ISSN : 0976-8505
Der Chemica Sinica
Investigation of the Behavior of Aminated Jojoba Derivatives as Green Corrosion Inhibitors for Mild Steel at 0.5 N HCl
1Petroleum Applications Department, Egyptian Petroleum Research Institute, Nasr City, Cairo, Egypt
2Jazan University, Faculty of Science and Arts, Samtah, Saudi Arabia
Abstract
Jojoba oil was brominated using bromine, then three aminated jojoba derivatives were synthesized via amination of the brominated jojoba oil, with different types of amines (aniline; 2, 4-dimethyl aniline, and 4-amino benzoic acid). The prepared compounds were elucidated using FT-IR and 1H-NMR. The behavior of the aminated jojoba derivatives was investigated as corrosion inhibitors at different temperatures (308, 318, 328 and 338K) for mild steel using 0.5N HCl via weight loss method, and chemical analysis method. The prepared compounds exhibit anti-corrosion properties for mild steel at acidic medium; while corrosion inhibitor II based on 2, 4- dimethyl aniline exhibits an excellent corrosion protection property.
Keywords
Aminated jojoba oil, Corrosion inhibitors, Mild steel, Weight loss method, Chemical analysis method
Introduction
Jojoba plant is well known in botanical literature as Simmondsia Chinensis [1-4]. It is a desert shrub that grows wild in Southern Arizona, north-western Mexico and neighboring areas. Greene and Foster [5] were the first to report that jojoba nuts contained about 46% of liquid oil which resembled sperm whale oil in its analytical characteristics and they are considered to be a liquid wax, which consist mainly of fatty acid esters of decyl alcohol, that contain two double bonds in each constituent molecule.
The potential importance of jojoba oil for industrial uses has stimulated research. Jojoba oil has been endorsed at the field of cosmetics; such as hair oil, creams, shampoo, conditioning rinse. Due to its higher thermal and oxidative stability, scientists all over the world increased there scientific searches on the industrial applications of jojoba oil. Jojoba oil has been used as lubricant [6-8], additives for lubricant [9,10], surfactant [11], micro-emulsion [12], antioxidants [13], corrosion inhibitors [14], friction modifiers [15], viscosity index improvers and pour point depressants [16-19], extreme pressure additives [20], bio-fuel [21-23], and bio-diesel [24-26].
In the present work, novel green aminated derivatives of jojoba oil were synthesized via brominating of jojoba oil using bromine, then amination of the brominated jojoba derivative using different types of amines. The prepared amines were investigated as corrosion inhibitors for mild steel at acidic medium, using different intervals of temperatures.
Materials and Methods
Materials
Jojoba oil was purchased from Dreams Mill for oils; Saudi Arabia, bromine, aniline, 4- amino benzoic acid and 2, 4-dimethyl aniline, were purchased from Aldrich.
Procedures
Brominating of Jojoba Oil
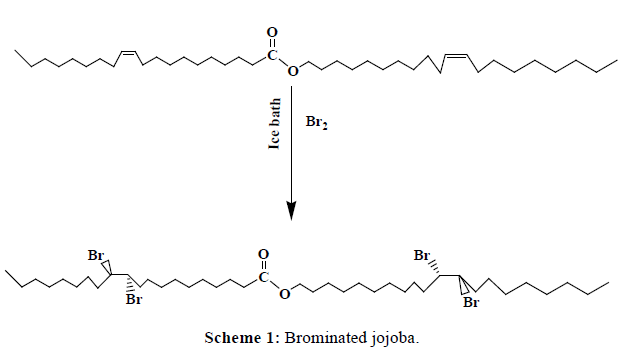
In an ice bath a 500 ml round bottom flask containing (1 mole of jojoba oil, 324 g) was connected directly to a dropping funnel containing 2 moles of bromine (319.62 g). The bromine was allowed to the reaction drop by drop till the end of addition (the color of the oil changed from yellow to dark brown), Scheme 1.
Amination of brominated jojoba with different types of amines
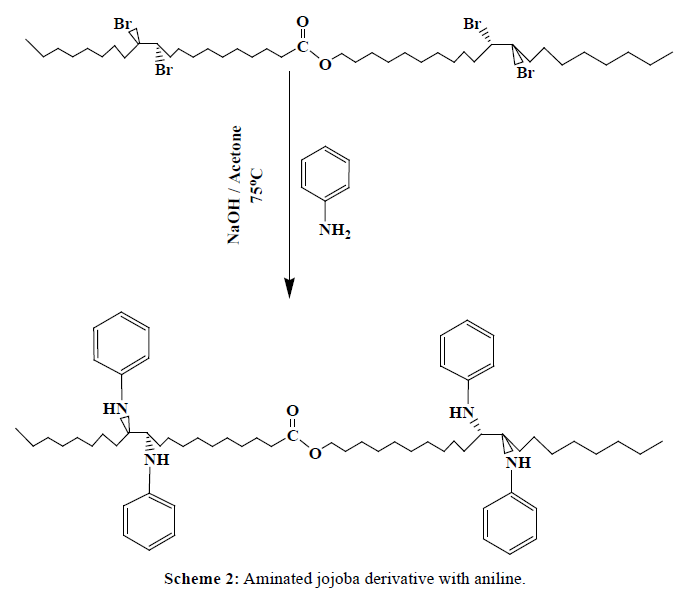
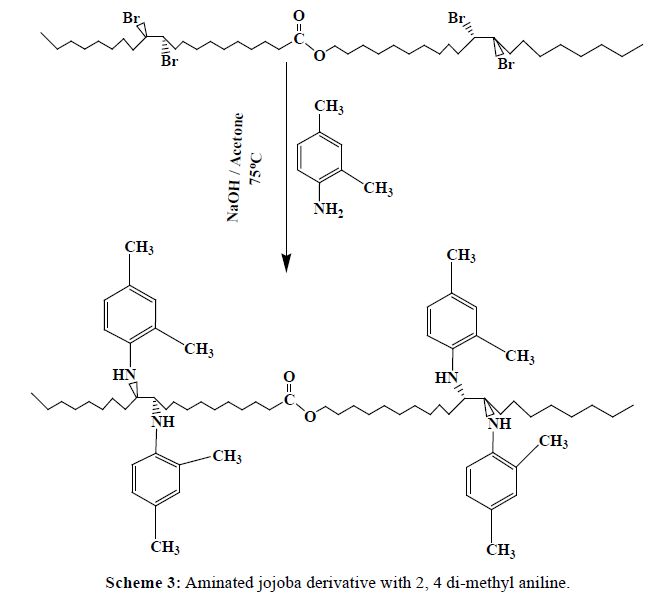
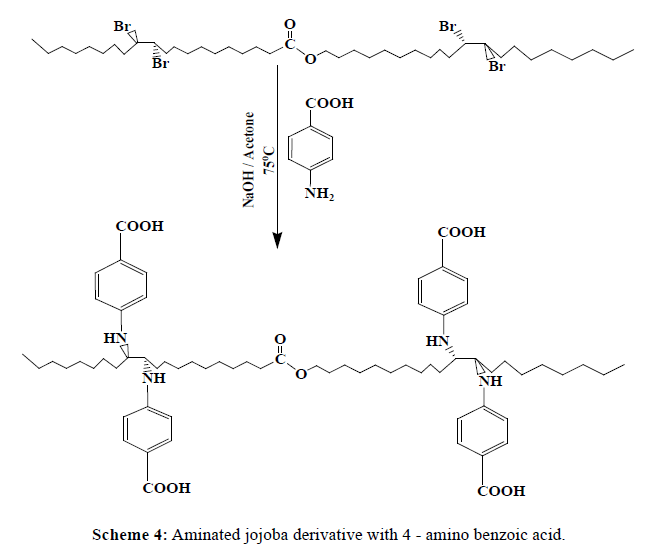
In 1000 ml round bottom flask, about 1 mole of brominated jojoba (643.62 g) was added to 4 mole of [aniline (372 g), 2,4-dimethyl aniline (484 g) , and 4-amino benzoic acid (548 g)] separately, 10 ml of Acetone, and 3 ml of 0.5N NaOH solution. Connect the flask directly to condenser and the reactants were allowed to condense at 75°C for one hour, Schemes 2-4. The products (J1-J3) were washed after cooling with distilled water separately, to remove the excess NaOH solution, and then wash with acetone. Table 1, indicates the designation and the chemical composition of the prepared compounds.
| Designation | Composition |
|---|---|
| J1 | Brominated jojoba with aniline |
| J2 | Brominated jojoba with 2, 4-di methyl aniline |
| J3 | Brominated jojoba with 4-amino benzoic acid |
Table 1: Chemical composition of the prepared (J1 – J3) compounds
Elucidation of chemical structure of the prepared compounds
Using FT-IR
The prepared compounds were elucidated using Nicolet iS10-FTIR Spectrophotometer, KBr, Jazan University, Saudi Arabia
Using 1H-NMR
The structure of the prepared compounds were elucidated using Proton Nuclear Magnetic Resonance "1H-NMR" spectra using a 300 MHz Varion NMR-300 spectrometer using DMSO as a solvent, NCR, Giza, Egypt.
Corrosion studies
Weight Loss Measurements: Mild steel coupons (CS37) having chemical composition mentioned in Table 2, with dimensions 7.0 × 5.0 × 0.3 cm were abraded using different grades of emery papers grades (310, 410, and 610), washed with bi-distilled water, degreased with acetone, dried and kept in a desiccator, weight accurately using a digital balance with high sensitivity. The coupons were immersed in 0.5N HCl solution with and without various concentrations (250, 500, 1000, 2000, and 3000) ppm of the prepared inhibitor for 3 hours, at different temperatures (308, 318, 328 and 338K). Weight loss experiments were carried out according to the ASTM G 31-72 standard procedure described in reference [27]. In brief, carbon steel specimens in triplicate were immersed in 100 ml 0.5N HCl containing various concentrations of the studied inhibitors. The mass of the specimens before and after immersion was determined using an analytical balance accurate to 0.01 mg. Reading were taken after each one hour, the coupons were washed with bi-distilled water, dried and weighed accurately [28-35]. The investigations carried out in the open air. For further data processing, the average of the three replicate values was used. The pre-cleaned and weighed coupons were suspended in beakers containing the test solutions using glass hooks and rods. The weight loss was calculated by equation (1):
| Element | C | Mn | P | S | Si | Fe |
|---|---|---|---|---|---|---|
| Ratio (%) | 0.11 | 0.45 | 0.04 | 0.05 | 0.25 | 99.10 |
Table 2: Chemical composition of mild steel (CS-37) alloy
ΔW=W1-W2 (1)
Where W1 and W2 are the weights of coupons before and after immersion.
The surface coverage area (θ) for the different concentrations of the prepared corrosion inhibitors in 0.5N HCl was calculated using equation (2):
θ=1-(W1/W2) (2)
The inhibition efficiency according to weight loss method IEw (%) was determined by equation (3):
IEw%=(Wcorr/W`corr) × 100 (3)
Where Wcorr and W`corr are the corrosion rate of coupon with and without corrosion inhibitors, respectively.
The corrosion rate (CR) was calculated by equation (4):
CR= (W1-W2)/At (4)
Where W1 and W2 are the weight of coupons before and after immersion in test solutions. A is the total area of the coupon (cm2), and t is the immersion time (h).
Chemical Analysis of Solution according to weight loss method
When a metal undergoes corrosion in an electrolyte of a fixed volume, the cations of corroding metal will accumulate in solution. Accordingly, the solution becomes more concentrated in the dissolved cations with the progression of time [36]. Thus, chemical analysis of withdrawn aliquots of the solution as a function of time allows determination of the corrosion rate according to equation (5).
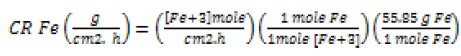 (5)
(5)
Results and Discussion
Electrophiles that have leaving groups (X) attached to C (sp3) usually undergo substitution or elimination reactions. Nucleophilic substitution at carbon has received exceptionally detailed mechanistic study by organic chemists. The reaction is of broad synthetic utility, and many individual observations as accumulated before systematic efforts to characterize the reaction mechanism began. In a nucleophilic substitution reaction, a nucleophile- electrophile σ bond replaces the electrophile -Xσ bond. Substitution reactions at 1°C and 2°C (sp3) usually proceed by the SN2 mechanism under basic or neutral conditions. In SN2 mechanism, the nucleophile Nu- approaches the electrophilic center opposite X and in line with the C-X bond. The lone pair on Nu- is used to form the C-Nu bond, and the pair of electrons in the C-X bond simultaneously leaves with X as the bond breaks. The other three groups on C move away from Nu and toward X as the reaction proceeds, so that when the reaction is complete, the stereochemistry of C is inverted [37].
Elucidation of the prepared compounds
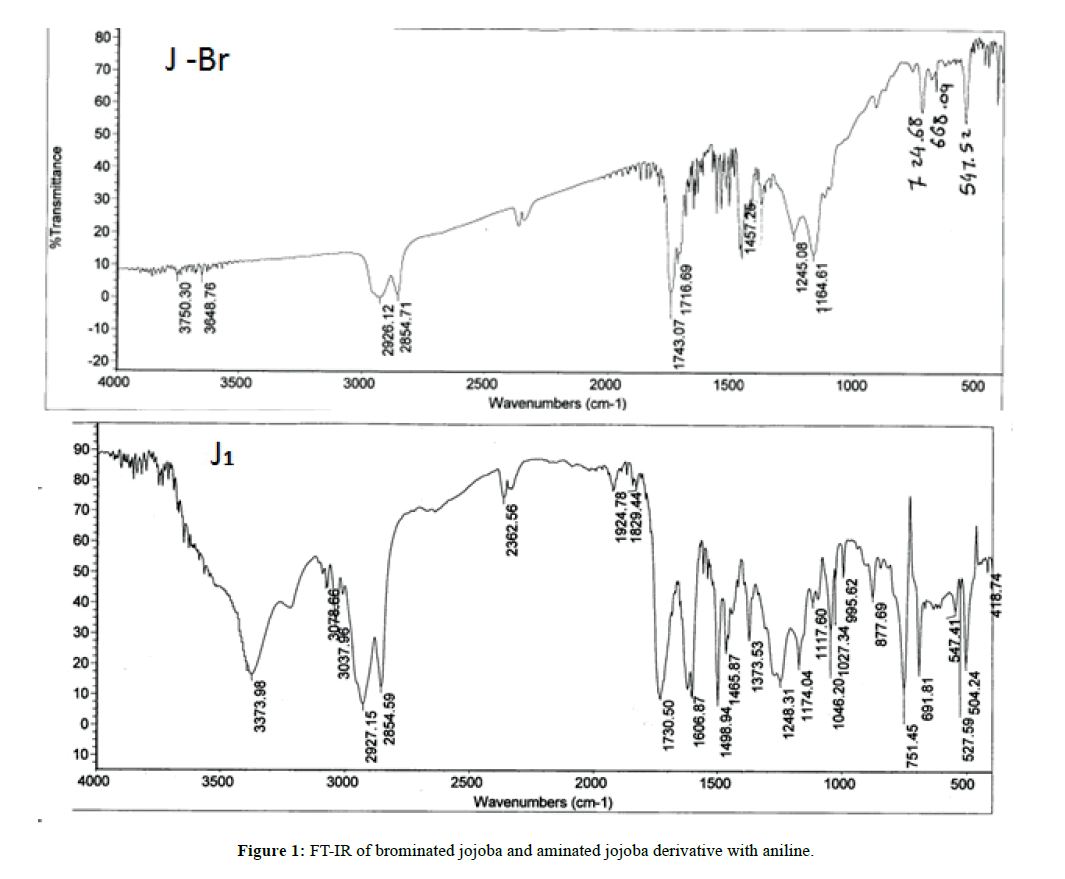
The prepared brominated jojoba derivative (J) was elucidated using Fourier Transform Infrared Spectroscopy (FT-IR), Figure 1. One can noticed the disappearance of (C=C) peak at 1638 cm-1 and appearance of peak corresponding to C-Br bond at 725 cm-1, this confirm the complete addition at C=C bond. FT-IR spectrum of J1 compound as an example of the prepared aminated jojoba derivatives indicates the disappearance of C-Br bond at 725 cm-1 and appearance of one peak at 3374 cm-1 crossponding to NH group. The presence of a peak at 1730 cm-1 insures the stability of the ester group toward amination reaction. 1H-NMR analysis of brominated jojoba (J) and aminated jojoba derivatives (J1-J3) are mentioned at Table 3.
| Compound digestion | Group | ppm |
|---|---|---|
| J | -CH3 -CH2 -(CH2)n -CH2-CH-Br -CH-Br -CO-CH2 -CH2-O -O-CO-C |
0.96 1.33 1.25 1.75 3.83 1.68 2.25 4.08 |
| J1 | -α, ε CH-Ph -β, δ CH-Ph -γ CH-Ph -NH -CO-O-CH2 -CO-O-CH2-CH2 -CO-CH2 -CO-CH2-CH2 -CH-NH -CH2-CH-NH -(CH2)n -CH2-CH3 -CH3 |
6.43 7.04 6.58 4.00 4.08 1.57 2.25 1.68 2.84 1.48 1.29 1.33 0.96 |
| J2 | -ε CH-Ph -δ CH-Ph -Ph-CH3 -β CH-Ph -CO-O-CH2 -CO-CH2 -NH -CH2-C-N -(CH2)n-CH2-CN -O-CH2-CH2 -(CH2)n -CH2-CH3 -CH3 |
6.19 6.65 2.35 6.64 4.08 2.25 4.00 1.48 2.84 1.57 1.29 1.33 0.96 |
| J3 | -COOH -α CH-Ph -β CH-Ph -NH -CH2-CN -CH2-CO -CH2-O-CO -O-CH2-CH2 -(CH2)n -CH2-CH3 -CH3 |
11 7.91 6.64 4.00 1.48 2.25 4.08 1.57 1.29 1.33 0.96 |
Table 3: 1H-NMR data of the prepared (J) and (J1-J3) compounds
Corrosion tests
Weight loss method: The inhibition efficiency of the organic compounds depends on many factors including the number of adsorption sites and their charge density, molecular size, heat of hydrogenation, mode of interaction with the metal surface and formation of metallic complexes [38]. Weight loss parameters were mentioned at Table 4.
| Inhibitor | Concentration "ppm" | Total weight loss (mg) | Corrosion Rate (mg.cm-2.h-1) |
Surface Coverage (q) | IE % |
|---|---|---|---|---|---|
| Bank | 0 | 650 | 6.19 | ……. | ……. |
| J1 | 250 | 80 | 0.7619 | 0.8769 | 87.69 |
| 500 | 78.77 | 0.7502 | 0.8788 | 87.88 | |
| 1000 | 68 | 0.6476 | 0.8953 | 89.53 | |
| 2000 | 47.57 | 0.4531 | 0.9268 | 92.68 | |
| 3000 | 39 | 0.3714 | 0.94 | 94.00 | |
| J2 | 250 | 59 | 0.5651 | 0.9087 | 90.87 |
| 500 | 41.20 | 0.3924 | 0.9366 | 93.66 | |
| 1000 | 26.712 | 0.2544 | 0.9589 | 95.89 | |
| 2000 | 15.20 | 0.145 | 0.9766 | 97.66 | |
| 3000 | 10.01 | 0.0953 | 0.9846 | 98.46 | |
| J3 | 250 | 94.50 | 0.90 | 0.8546 | 85.46 |
| 500 | 84.76 | 0.8071 | 0.8696 | 86.96 | |
| 1000 | 78 | 0.74 | 0.8800 | 88.00 | |
| 2000 | 53.10 | 0.5057 | 0.9183 | 91.83 | |
| 3000 | 41.21 | 0.3924 | 0.9366 | 93.66 |
Table 4: Weight loss parameters of mild steel (CS-37) treated without and with the prepared corrosion inhibitors (J1-J3) at 308K
Effect of (J1-J3) concentrations
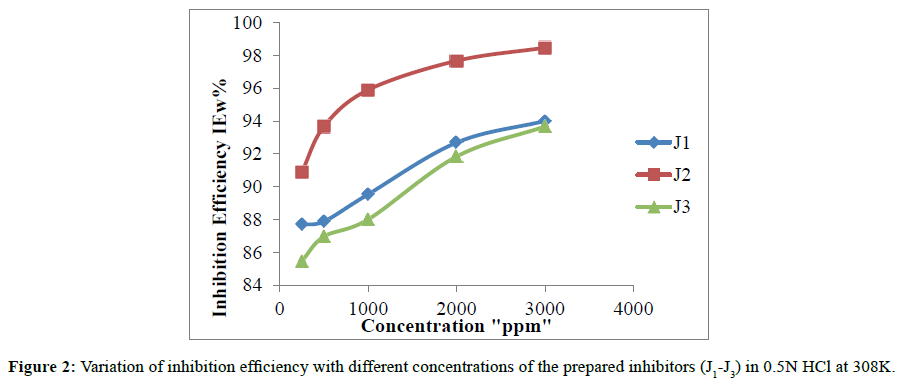
The variation of inhibition efficiency (IEw%) from weight loss measurements with (J1-J3) concentrations is shown in Figure 2, while the weight loss parameters; corrosion rate CR, surface coverage θ, and percentage of inhibition efficiency increases with increasing (J1-J3) concentration and record the maximum value (94.00% J1, 98.46% J2, and 93.66% for J3) of inhibition efficiency at 3000 ppm concentration. The inhibition efficiency of the prepared additives increases in the order J2>J1>J3. The highest inhibition efficiency of inhibitor J2 may be attributed to the presence of two electron releasing groups (methyl group).
Effect of temperature
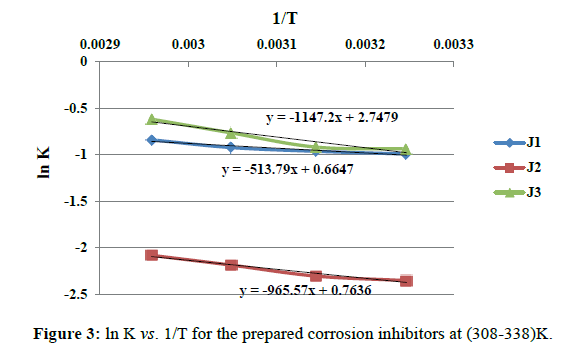
In order to calculate the activation energy (Ea) of the corrosion process and investigate the mechanism of inhibition, weight loss measurements were performed in the temperature range of 308-338K in absence and presence of 3000 ppm of the prepared inhibitors (J1-J3) at 0.5N HCl. It was found that the corrosion rate of mild steel CS-37 increases with increasing temperature. The results indicates that increasing temperature leads to a decrease of RT, hence increasing the corrosion rate of carbon steel as shown in Figure 3. A plot of ln corrosion rate (ln k) against the reciprocal of absolute temperature (1/T) was drawn graphically to obtain activation energy Ea, according to Arrhenius equation:
ln K=-(Ea/RT)+constant (5)
Where Ea equal to slope of this equation.
Adsorption isotherm
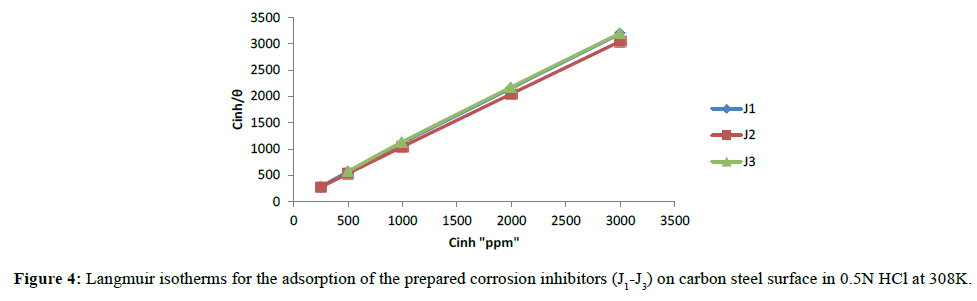
To understand the corrosion inhibition mechanism, the organic compound’s adsorption behaviour on the carbon steel surface must be known. The plot of Cinh/θ vs. Cinh, Figure 4 yielded a straight line, proved that the adsorption of the prepared corrosion inhibitors from the hydrochloric acid solution obeys Langmuir adsorption isotherm, which is presented by equation (6) [34].
Cinh/θ=1/Kads+Cinh (6)
Where Cinh is the inhibitor concentration and Kads is the equilibrium constant for the adsorption/desorption process. From the intercepts of the straight lines on the Cinh/θ-axis, one can calculate Kads, which is related to the standard free energy of adsorption, ΔGads, as given by equation (7) [35]:
ΔGads=-RT(ln Kads × 55.5) (7)
The calculated free energy of adsorption (ΔGads) is given in Table 5. The negative values of ΔGads indicated that the adsorption of the inhibitors on the metal surface is spontaneous. Generally, values of ΔGads around 20 kJ mol-1 or lower are consistent with the electrostatic interaction between charged molecules and the charged metal surface (physisorption and chemisorption); It can be seen from Table 5 that, calculated ΔGads values indicated that the adsorption mechanism of the prepared corrosion inhibitors on carbon steel in 0.5N HCl solution is physical and chemical adsorption. The large values of ΔGads and its negative sign are usually characteristic of strong interaction and a highly efficient adsorption. The aminated derivatives of soybean oil were generally chemisorbed at the metal surface and displace the adsorbed water and electrolytes from the surface. It is assumed that these N-containing functional groups act as electron pair donors to the electron depleted dehydrated metal surface. This interaction between the inhibitor and the metal surface is due to the formation of a bond between the N electron pair and the electron cloud at the metal surface.
| Inhibitor | Kads | ∆Gads (KJ/mole) |
|---|---|---|
| J1 | 42.272 | -19.549 |
| J2 | 29.684 | -18.659 |
| J3 | 51.043 | -20.024 |
Table 5: Adsorption parameters for the prepared corrosion inhibitors (J1-J3) on CS-37 in 0.5N HCl at 308K
Corded metal concentration
The concentration of the corroded metal was calculated using chemical analysis method according to weight loss method at 308K. It was found that the concentration of the corroded metal decreases with increasing the concentration of corrosion inhibitors, while at 3000 ppm concentration of J2 corrosion inhibitor has the lowest concentration of corroded metal “1.01 × 10-5” (Table 6).
| Inhibitor | Concentration "ppm" | Concentration of [Fe+3] Ion (mol/cm2.h) |
|---|---|---|
| Blank | 0 | 0.0000726 |
| J1 | 3000 | 6.65 ´ 10-6 |
| 2000 | 8.11 ´ 10-6 | |
| 1000 | 1.159 ´ 10-5 | |
| 500 | 1.343 ´ 10-5 | |
| 250 | 1.36 ´ 10-5 | |
| J2 | 3000 | 1.706 ´ 10-6 |
| 2000 | 2.596 ´ 10-6 | |
| 1000 | 4.55 ´ 10-6 | |
| 500 | 7.026 ´ 10-6 | |
| 250 | 1.0119 ´ 10-5 | |
| J3 | 3000 | 7.026 ´ 10-6 |
| 2000 | 9.055 ´ 10-6 | |
| 1000 | 1.325 ´ 10-5 | |
| 500 | 1.445 ´ 10-5 | |
| 250 | 1.612 ´ 10-5 |
Table 6: Concentration of corroded iron by chemical analysis according to weight loss method
Conclusion
Three green corrosion inhibitors were prepared via reaction of brominated jojoba oil with different types of amines "aniline, 2,4- dimethyl aniline and 4-amino benzoic acid". The aminated derivatives were elucidated using FT-IR and 1H-NMR, and they were evaluated as corrosion inhibitors for mild steel CS-37 at 0.5N HCl, using weight loss method and chemical analysis method at different temperatures "308-338"K. It was found that the inhibition efficiency "IEw%" increases with increasing the inhibitor concentration, while decrease with rising of temperature and the order of increasing the inhibition efficiency "IEw%" using the prepared corrosion inhibitors was as fellow: J3<J1<J2. The adsorption of the prepared inhibitors on metal surfaces from 0.5N HCl solution obeys Langmuir adsorption isotherm. The high value of adsorption equilibrium constant suggested that the prepared organic corrosion inhibitors were strongly chemically and physically adsorbed on the carbon steel surfaces. Chemical analysis method was used for the determination of concentration of the corroded iron according to weight loss calculations and it was found that the concentration of the corroded iron decreases with increasing concentration of the inhibitor, and the order of increasing the corroded iron was as fellow: J2>J1>J3.
Acknowledgements
I would like to thank the management of Faculty of Science and Arts, Samtah, Jazan University for providing me with chemicals and analysis.
References
- Jaime W (1977) Jojoba Oil and Derivatives. Prog Chem Fats other Lipids 55: 167-218.
- Daugherty PM, Sineath HH, Wastler TA (1958) Industrial raw materials of plant origin. IV. A survey ofSimmondsia chinensis (Jojoba). Econ Bot 12: 296-304.
- Gentry JS (1958) The natural history of Jojoba (Simmondsia chinensis) and its cultural aspects. Econ Bot 12: 261-295.
- Mirov NT (1952) Simmondsia or Jojoba-a problem in economy Botany. Econ Bot 6: 41-47.
- Greene RA, Foster ED (1933) The liquid wax of seeds of Simmondsia californica. Bot Gazette 94: 826-828.
- Allawzi M, Abu-Arabi MK, Al- Zoubi HS, Tamimi A (1998) Physicochemical characteristics and thermal stability of Jordanian jojoba oil. J Am Oil Chem Soc 75: 57-62.
- Phillip SL, Frank E (1989) Jojoba oil and jojoba oil derivative lubricant compositions. US patent 4,873, 008.
- Biresaw G (2006) Elastohydrodynamic Properties of Seed Oils. J Am Oil Chem Soc 83: 559-566.
- Bisht RPS, Sivasankaran GA, Bhatia VK (1992) Additive properties of jojoba oil for lubricating oil formulations. Wear 161: 193-197.
- George A (1987) Lubricant additive concentrate containing isomerized jojoba oil. US patent 4,664,821.
- Arnon S, Horowitz E (1980) Quaternary ammonium salts derived from jojoba oil. J Am Oil Chem Soc pp.161-166.
- Shevachman M, Shani A, Garti N (2004) Formation and investigation of microemulsions based on jojoba oil and nonionic surfactants. J Am Oil Chem Soc 81: 1143-1152.
- Fox NJ, Stachowiak GW (2007) Vegetable oil based lubricants-A review of oxidation. Tribol Int 40: 1035-1046.
- Chetouani A, Hammouti B, Benkaddour M (2004) Corrosion inhibition of iron in hydrochloric acid solution by jojoba oil. Pigm Resin Technol, 33: 26-31.
- Biresaw G, Adhvaryu A, Erhan SZ (2003) Friction properties of vegetable oils. J Am Oil Chem Soc 80: 697-704.
- Nasser RM, Ahmed NS, Nasser RM (2014) Terpolymers for modifying the performance properties of engine oil. Appl Petrochem Res 5: 61-69.
- Nassar AM, Ahmed NS, Nasser RM (2015) Green lubricating oil additives. Chem Sin 6: 100-107.
- Nassar AM, Ahmed NS, Nasser RM (2015) Jojoba polymers as lubricating oil additives. Pet Coal 57: 120-129.
- Ahmed NS, Nassar AM, Nasser RM (2015) Jojoba modified polymers as performance modifiers additives for engine oil. Ind Lubr Tribol 67: 425-433.
- Miwa TK, Rothfus JA, Dimitroff E (1979) Extreme pressure lubricant tests on jojoba and sperm whale oils. J Am Oil Chem Soc 56: 765-770.
- Radwan MS, Ismail MA, Elfeky SMS, Abu-Elyazeed OSM (2007) Jojoba methyl ester as a diesel fuel substitute: preparation and Characterization. Appl Therm Eng 27: 314-322.
- Mohamed YES, Radwan MS, Saleh HE (2008) Improving the performance of dual fuel engines running on natural gas/LPG by using pilot fuel derived from jojoba seeds. Renew Energy 33: 1173-1185.
- Shah SN, Sharma BK, Moser BR, Erhan SZ (2010) Preparation and evaluation of jojoba oil methyl esters as biodiesel and as a blend component in Ultra-low sulfur diesel fuel. Bioenerg Res 3: 214-223.
- Saleh HE (2009) Experimental study on diesel engine nitrogen oxide reduction running with jojoba methyl ester by exhaust gas recirculation. Fuel 88: 1357-1364.
- Shehata MS, Abel Razek SM (2011) Experimental investigation of diesel engine performance and emission characteristics using jojoba/diesel blend and sunflower oil. Fuel 90: 886-897.
- Mohamed YES, Radwan MS, Elfeky SMS (2003) Combustion of jojoba methyl ester in an indirect injection diesel engine. Renew Energy 28: 1401-1420.
- ASTM G 31-72, standard practice for laboratory immersion corrosion testing of metals (1990) West Conshohocken, PA, USA.
- Mthar M, Ali H, Quraishi MA (2002) Corrosion inhibition of carbon steel in hydrochloric acid by organic compounds containing heteroatoms. Br Corros J 37: 155-158.
- Selvi ST, Raman V, Rajendran N (2003) The performance of a migrating corrosion inhibitor suitable for reinforced concrete. J Appl Electrochem 33: 1182-1189.
- Bentiss F, Traisnel M, Vezin H, Hildebrand HF, Lagrenee M (2004) 2,5-Bis(4-dimethylaminophenyl)-1,3,4- oxadiazole and 2,5-bis(4-dimethylaminophenyl)- 1,3,4-thiadiazole as corrosion inhibitors for mild steel in acidic media. Corros Sci 46: 2781-2792.
- Noor EA (2005) The inhibition of mild steel corrosion in phosphoric acid solutions by some N-heterocyclic compounds in the salt form. Corros Sci 47: 33-55.
- Yurt A, Bereket G, Balaban AB, Erk E (2005) Effect of schiff bases containing pyridyl group as corrosion inhibitors for low carbon steel in 0.1 M HCl. J Appl Electrochem 35: 1025-1032.
- Li X, Tang L, Li L, Mu G, Li G (2006) Synergistic inhibition between o-phenanthroline and chloride ion for steel Corrosion in sulphuric acid. Corros Sci 48: 308-321.
- Al-Sabagh AM, Kandile NG, Nasser NM, Mishrif MR, El-Tabey AE (2013) Novel surfactants incorporated with 1,3,5-triethanolhexahydro-1,3,5-triazine moiety as corrosion inhibitors for carbon steel in hydrochloric acid: Electrochemical and quantum chemical investigations. Egypt J Pet 22: 351-365.
- Al-Sabagh AM, Abd-El-Bary HM, El-Ghazawy RA, Mishrif MR, Hussein BM (2011) Corrosion inhibition efficiency of linear alkyl benzene derivatives for carbon steel pipelines in 1M HCl. Egypt J Pet 20: 33-45.
- McCafferty E (2010) Introduction to corrosion Science; Springer Science and Business Media LLC, 119-175.
- Robert BG (2003) The Art of Writing Reasonable Organic Reaction Mechanism, 2nd Edition; Springer Science and Business Media, New York, pp. 50-57.
- Mouayed YK, Abdulsada AM, Alshawi JMS (2014) synthesis, identification and the study of some new azo dyes as corrosion inhibitors for copper in (1m) HCl. Journal of Basrah Researches (Sciences) 40: 108-127.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences