high atmospheric temperature of 27.5°C and relative humidity fluctuating between 70-90% .The annual rainfalls of the Niger Delta is between 2000-3000 mm per year [11]. Dry season lasts for about six (6) months between November-April with occasional rainfall. Generally, rainfalls throughout the year in Niger Delta particularly Port Harcourt.
Stock density
A total of 200 larvae of 4.8 ± 0.16 mg weight and 6.16 ± 0.30 mm lengths were transferred to each of the experimental tanks (40 × 25 × 25 cm3) that were properly labeled. The weight was obtained by the use of a Rohr sensitive electric weighing balance (Model no 3002N, by Want Instrument Co. Ltd., Shanghai, China). A wet filter paper was placed in the balance and reduced to zero, twenty five larvae were collected randomly from the hatchery at the end of endogenous feeding and placed at the zero weight filter paper and the weight was taken, and its average determined. This was repeated five times, and the average of the results of the five sets was taken as the weight of the individual larvae in the hatchery. Five set of five larvae each was measured using a transparent millimeter calibrated ruler and a magnifying hand lens. The average lengths of each of the sets were taken and the mean of the various set was taken as length of each larva in the hatchery. Feeding commenced 12 h after stocking.
Physico-chemical parameters
The temperature was taken by the use of mercury in glass thermometer calibrated in degree centigrade (0-100°C). The thermometer was immersed in the experimental water column and was allowed to stand for 5 min. The reading was taken immediately the thermometer was removed from the water. An average of three (3) measurements was taken per tank, during reading. The pH value of the water was determined by the use of a pH meter, pocket pen pH meter model 700, made in Japan. The dissolved oxygen (D.O), ammonia and Nitrite were determined using a 9-series multi-parameter water quality meter (Bante 980 Precision Meter, Bante Instruments, Beijing China) Version Number: 2009070200. The ammonia, nitrate and nitrite test was conducted using La Motte Aquaculture test kit MODEL AQ-4, CODE 3635-04, Chester town, Maryland, 21620. USA.
Feeding and evaluation of growth parameters
The larvae were fed 10% of their body weight of feeds per day. They were fed six times daily.
The length of the fish was measured by the use of a transmitted millimeter calibrated ruler and a magnifying hand lens. The initial larva length was 6.16+0.30 mm and measurements were done at days 7, 14 and 21.
While the weight was determined by the use of an electric sensitive weighing balance (model: 3002N, No.110628014, made in Shangai, China by Wart Instrument Co. Ltd). The initial larva weight before stocking was 4.8+0.16 mg and weighing was done at 7, 14 and 21 day.
The survival of the larvae during the trial rate was determined using the formulal:
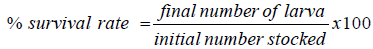
Specific growth rate (SGR) [12]
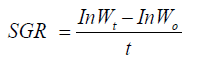
Where: W1=Final body weight, Wo=Initial body weight, t=Time (days)
The Condition Factor (K) [13]: This was calculated using the formula:
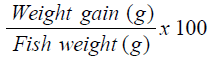
W=Weight (g) and L=Length (cm)
Percentage Weight Gain (PWG) [14]
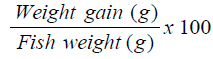
Absolute growth rate (AGR) [15]
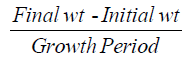
Daily weight gain (DWG)
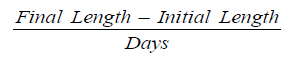
Average daily length gain (ADLG) [15]
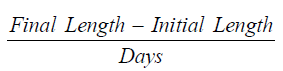
Relative weight gain (RWG) [16]
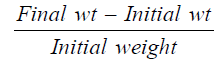
Proximate analysis
Proximate analysis was conducted to determine the percentage composition of the test feeds, using the standard of analysis of the Association of Official Analytical Chemist [17].
Microbiological analysis
The total number of coliforms, Salmonella shigella and Vibro sp. in a water sample was determined by the most probable number (MPN) test [18].
Statistical analysis of data
Statistical analysis was carried out on all data using the SPSS VERSION 12 for windows. Data was pooled by treatment and presented as mean + standard deviation (SD) and standard error (SE).Data was analyzed for treatment effect by one way analysis of variance (ANOVA). The Turkey Post hoc test was used to 95% confidence level to produce specific information on which means are significantly different from each other.
Results
Physiochemical parameter of water in experimental tanks
The result of the physicochemical parameters of water in experimental tanks is shown in Table 1. There were no significant difference (P>0.05) in the values of temperature, pH and dissolved oxygen of the water in all the experimental tanks. While ammonia, nitrate and nitrite were 0.00 (zero) in all the experimental waters.
| Parameters | Experimental Weeks | ||
|---|---|---|---|
| Week 1 | Week 2 | Week 3 | |
| Temperature (°C) | 27.85 ± 1.15 | 27.49 ± 1.29 | 27.96 ± 1.34 |
| pH | 6.11 ± 0.45 | 6.13 ± 0.48 | 6.26 ± 0.19 |
| Dissolved Oxygen (mg/l) | 6.32 ± 0.16 | 6.37 ± 0.16 | 6.09 ± 0.11 |
| NH3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Nitrate | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Nitrite | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Table 1 Values of physiochemical parameters of water in the experimental container (Mean ± SD).
Growth response in C. gariepinus fed experimental diets
The growth responses of larvae to the experimental diets are shown in Table 2. The final length and weight of the larva differs significantly (P>0.05) within the experimental period. The highest value of length and weight were recorded in the third week and the lowest values were observed in the first week of the experimental period. The percentage survival differs significantly (P<0.05) among the experimental period. The highest survival rate was recorded in week 1, while the lowest percentage survival was recorded in week 3. The specific growth rate of the larvae also differs significantly (P<0.05) among the experimental period. The highest rate was recorded in week 2 and the lowest in week 1. The condition factor differs significantly (P<0.05) among the larvae fed with Acartia tonsa in the experimental periods. There were significant differences (P<0.05) in percentage weight gain, absolute growth rate and relative weight gained among the larvae fed experimental diets during the experimental period. There was significance difference (P<0.05) in average daily growth rate in C. gariepinus fed Acatia tonsa.
| Parameters | Experimental Weeks | ||
|---|---|---|---|
| Week 1 | Week 2 | Week 3 | |
| Final Length (mm) | 2.86 ± 0.56a | 5.433 ± 0.56 b | 27.51 ± 4.73 c |
| Final Weight (g) | 11.20 ± 3.46 a | 30.20 ± 6.55 b | 70.20 ± 6.00 c |
| Survival (%) | 81.00 ± 9.01 c | 67.667 ± 2.92 b | 65.53 ± 11.33 a |
| Specific Growth Rate (%) | 9.913 ± 1.06 a | 13.87 ± 0.53 b | 12.76 ± 0.41 b |
| Condition Factor | 1.87 ± 0.04 b | 2.49 ± 0.63 c | 0.19 ± 0.01 a |
| Daily Weight Gained (g) | 0.14 ± 0.01 a | 0.08 ± 0.01 a | 0.03 ± 0.02 a |
| Percentage Weight Gained | 58.25 ± 12.93 a | 87.44 ± 0.82 b | 93.57 ± 0.52 c |
| Absolute Growth Rate | 1.12 ± 0.33 a | 2.39 ± 0.19 b | 3.34 ± 0.28 c |
| Average Daily Growth Rate | 0.41 ± 0.08 a | 0.39 ± 0.05 a | 1.30 ± 0.22 b |
| Relative Weight Gained | 1.64 ± 0.48 a | 6.94 ± 0.54 b | 14.63 ± 1.25 c |
Table 2: Growth response in C. gariepinus fry fed experimental diets for 21 days (Mean ± SD).
Microbial analysis of water in experimental containers
The experimental water for the larvae fed Acartia tonsa during the periods of the experiment was analyzed for the four microbial floras (Table 3). There were 5.0 × 102 total heterotrophic at the end of the first week. At the end of the second week, Total heterotrophic count was 3.5 × 102 cfu/ml and it reduced to 2.963 × 102cfu/ at the third week. The counts of the other three microbial flora (Vibro Count, Total Coliform Count and Salmonela/Shigella) were less than thirty (<30) in the experimental waters.
| Parameters | Experimental Weeks | ||
|---|---|---|---|
| Week 1 | Week 2 | Week 3 | |
| Total Heterotrophic Count (cfu/ml) | 5.00 × 102 | 3.50 × 102 | 2.65 × 102 |
| Vibro Count (cfu/ml) | <30 | <30 | <30 |
| Total Coliform Count (cfu/ml) | <30 | <30 | <30 |
| Salmonela/Shigella (cfu/ml) | <30 | <30 | <30 |
Table 3 Microbial analysis of water in experimental containers
Discussion
The application of copepods as live feed in larviculture has been developed since 1980s [19]. In nature, copepods is probably the most important factor in the diet of marine and fresh water fish larvae, so it could generally be concluded that the nutritional composition of copepods satisfy the requirements of fish larvae [3]. Many studies showed that using copepods alone or in combination with rotifers or Artemia, improved the growth, survival rate and frequency of normal pigmentation of fish larvae when compared to using only rotifers or Artemia, as reported for Atlantic halibut (Hippoglossus hippoglossus), Golden snapper (Lutjanus johnii), grouper (Epinephelus coioides)) and Australian seahorse (Hippocampus subelongatus) [5,11,20]. In this study, using the calanoid copepods (Acartia tonsa) during larval first feeding period of C. gariepinus larvae resulted in a better survival and growth, of the fish. This result is in line with the observations made in Atlantic cod (Gadus morhua) larvae), where a better growth and stress tolerance, were observed [21]. Also, the same growth response was observed in Ballan wrasse (Labrus bergylta) larvae [22].This can be due to superior nutritional value of Acartia and higher digestibility in comparison to enriched rotifer and Artemia [4]. Furthermore, small sizes (<100 μm) of nauplii and/or first copepodite stages can make them become a first feed for small larvae with small mouth gape. With zigzagging swimming pattern, most copepod nauplii and copepodites strongly stimulated the feeding behavior of fish larvae [23,24].
At the end of 21 days experimental period, growth and survival obtained in larvae fish were significantly higher, indicating that feeding of fish with copepods resulted in an increased larval development and satisfactory survival rates, as corroborated by advanced morphological changes and dark pigmentation. This same trend was observed in dusky grouper (Epinephelus marginatus), larviculture fed with Acartia tonsa in a semi intensive conditions [25]. Pigmentation is also an important parameter for fresh water fish larvae, indicating good health and nutrition, since copepods are also a source of vitamins C and E, astaxanthin and polar lipids, which are more available to fish larvae [26,27]. Improved survival and growth of fish larvae fed copepods associated with improved nutritional composition have been well documented [2,7,28]. Moreover, Schipp et al. also reported that Acartia tonsa are small in size (about 100 μm), easily digested and are still a good source of antioxidants [29]. Another important advantage of copepods is their constant jerking swimming motion, providing a visual stimulus for the larvae of catfish fish, which are recognized as visual predators.
Conclusion
The results obtained from this experiment shows that the Acartia tonsa considered in this experiment showed commendable response to growth parameters. Before now, Artemia have been known as one of the best starter feed, when it concerns general growth rate, survival rate and well-being of Bacterial larvae, but the result of this work indicates that the larvae fed Acartia tonsa performed very well, an indication that Acartia can effectively replace some starter feeds used in aquaculture.
References
- David AB (2003) Status of Marine aquaculture in relation to live pray: Past, present and future. Live feeds in Marine aquaculture. Blackwell Publishing, pp: 1-16.
- Evjemo JO, Reitan KI, Olsen Y (2003) Copepods as live food organism in the larval rearing of halibut larva (Hippoglossus hippoglossus L.) with special emphasis on the nutritional value. Aquaculture 227: 191-210.
- Adigun BA (2005) Water quality management in aquaculture and freshwater zooplankton production for use in fish hatcheries. (pp. 12-13). New Bussa, Niger State, Nigeria.
- Adewumi AA (2015) Growth performance and survival of Clarias gariepinus hatchlings fed different starter diets. Eur J Exp Bio 5: 1-5.
- Adewolu MA, Adenij CA, Adejobi B (2008) Feed utilization, growth and survival of Clarias gariepinus (Burchell 1822) fingerlings cultured under different photoperiod. Aquaculture 283: 64-67.
- Drillet G, Iversen MH, Sorensen TF, Ramlov H, Lund T, et al.(2006) Effect of cold storage upon eggs of calanoid copepod Acartia tonsa and their offspring. Aquaculture 254: 714-729.
- Chesney EJ (2005) Copepods as live prey: A review of factors that influence the feeding success of marine fish larvae. In: Lee CS, O’Bryen PJ, Marcus NH. (Eds.), copepods in Aquaculture. Blackwell Publishing Ames, pp: 133-150.
- Craig SR, Connie R, Holt JG (1994) Effects of enriching live foods with unsaturated fatty acids on the growth and fatty acids composition of larva red drum Sciannops ocellatus. J World Aquacult Soc 25: 424-434.
- Adewolu MA, Akintola SL, Akinwunmi OO (2009) Growth performance and survival of hybrid African catfish larvae (Clarias gariepinus × Heterobranchus bidorsalis) fed different diets. Zoologists 7: 45-51.
- Ajah PO (2010) Prey selection and predation behavior of Heterobranchus longifilis Vol. (1840) larva during first exogenous feeding. Afr J Food Sci 4: 73-79.
- Gobo A (1988) Relationship between Rainfall Trends and flooding in Niger Delta – Benue River Basin. J Meteorol UK 13: 220-224.
- Arimoro F (2007) First feeding in African catfish Clarias anguillaris fry in tanks with fresh water rotifer Branchinus calyciflorus cultured in a continuous feedback mechanism in comparison with mixed zooplankton diet. J Fish Aquat Sci 2: 275-284.
- Peanase P, Mengumphan K (2015) Growth performance length-weight relationship and condition factor of backcross and reciprocal hybrid catfish reared in Ned cages. Int J Zoo Res 11:57-64.
- Richinr WE (1979) Growth rates and models in: W.S. Hoar, Bionenergeic and Growth. Academic Press, New York, pp: 682-743.
- Orisamuko EA.(2006) Influence of diets on the growth of the African river prawn, Macrobranchuim vollenhoveni. Nig J Fish 2: 110-126.
- AOAC (1990) Official Methods of Analysis (15th edn; K. Holdrick, Editor). Association of Official Analytical Chemists, Virginia, USA, pp: 125-291.
- Mbagwu IG, Adeniji HA (1988) The nutritional content of duck weed (Lemna paucicostata hegelm) in the Kainj lake area, Nigeria. Aqua Bot 29: 357-366.
- APHA (1998) Standard methods for the extermination of water and waste water, 20th edn. Washington D.C., p: 1193.
- Overrien I (2010) Copepods lipids in aquaculture. Department of Biotechnology, NTNU (Norwegian University of Science and Technology.
- Patra SK, Mohamed KS (2003) Enrichment of Artemia nauphlii with the probiotic yeast Sacccaromyces boulardii and its resistance against a pathogenic vibrio. Aquacult Int 11: 505-514.
- Hansen GH, Olafsen JA (1989) Bacterial colonization of cod (Gadus morhua L.) and halibut (Hippoglosus hippoglossus) eggs in marine aquaculture. Appl Environ Microbiol 55: 1435-1445.
- Al-Harbbi AH, Uddin MN (2008) Aerobic bacteria flora of common carp (Cyprinus carpio L) cultured in earthen ponds in Saudi Arabia. J Appl Aquacult 20: 108-119.
- Abelin P, Tackaert W, Sorgeloos S (2011) Ensiled Artemia biomass: A promising and practical feed for Penaeid shrimp post larvae. In: Lavens P, Sorgeloos P, Jaspers E, Ollevier F, 2 Eds, Larvi 91: Fish and Crustacean Larviculture Symposium. European Aquaculture Society, pp: 125-127.
- Abdulraheen I, Otubusin SO, Agbebi OT, Olowofeso O, Alegbeleye WO, et al. (2012). The growth response of Clarias gariepinus hatchlings to different dry feeds. J Agricult Sci 4: 75-80.
- Russo T, Boglione C, Marzi P, Cataudella S (2009) Feeding preferences of the dusky grouper (Epinephelus marginatus, Lowe, 1834) larvae reared in semi-intensive conditions: A contribution addressing the domestication of this species. Aquaculture 289: 289-296.
- Sargent, J, Bell G, McEvoy L, Tocher D, Esteves A (1999)Recent developments in the essential fatty acid nutrition of fish. Aquaculture 177: 191-199.
- Sargent, J, McEvoy L, Esteves A, Bell G, Bell M, et al. (1999) Lipid nutrition of marine fish during early development: Current status and future directions. Aquaculture 179: 217-229.
- Schipp G (2006) The use of calanoid copepod in semi-intensive, tropical marine fish larviculture. Avances em nutrición acuícola. VIII Simposium Internacional de Nutrición Acuícola, Monterrey, Nuevo León, pp: 84-94.
- Schipp, GR, Bosmans JMP, Marshall AJ (1999) A method for hatchery culture of tropical calanoid copepods, Acartia spp. Aquaculture 174: 81-88.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
