ISSN : 0976 - 8688
Der Pharmacia Sinica
Insights on Analytical Methods for Determination of Risperidone, Levetiracetam, Sodium Valproate and Oxcarbazepine
Mahmoud M Sebaiy1*,Hisham Elrefay2, Omnia A Ismaiel2, Wafaa S Hassan2, Abdalla Shalaby2 and Ali Fouad3
1Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
2Department of Analytical Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt
3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Al-Azhar University, Assiut, Egypt
- *Corresponding Author:
- Mahmoud M Sebaiy, Department of Medicinal Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt, Tel: 1062780060; E-mail: mmsebai@zu.edu.eg
Received: 18-Feb-2022, Manuscript No. IPDPS-22-12238; Editor assigned: 21-Feb-2022, Pre QC No. IPDPS-22-12238 (PQ); Reviewed: 7-Mar-2022, QC No. IPDPS-22-12238; Revised: 11-Mar-2022, Manuscript No. IPDPS-22-12238 (R); Published: 18-Mar-2022, DOI: 10.36648/0976-8688/22.13.13
Citation: Sebaiy MM, Elrefay H, Ismaiel OA, HassanWS, Shalaby A , et al. (2022) Insights on Analytical Methods for Determination of Risperidone, Levetiracetam, Sodium Valproate and Oxcarbazepine. Der Pharmacia Sinica Vol:13 No:2
Abstract
In this literature review, we will introduce mode of action and most of up-to-date reported methods that have been developed for determination of certain antipsychotic drugs such as risperidone, levetiracetam, sodium valproate and oxcarbazepine in their pure form, combined form with other drugs, combined form with degradation products, and in biological samples. Most of reported methods include spectrophotometric and chromatographic methods in addition to some electrochemistry methods.
Keywords
Review; Antipsychotic; Risperidone; Levetiracetam; Sodium valproate; Oxcarbazepine.
Introduction
Epilepsy, which affects approximately 1% of the world’s population, is a chronic disorder that usually persists for many years and often for a lifetime [1]. Antiepileptic Drugs (AEDs) are the mainstay of epilepsy treatment, and complete seizure control can be achieved in the majority (65%) of newly diagnosed patients by prescribing a single AED, and this is the ideal situation [2]. For the remaining 35% of patients, the prescribing of polytherapy regimens (the use primarily of two AEDs but often three or four AEDs), so as to achieve optimal seizure control, is a common practice.
However, for the majority of these patients, little additional benefit is achieved from the use of polytherapy AEDs as intolerable adverse effects commonly occur as a consequence of pharmacokinetic and/or pharmacodynamic interactions. Furthermore, for those patients that respond to monotherapy, they too may experience the consequences of AED interactions as AEDs are added and withdrawn during the optimization of their monotherapy drug regimen [3-5]. A further confounding factor is that since epilepsy is a chronic condition many patients will inevitably develop co-morbid diseases or other debilitating conditions and disorders, which will require the co-administration of non-AEDs. In this setting the potential for drug interactions is considerable [6]. A further source of potential clinically significant interactions that is being increasingly recognized relates to the increasing use of over-the-counter medications and supplements, many of which have unknown constituents and inconsistent quality [7]. Finally, AEDs are increasingly used to treat
Antipsychotic Drugs (APDs) can be separated into two classes: ‘‘conventional’’ APDs (CAPDs) and ‘‘atypical’’ APDs (AAPDs). The former includes 18 drugs developed between the 1950s and the 1970s. Their mechanism of action resides in their ability to block dopamine (DA-2) receptors, at the level of Meso-cortical, Nigrostriatal and Tuberoinfundibular DA pathways [8].
As such, in this review article, four antipsychotic drugs have been studied in respect of physical, chemical characters, mode of action and most reported analytical methods that have been developed for determination of these drugs in different matrices.
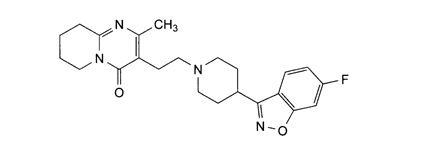
Risperidone (RSP)
- Chemical name: benzisoxazole derivatives and chemically, it is 3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one [9].
- Molecular formula: C23H27FN4O2
- Molecular weight:5 gm/mol.
- Physical properties: It is White or almost white powder. It is practically insoluble in water, freely soluble in methylene chloride, sparingly soluble in ethanol (96 per cent). It dissolves in dilute acid solutions. It shows polymorphism [9].
- Melting point: 170°C.
Pharmacological action: RSP is a dopamine D2 receptor antagonist; serotonin 5HT2 receptor antagonist; neuroleptic [9]. RSP is mostly metabolized by alicyclic hydroxylation and oxidative N-dealkylation [10]. RSP is used to treat schizophrenia, symptoms of bipolar disorder (manic depression) and irritability in autistic children [11,12].
Methods of determination
Official methods
The BP [9] proposes a non-aqueous titration for the determination of RSP. The drug was dissolved in anhydrous acetic acid followed by addition of methyl ethyl ketone then titration with 0.1 M perchloric acid, determining the end-point potentiometrically [9].
Spectroscopic methods
Literature describes different spectroscopic methods for determination of RSP. A simple UV spectroscopic determination was carried out at an absorption maximum of 238 nm using 0.1 NHCl as solvent [13]. A simple, non-destructive, methodology based on FT-Raman spectroscopy was developed for the quantitative analysis of RSP in commercially available film-coated tablets. A simple linear regression model was constructed based on standard tablets, prepared using the same manufacturing process as the commercially available [14]. A simple, sensitive, specific, spectrophotometric method developed for the detection of RSP in bulk drug and Pharmaceutical formulation. The optimum conditions for the analysis of the drug were established. The λ max of the RSP was found to be 280 nm [15]. A simple, sensitive, specific, spectrophotometric using methanol as a solvent method has been developed for the detection of RSP in pure form and pharmaceutical dosage forms. The optimum condition for the analysis of the drug were established. RSP exhibiting absorption both at 240 and 280 nm [16].
Chromatographic methods
Several chromatographic methods were described for the determination of the proposed drug either in pure or in combination with other drugs summarized in Table 1.
Table 1: Chromatographic methods for the determination of RSP in pure form or in combination with other drugs.
| Drugs | Method | Column | Mobile phase | Detector | λ |
|---|---|---|---|---|---|
| RSP | HPLC | Waters Xterra RP C8 column (250* 4.6 mm, 5 µ) | Mixture of solution (10 mM potassium dihydrogen phosphate, pH 3.5 ± 0.05): acetonitrile: methanol (65: 20: 15) |
UV | 276 nm |
| RSP, paliperidone and olanzapine | HPLC-MS | Xbidge™ C18 column (3.5 μm, 100 × 2.1 mm) | 70% acetonitrile and 30% ammonium hydroxide 1% solution | Mass spectrometry | Product ion191.2 (m/z) |
| RSP | LC/DAD | Purosphere STAR RP-C18 250×4.5 mm (5 μ) | Mixture of water: glacial acetic acid 0.50%: triethylamine 0.80%: acetonitrile (65.00: 0.32: 0.52: 34.16, v/v), | DAD | 294 nm |
| RSP, Olanzapine, Quetiapine, Clozapine, Ziprasidone, Perospirone, Aripiprazole and Blonanserin | LC/MS/MS | Mightysil-RP-C18 MS column (2.0 mm × 150 mm, particle size 3 μm) | 10 mM formic ammonium buffer (pH 6.0) and acetonitrile | Mass spectrometry | 411 m/z →191 m/z |
| RSP and Benzoic acid | HPLC | Waters Xterra C18 column (150 mm × 4.6 mm, 5 μm pore size) | Methanol: water (50:50% v/v) | UV | 275 nm |
| RSP | HPLC | C18 BDS Hypersil analytical column (3 μm, 100 × 4.6 mm I.D.) | Phosphate buffer (0.05 M, pH 3.7 with 25% H3PO4)–acetonitrile (70:30, v/v), | UV | 278 nm |
| RSP | HPLC-MS/MS | Alltima-C18 column (2.1 mm × 100 mm, 3 μm) | 0.1% formic acid-acetonitrile (40:60, v/v) | MS/MS | 411.3→191.1 m/z |
| RSP, a benzisoxazole derivative | HPLC-MS/MS | Inertsil HPLC column (2.1 × 150 mm, 5-µm particle size) | Solvent A was water with 0.1% formic acid, and solvent B was in gradient ratioAcetonitrile with 0.1% formic acid. | MS/MS | 411 to 191 m/z |
| RSP, Citalopram, Clozapine, Quetiapine, Levomepromazine, Perazine and Aripiprazole | HPLC-DAD | AXselect CSH Phenyl-Hexyl | Ethanol, acetate buffer at pH 3.5 and 0.025 mL−1 diethylamine | DAD | 200-400 nm range |
| HPLC-MS | Phenyl-Hexyl analytical column | Methanol, acetate buffer at pH 3.5 and 0.025 mL−1 mixed | MS/MS | 50–1000 m/z | |
| RSP | HPLC | Symmetry C18 column (5 μm size, 250 mm × 4.6 mm i.d.) | Methanol: acetonitrile (80: 20, v/v) | UV | 280 nm |
| RSP | HPLC | Gemini analytical column(250 × 4.6 mm, particle size 5 μm) | acetonitrile-potassium dihydrogen phosphate (45:55, v/v, pH 6.5; 0.05 M) | PDA | 237 nm |
| RSP | HPTLC | Silica gel 60 F254 | Methanol-ethyl acetate 80:20 (v/v) | densitometry | 285 nm |
Voltammetric methods
A voltammetry method has been reported for determination of RSP in pharmaceutical formulations using multiwalled carbon nanotube paste electrode as an easy, inexpensive and highly selective sensor [28]. Another adsorptive stripping differential pulse voltammetric determination of RSP with a multi walled carbon nanotube ionic liquid paste modified glassy carbon electrode was described [29].
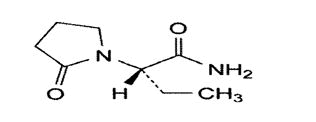
Levetiracetam (LVT)
- Chemical name: (2S)-2-(2-Oxopyrrolidin-1-yl)butanamide [9]
- Molecular formula: C8H14N2O2
- Molecular weight:2 gm/mol.
- Physical properties: It is White or almost white powder. It is Very soluble in water, soluble in acetonitrile, practically insoluble in hexane [9].
- Melting point: 112-115 °C [30].
Pharmacological action: LVT is a novel antiepileptic agent. It is used as an adjunctive therapy in the treatment of partial seizures [31]. LVT can prevent myoclonic jerks and generalizes epileptiform activity in patients with photosensitive epilepsy [32]. It is also used in veterinary medicine for similar purpose [33]. It is also used to treat neuropathic pain [34]. The bioavailability of LVT after oral administration is almost equal to 100% [35]. The biotransformation occurs by the enzymatic hydrolysis of acetamide group [36]. The metabolized drug is excreted through urine [35].
Methods of determination
Official methods
The BP proposes a HPLC-UV method using 0.25 m end-capped octadecyl silyl silica gel for chromatography column and mobile phase of 4:96 V/V 1.96 g/L solution of sulfuric acid, and acetonitrile, the retention time of LVT is about 10 minutes the detection was at 205 nm [9].
Spectroscopic methods
Literature describes different spectroscopic methods for determination of LVT, In the present study simple, precise, accurate, economical and reliable UV spectrophotometric method was developed for the estimation of LVT in tablet dosage form. The drug shows maximum absorption (maximum λmax) at 265.0 nm in distills water [37]. A simple UV spectrophotometric method has been developed for the estimation of LVT in tablet dosage form. The drug shows maximum absorption (maximum λmax) at 209.0 nm in triple distilled water [38]. Three simple, economical, precise, reliable and reproducible visible spectrophotometric methods (A, B and C) have been developed for the estimation of LVT in bulk as well as in Tablet formulation. The developed methods A, B and C are based on the formation of chloroform extractable complex of LVT with Bromocresol green (method A), Bromophenol blue (method B) and Bromothymol blue (method C) which shows absorbance maxima at 435 nm, 454 nm and 415 nm respectively [39]. Two simple, sensitive, reproducible, rapid and economical spectrophotometric methods are described for the determination of LVT in bulk and formulations. Both the methods are based on the formation of colored complexes of LVT with 2-chlorophenylhydrazine (method-A) and anthranilic acid (method-B) in alcoholic medium. Under the optimized conditions the complexes show an absorption maximum at 560 and 485 nm [40-50].
Chromatographic methods
Several chromatographic methods were described for the determination of the proposed drug either in pure or in combination with other drugs summarized in Table 2.
Table 2: Chromatographic methods for the determination of LVT in pure form or in combination with other drugs.
| Drugs | Method | Column | Mobile phase | Detector | λ |
|---|---|---|---|---|---|
| LVT and pyridoxine HCl | RP-HPLC | BDS Hypersil C8 (250×4.6â?¯mm, 5â?¯μm) | methanol and 25â?¯mm KH2PO4 buffer pHâ?¯3 (38.4:61.6, v/v) | UV | 214â?¯nm |
| LVT, lamotrigine, oxcarbazepine and carbamazepine | HPLC | Nova pack C18 reversed-phase column (4.6×250 mm, 5 μm) | Methanol:acetonitrile:water (pH of the aqueous phase, adjusted to pH 5 with 0.2 M phosphoric acid) in a ratio of 30:10:60 (v/v) | DAD | 230 nm |
| LVT, Methylparaben and propyl paraben | HPLC | RP C18 Hypersil BDS analytical column (150 mm×4.6 mm ID) | 0.05 M phosphate buffer pH 3.5: acetonitrile gradient elution | UV | 240 nm |
| LVT | LC-ESI-MS | 250×4.6 mm (i.d.) Discovery® (5 µm particle size) reversed-phase C18 | 0.1% formic acid in water–acetonitrile, in ratio of 85:15, v/v | UV for Quantification and Mass spectroscopy for Identification | 210 nm & m/z 50–190 |
| LVT | HPLC | Synergi 4-_m Hydro-RP, 150 mm×4 mm I.D. | Mixture of potassiumdihydrogen phosphate buffer (50 mM, pH 4.5) and acetonitrile (94:6, v/v) | UV-DAD | 205 nm |
| LVT | GC | Rtx-5 capillary column (cross bond 5% diphenyl/95% dimethyl polysiloxane) with a length of 30 meters and an internal diameter of 0.25 mm | Nitrogen | Flame ionization detector at 250°C using Synthetic air (flow rate of 100 ml/min),hydrogen (25 ml/min) | |
| Gabapentin, Lamotrigine, LVT, Monohydroxy Derivative of Oxcarbazepine, and Zonisamide | U-HPLC-MS/MS | Waters ACQUITY UPLC BEH C18 Column (2.1×30 mm, 1.7 μm particle size), | Solvent A (2 mmol/Lammonium acetate in Milli-Q filtered water with 0.1% formic acid) and solvent B (2mmol/L ammonium acetate in MeOH with 0.1% formic acid) in gradient elution | Mass spectrometry | 171 →69 m/z |
| LVT | UPLC-PDA | BEH C18 column (1.7 µm particle size and 100×2.1 mm i.d.) | Acetonitrile-phosphate buffer (pH=6.6; 0.01 M) (10/90 v/v) | UV-PDA | 215 nm |
| Lamotrigine, Zonisamide and LVT | HPTLC | Silica gel 60 F254 plates | Ethylacetate: methanol:ammonia (91:10:15 v/v/v) | densitometry | 312, 240 and 210 nm |
| LVT and oxcarbazepine | HPTLC | Silica gel 60 F 254 HPTLC plates | Toluene-acetone-methanol, 6:2:2 (v/v ), | densitometry | 200 and 261 nm |
Miscellaneous methods
A voltammetry method have been reported for determination of LVT by screen-printed based biosensors [51], and based on a silver nanoparticle modified carbon ionic liquid electrode [52]. Another capillary electrophoresis method has been reported [53].
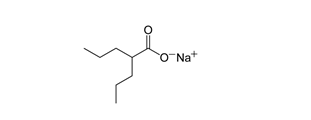
Sodium Valproate (VLP)
- Chemical name: Sodium 2-propylpentanoate [9].
- Molecular formula: C8H15NaO2
- Molecular weight:2 gm/mol.
- Physical properties: It is White or almost white, crystalline, hygroscopic powder. It is Very soluble in water, freely soluble in ethanol (96 per cent). It shows polymorphism [9].
- Melting point: 300°C.
Pharmacological action: VLP is the first line drug used for its unique anticonvulsant properties in the treatment of primary generalized seizures, partial seizures and myoclonic seizures. The mode of action is to stabilize the electrical activity in the brain by increasing the synthesis and decreasing the metabolism of gamma amino butyric acid [54].
Methods of determination
Official methods
The BP and USP proposes a Gas Chromatography (GC) and HPLC methods for quantitative analysis of VLP in formulation, respectively [9,55].
Spectroscopic methods
Literature describes different spectroscopic methods for determination of VLP. Four simple and direct spectrophotometric methods for determination of VLP were developed through charge transfer complexation reactions. The first method was based on the reaction of the drug with p-chloranilic acid in acetone to give a purple colored product with maximum absorbance at 524 nm. The second method was depending on the reaction of VLP with dichlone in dimethylformamide forming a reddish orange product measured at 490 nm. The third method was based upon the interaction of VLP and picric acid in chloroform resulting in the formation of a yellow complex measured at 415 nm. The fourth method involved the formation of a yellow complex peaking at 361 nm upon the reaction of the drug with iodine in chloroform. Experimental conditions affecting the color development were studied and optimized. Stoichiometry of the reactions was determined [56-63].
Chromatographic methods
Several chromatographic methods were described for the determination of the proposed drug either in pure or in combination with other drugs summarized in Table 3.
Table 3: Chromatographic methods for the determination of VLP in pure form or in combination with other drugs.
| Drugs | Method | Column | Mobile phase | Detector | λ |
|---|---|---|---|---|---|
| VLP and its five metabolites | HPLC–MS/MS | Hypersil GOLD C18 | Acetonitrile: 10 mm ammonium acetate solution (90:10, v/v) | Mass spectroscopy | m/z 143.183 → 143.183 |
| VLP and 2-propyl-4-pentenoic acid | HPLC-UV | Waters Xterra MS C18 column (5 μm, 4.6 mm × 150 mm, Waters, Milford, USA) | Methanol/water (76:24, v/v) | UV | 254 nm |
| Phenobarbital, VLP, phenytoin sodium and carbamazepine |
HPLC-MS | XDB-C18 (2.1mm×150.0 mm, 3.5 μm) | 0.05% ammonium acetate in water methanol (47â?¶ 53) | Mass spectroscopy | |
| 9-Fluorenylmethyl chloroformate as a fluorescence-labeling reagent for derivatization of carboxylic acid moiety of VLP | HPLC | Shim pack CLC-ODS (Shimadzu, Kyoto, Japan), 150 mm×4.6 mmI.D., 5 _m particle size, which was protected by a Shim-pack G-ODSguard column (1 cm×4.0 mm I.D., 5 _m particle size). | A mixture ofacetonitrile and distilled water (78:22) | Spectro-fluorometric | 265 and 315 nm |
| VLP and two metabolites | GC-MS | HP-lcross-linked methyl siloxane, 60 mxO.25 mm i.d., | Helium | Mass spectroscopy | m/z 199 |
| VLP | GCâ?FID | 30 m×0.32 mm i.d.×0.25 µm film thickness BPâ?10 | Carrier (Helium, 99.999%) 2.0 mL/min, makeâ?up (Nitrogen, 99.999%) 30 mL/min | Flame ionization | Temperatures were set at 250 °C and 280 °C respectively using hydrogen and air (for FID) 40 and 300 mL/min, respectively. |
Miscellaneous methods
A potentiometric method for quantitative analysis of VLP in pharmaceutical preparations by a Valproate-Selective Electrode [63] and by ion selective sensor based on conducting polypyrrole films [64]. A direct method for determination of VLP acid in biological fluids by capillary electrophoresis with contactless conductivity detection has been reported [65].
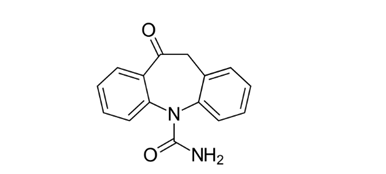
Oxcarbazepine (OXZ)
- Chemical name: 10,11-dihydro-10-oxo-5H-dibenz [b,f]azepine-5-carboxamide [9].
- Molecular formula: C15H12N2O2
- Molecular weight:3 gm/mol.
- Physical properties: It is White to faintly orange crystalline powder. It is Slightly soluble in chloroform, dichloromethane, acetone, and methanol and practically insoluble in ethanol, ether, and water [9].
- Melting point:5 °C.
Pharmacological action: OXZ is a new antiepileptic drug that has been registered in more than 50 countries worldwide since 1990 and recently received approval in the United States and the European Union. OXZ has a more favorable pharmacokinetic profile than carbamazepine [66]. It is rapidly absorbed after oral administration and undergoes rapid and almost complete reductive metabolism to form the pharmacologically active 10-monohydroxy derivative [66].
Methods of determination
Official methods
The BP and USP proposes a HPLC method for quantitative analysis of OXZ [9][67].
Spectroscopic methods
Literature describes different spectroscopic methods for determination of OXZ. A Simple, accurate, rapid and sensitive method was reported for the estimation of OXZ in tablets. The estimation is based on the reduction of ferric ions in its salt form to ferrous ions by the drug, which in presence of potassium ferricyanide produces green colored chromogen measured at 770 nm against reagent blank [68]. Two simple, selective and stability indicating UV-spectrophotometric methods have been developed and validated for assay of OXZ in bulk drug and in its dosage forms. Proposed methods were based on measurement of absorbance of OXZ either in methanol (method A) or in acetonitrile (method B) at 254 nm [69]. Spectrophotometric methods were described for the determination of OXZ in bulk drug and in tablets. The methods used N-bromosuccinimide and bromopyrogallol red as reagents. The method involved the addition of known excess of N-bromosuccinimide to an acidified solution of OXZ followed by the determination of the unreacted N-bromosuccinimide by reacting with bromopyrogallol red and measuring the absorbance of the unreacted dye at 460 nm [70].
Chromatographic methods
Several chromatographic methods were described for the determination of the proposed drug either in pure or in combination with other drugs summarized in Table 4.
Table 4: Chromatographic methods for the determination of OXZ in pure form or in combination with other drugs.
| Drugs | Method | Column | Mobile phase | Detector | λ |
|---|---|---|---|---|---|
| OXZ | HPLC | Hypersil BDS C18 column (250 mm × 4.6 mm, 5 μ) | Mixture of Acetonitrile, methanolAnd pH adjusted by Triethylamine | 215 nm | UV |
| OXZ | HPLC | Diamonsil C18 column | acetonitrile, potassium phosphate monobasic buffer (pH 6.8) and water (36:8:56, v/v) | UV | 255 nm |
| OXZ and its main metabolites | HPLC-UV | a X-TERRA C18 column | 20 mM KH2PO4, acetonitrile, and n-octylamine (76:24:0.05, v/v/v) | UV | 237 nm |
| Carbamazepine, OXZ, and Eslicarbazepine | HPLC-UV | Lichrocart®Purospher® Star (C18, 3 µm, 55 mm × 4 mm) | Water, methanol, and acetonitrile in the ratio 64:30:6 | UV | 235 nm |
| OXZ | HPTLC | 20 cm × 20 cm aluminum foil platescoated with silica gel G60F254 | Ethyl acetate-toluene-methanol 7.0:2.0:1.0 (v/v) | densitometry | 254 nm |
| OXZ | HPTLC | Precoated silica gel aluminum plate 60 F254, (20 × 10 cm with 250 mm thickness; | Ethyl Acetate: Methanol (6:4 v/v) | Densitometryin the fluorescence mode | 366 nm |
| Carbamazepine, OXZ, and Eslicarbazepine acetate | UPLC-MS/MS | Intersil® ODS column (250 x 4.6 mm, 5 μm) | acetonitrile, methanol, 0.5% formic acid in water (5:40:55, v/v/v) (i.e. 45% organic: 55% aqueous) | Tandem mass spectrometry | 208.14m/z |
Miscellaneous methods
Titrimetric method was described for the determination of OXZ in bulk drug and in tablets. The methods use N-bromosuccinimide and bromopyrogallol red as reagents. An acidified solution of OXC was titrated directly with N-bromosuccinimide using methyl orange as indicator [70]. The catalytic effect of silver nanoparticles in the development of disposable screen-printed sensors for the analysis of the drug OXZ has been demonstrated. Mercury film and metallic nanoparticles modified screen-printed carbon electrodes have been tested for the analysis of OXZ using differential pulse adsorptive stripping voltammetry. Mercury coated screen-printed electrodes were obtained by means of electrodeposition at a fixed potential from a Hg(II) solution. Nanoparticle modified electrodes were obtained also by electrode position on the screen-printed carbon surface of silver and gold nanoparticles. Among the electrodes tested only silver nanoparticle modified screen-printed carbon electrodes gave rise to an analytical signal viable for the determination of OXZ [71-77].
Conclusion
This literature review represents an up to date survey about all reported methods that have been developed for determination of certain antipsychotic drugs such as risperidone, levetiracetam, sodium valproate and oxcarbazepine in their pure form, combined form with other drugs, combined form with degradation products, and in biological samples such as liquid chromatography, spectrophotometry, and voltammetry.
References
- Kwan P, Sander J (2004) The natural history of epilepsy: an epidemiological view. J Neurol Neurosurg Psychiatry 75:1376-1381
- Kwan P, Schachter S, brodie MJ (2000) Early identification of refractory epilepsy. N Engl J Med 342:314-319
- Patsalos PN (2002) The importance of drug interactions in epilepsy therapy. Epileps 4:365-385
- Patsalos PN, Perucca E (2003) clinically important drug interactions in epilepsy: general features and interactions between antiepileptic drugs. Lancet Neurol 2:347-356
- Johannessen Landmark C, Patsalos PN (2010) Drug interactions involving the new second-and third-generation antiepileptic drugs. Expert Rev Neurother 10:119-140
[Crossref] [Google Scholar] [Indexed]
- Patsalos PN, Perucca E (2003) clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet Neurol 2:473-481
- Landmark CJ, Patsalos PN (2008) Interactions between antiepileptic drugs and herbal medicines. Bol Latinoam Caribe Plantas Med Aromat 7:109-118[Crossref]
- Stahl SM (2000) Essential psychopharmacology: Neuroscientific basis and practical applications: Cambridge university press
- The British P, Volumes II and III, Her Majesty’s Stationery Office2013, London, UK.
- Suthar AP (2009) Determination of Risperidone and forced degradation behavior by HPLC in tablet dosage form. Int J Pharmtech Res 1:568-574
- Haas M (2009) Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: A randomized, double-blind, placebo-controlled study. Bipolar Disord 11:687-700
- Shea S (2004) Risperidone in the Treatment of Disruptive Behavioral Symptoms in Children With Autistic and Other Pervasive Developmental Disorders. Pediatrics114:634-641
- Kulkarni S, Chhabra G, Shivani M (2012) Development and validation of UV spectrophotometric method for the determination of risperidone in bulk and tablets formulation. Int J Pharmac Chem Res 1:1-5
- Orkoula MG, Kontoyannis CG (2008) Non-destructive quantitative analysis of risperidone in film-coated tablets. J Pharm Biomed Anal 47:631-635
- Kumar MS (2010) Development of analytical method for risperidone by UV spectrophotometry. Int J Pharmac Chem Res 1:122-126
- Vasagam GA (2010) Development of analytical method for Risperidone by UV Spectrophotometry using methanol as a solvent. Sch Res J 2:3-4
- Ruan CJ (2018) Quantitative determination of risperidone, paliperidone and olanzapine in human serum by liquid chromatography tandem mass spectrometry coupled with on-line solid-phase extraction. Biomed Chromatogr 32:4209-4209
- Mennickent S (2018) Stability indicating hplc method for quantification of risperidone in tablets. J Chil Chem Soc 63:4150-4154
- Tonooka K (2018) Sensitive Liquid Chromatography/Tandem Mass Spectrometry Method for the Simultaneous Determination of Risperidone, Olanzapine, Quetiapine, Clozapine, Ziprasidone, Perospirone, Aripiprazole and Blonanserin in Human Serum. Am J Analyt Chem 9:88-89
[Crossref] [Google Scholar] [Indexed]
- Bhusnure OG (2015) QbD approach for analytical method development of anti-pschotic drug. Der Pharmacia Lettre 7:62-70
- Avenoso A (2000) Determination of risperidone and its major metabolite 9-hydroxyrisperidone in human plasma by reversed-phase liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Appl 746:173-181
- Huang Mz (2008) Determination of risperidone in human plasma by HPLC-MS/MS and its application to a pharmacokinetic study in Chinese volunteers. J Zhejiang Univ Sci B 9:114-120
- Moody DE (2004) A high-performance liquid chromatographic-atmospheric pressure chemical ionization-tandem mass spectrometric method for determination of risperidone and 9-hydroxyrisperidone in human plasma. J Anal Toxicol 28:494-497
- Petruczynik A (2016) Determination of some psychotropic drugs in serum and saliva samples by HPLC-DAD and HPLC MS. J Pharm Biomed Anal 127:68-80
- Dedania ZR (2011) Stability indicating HPLC determination of risperidone in bulk drug and pharmaceutical formulations. Int J Anal Chem 2011:124917-124923
[Crossref] [Google Scholar] [Indexed]
- Svirskis DJ,Travas-Sejdic, and S. Garg, A (2011) stability indicating HPLC method for the determination of electrochemically controlled release of risperidone. J Chromatogr Sci 49:780-785
- Patel R (2010) HPTLc method development and validation for analysis of risperidone in formulations, and in-vitro release study. Acta Chromatographica 22:549-567
- Afkhami A, Ghaedi H (2012) Multiwalled carbon nanotube paste electrode as an easy, inexpensive and highly selective sensor for voltammetric determination of Risperidone. Anal Methods 4:1415-1415
- Arvand M, Pourhabib A (2013) Adsorptive Stripping Differential Pulse Voltammetric Determination of Risperidone with a Multi-Walled Carbon Nanotube-Ionic Liquid Paste Modified Glassy Carbon Electrode. J Chin Chem Soc 60:63-72
- Franco AS (2008) Bioequivalence of two formulations of levetiracetam. Int J Clin Pharmacol Ther 46:591-596
- Glauser TA (2006) Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology 66:1654-1660
- Grünewald, R (2005) Levetiracetam in the treatment of idiopathic generalized epilepsies. Epilepsia 46:154-160
- Moore SA (2010) Levetiracetam pharmacokinetics in healthy dogs following oral administration of single and multiple doses. Am J Vet Res 71:337-341
- Wiffen PJ (2014) Levetiracetam for neuropathic pain in adults. Cochrane Database Syst Rev 2014:10943-1094
- Patsalos PN (2004) Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet 43:707-724
- Mirsonbol SZ (2014) Antimicrobial efficacy of the methylparaben and benzoate sodium against selected standard microorganisms. India j fundam appl life sci 4:363-367
- Bhusnure omprakash CS, Gholve SB, Kumar JS (2018) Development of the up spectrophotometric method of levetiracetam in bulk drug dosage form and stress degradation study. Int J Pharm Sci Res 8:532-539
- Ravisankar P (2015) A simple validated UV spectrophotometric method for quantitative analysis of Levetiracetam in pharmaceutical dosage form. IJRPB 3:380-381
- Srinivasu KJVR (2008) Extractive spectrophotometric determination of levetiracetam in pharmaceutical formulations. Orient J Chem 24:1013-1016
- Muralikrishna C (2012) Spectrophotometric Determination of Levetiracetam by Developing Coloured Complexes with 2-Chlorophenylhydrazine and Anthranilic Acid. Asian J Chem 24:1855-185
- Hashem H, El-Sayed HM (2018) Quality by design approach for development and validation of a RP-HPLC method for simultaneous determination of co-administered levetiracetam and pyridoxine HCl in prepared tablets. Microchem j 143:55-63
- Ibrahim FA (2016) Sensitive inexpensive HPLC determination of four antiepileptic drugs in human plasma: application to PK studies. Bioanalysis 8:2219-2234
- Abd el-hay S, Mohram M S (2016) Development and Validation of New RP-HPLC Method for Simultaneous Determination of Methyl and Propyl Parabens with Levetiracetam in Pure Form and Pharmaceutical Formulation. Chrom Res Inter 2016:1-5
- Hadad GM, Abdel Salam RA, Abdel Hameed EA (2013) Quantitative determination of Levetiracetam in human urine using HPLC-UV and its identification by LC-ESI-MS. J Liq Chromatogr Relat 36:2568-2579
- Contin M (2008) Simple and validated HPLC–UV analysis of levetiracetam in deproteinized plasma of patients with epilepsy. J Chromatogr B:Anal Technol Biomed Life Sci 873:129-32
- Indupriya M (2011) Quantitative determination of levetiracetam by gas chromatography using ethyl chloroformate as a derivatizing reagent in pure and pharmaceutical preparation. Int J Pharm Technol 3:1694-1701
- Palte MJ (2018) Development and Validation of an Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry Method for the Concurrent Measurement of Gabapentin, Lamotrigine, Levetiracetam, Monohydroxy Derivative of Oxcarbazepine, and Zonisamide Concentrations in Serum in a Clinical Setting. Ther Drug Monit 40:469-476
- Olah E (2012) Determination of ng/mL levetiracetam using ultra-high-performance liquid chromatography–photodiode absorbance. J Chromatogr Sci 50:253-258
- Antonilli L (2011) Development and validation of an analytical method based on high performance thin layer chromatography for the simultaneous determination of lamotrigine, zonisamide and levetiracetam in human plasma. J Pharm Biomed Anal 56:763-770
- Sheshashena Reddy T, Sita Devi P (2007) Validation of a high-performance thin-layer chromatographic method with densitometric detection for quantitative analysis of two anticonvulsants in tablets. JPC -Modern TLC 20:451-456
- Alonso-Lomillo MA (2009) Electrochemical determination of levetiracetam by screen-printed based biosensors. Bioelectrochem 74:306-309
- Arkan E (2014) A novel electrochemical sensor based on a silver nanoparticle modified carbon ionic liquid electrode for selective and sensitive determination of levetiracetam in pharmaceutical tablets and blood plasma samples. Anal Methods 6:2197-2197
- Shihabi ZK, Oles K, Hinsdale M (2003) Analysis of the antiepileptic drug keppra by capillary electrophoresis. J Chromatogr A 1004:9-12
- Willmore LJ (2003) Divalproex and epilepsy. Psychopharmacol Bull 37:43-53
- United States Pharmacopeial Convention (2013) USP36 NF31, United State Pharmacopoeia National Formulary. United States Pharmacopeial, Montgomery
- Belal TS (2016) Validated spectrophotometric methods for determination of sodium valproate based on charge transfer complexation reactions. Spectrochim Acta A Mol Biomol 155:47-53
- Wen D (2018) A rapid and simple HPLC–MS/MS method for the simultaneous quantification of VLP acid and its five metabolites in human plasma and application to study pharmacokinetic interaction in Chinese epilepsy patients. J Pharm Biomed Anal 149:448-456
- Chen Zj (2012) Simultaneous determination of VLP acid and 2-propyl-4-pentenoic acid for the prediction of clinical adverse effects in Chinese patients with epilepsy. Seizure 21:110-117
- Dai B (2011) Determination the concentrations of phenobarbital, sodium valproate, phenytoin sodium and carbamazepine in human plasma for simultaneous quatification by HPLC-MS. Chinese J Pharmacol Toxicol 3:14-14
- Mohammadi B (2012) 9-Fluorenylmethyl chloroformate as a fluorescence-labeling reagent for derivatization of carboxylic acid moiety of sodium valproate using liquid chromatography/tandem mass spectrometry for binding characterization: A human pharmacokinetic study. J Chromatogr B 880:12-18
- Amini-Shirazi N (2010) Determination of VPA and its two important metabolites in Iranian overdosed patients. Inter J Pharm 6:854-862
- Sobhi HR (2010) Quantitation of VLP acid in pharmaceutical preparations using dispersive liquid liquid microextraction followed by gas chromatography flame ionization detection without prior derivatization. Drug Test Anal 2:362-366
- Suzuki H, Akimoto K, Nakagawa H (1991) Quantitative analysis of sodium valproate in pharmaceutical preparations by a valproate-selective electrode. Chem Pharm Bull 39:133-136
- Sabah SM, Aghamohammadi N, Alizadeh (2006) Solid-State valproate ion selective sensor based on conducting polypyrrole films for determination of valproate in pharmaceutical preparations. Sensors and Actuators B: Chemical 114:489-496
- Belin GK, Krähenbühl S, Hauser PC (2007) Direct determination of VLP acid in biological fluids by capillary electrophoresis with contactless conductivity detection. J Chromatogr B 847:205-209
- Glauser TA (2001) Oxcarbazepine in the treatment of epilepsy. Pharmaco: J Hum Pharmacol Drug Thera 21:904-919
- Ramaa C (2006) Spectrophotometric method for the estimation of oxcarbazepine in tablets. India j pharma sci 68:265-266
- Basavaiah K (2011) Development and validation of stability indicating spectrophotometric methods for determination of oxcarbazepine in pharmaceuticals. J Sci Indus Rese (JSIR)70:346-351
- Rajendraprasad N, Basavaiah K, Vinay KB (2011) Titrimetric and spectrophotometric assay of oxcarbazepine in pharmaceuticals using N-bromosuccinimide and bromopyrogallol. Red Int J Anal Chem Rajendraprasad N, Basavaiah K, Vinay KB (2011) Titrimetric and spectrophotometric assay of oxcarbazepine in pharmaceuticals using N-bromosuccinimide and bromopyrogallol. Red Int J Anal Chem 2011(1687-8760):138628
- Pradhan DP, Annapurna MM (2019) Stability indicating HPLC-DAD method for the determination of Oxcarbazepine-An Anticonvulsant. Res J Pharm Technol 12:723-728
- Qi ML (2003) LC method for the determination of oxcarbazepine in pharmaceutical preparations. J Pharm Biomed Anal 31:57-62
- Kimiskidis V (2007) Development and validation of a high performance liquid chromatographic method for the determination of oxcarbazepine and its main metabolites in human plasma and cerebrospinal fluid and its application to pharmacokinetic study. J Pharm Biomed Ana 43:763-768
- Fortuna A (2010) Development and validation of an HPLC-UV method for the simultaneous quantification of carbamazepine, oxcarbazepine, eslicarbazepine acetate and their main metabolites in human plasma. Anal Bioanal Chem 397:1605-1615
- Gandhimathi M, Ravi T (2008) Rapid HPTLC analysis of oxcarbazepine in human plasma. JPC-Modern TLC 21:437-439
- Bhoite DS (2013) Development and validation of stability indicating HPTLC method for determination of oxcarbazepine in bulk and pharmaceutical formulation. Int J Pharm Pharm Sci 5:127-132
- Farouk F, ElKady EF, Azzaz HME Y (2017) Simultaneous UPLC-MS/MS determination of antiepileptic agents for dose adjustment. Biomed Chromatogr 31:31
- Domínguez-Renedo O (2010) Oxcarbazepine analysis by adsorptive stripping voltammetry using silver nanoparticle-modified carbon screen-printed electrodes. Sensor Letters 8:268-272
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences